Abstract
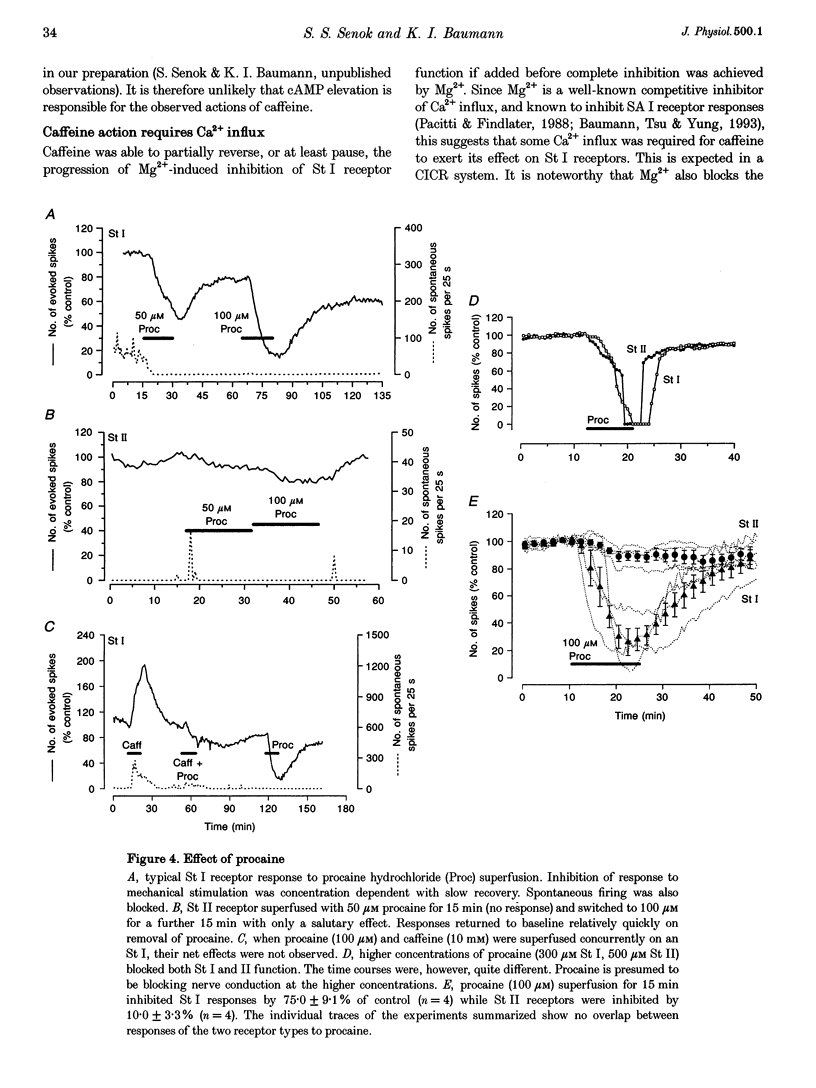
1. Single unit recordings were made from Merkel cell (sinus hair type I; St I) and sinus hair type II (St II) mechanoreceptors in isolated rat vibrissae. Responses were determined as the number of spikes evoked by controlled mechanical displacement of the hair shaft for 5 s every 30 s. 2. Superfusion of caffeine (10 mM) increased the responses of Merkel cell receptors by 50-180% of control (mean +/- S.E.M., 64 +/- 12.6%, n = 6, P < 0.001). Similar concentrations of caffeine inhibited St II receptor responses by 20-60% (mean +/- S.E.M., 35 +/- 8%, n = 5, P < 0.01). In both receptor types, caffeine induced a low-frequency increase in spontaneous firing. 3. When Merkel cell receptor responses were completely blocked by superfusion of high Mg2+-containing solution (to competitively block Ca2+ influx) caffeine had no effect when added after complete inhibition, but when added during partial inhibition of responses, the Mg2+-induced inhibition was transiently reversed or halted. This suggests that Ca2+ influx was a prerequisite for the action of caffeine. 4. Ryanodine (1 microM) increased the responses of Merkel cell receptors to mechanical stimulation by 7-60% (mean +/- S.E.M., 32 +/- 10.9 %, n = 5, P < 0.05) but had no effect on St II receptor responses. 5. The Ca2+-induced Ca2+ release (CICR) inhibitor procaine inhibited St I receptor responses in a concentration-dependent manner. Near-maximal inhibition was attained with 100 microM procaine. In four St I units, mean responses were depressed to 25% of control values. When both procaine (100 microM) and caffeine (10 mM) were introduced together, no net effect was seen. The responses of St II receptors were little affected by up to 100 microM procaine superfusion. 6. It is concluded that the mechano-electrical transduction process in St I receptors (but not St II) includes a CICR pathway. Taken with previous findings on the role of Merkel cells, it is likely that CICR is occurring in the Merkel cells.
Full text
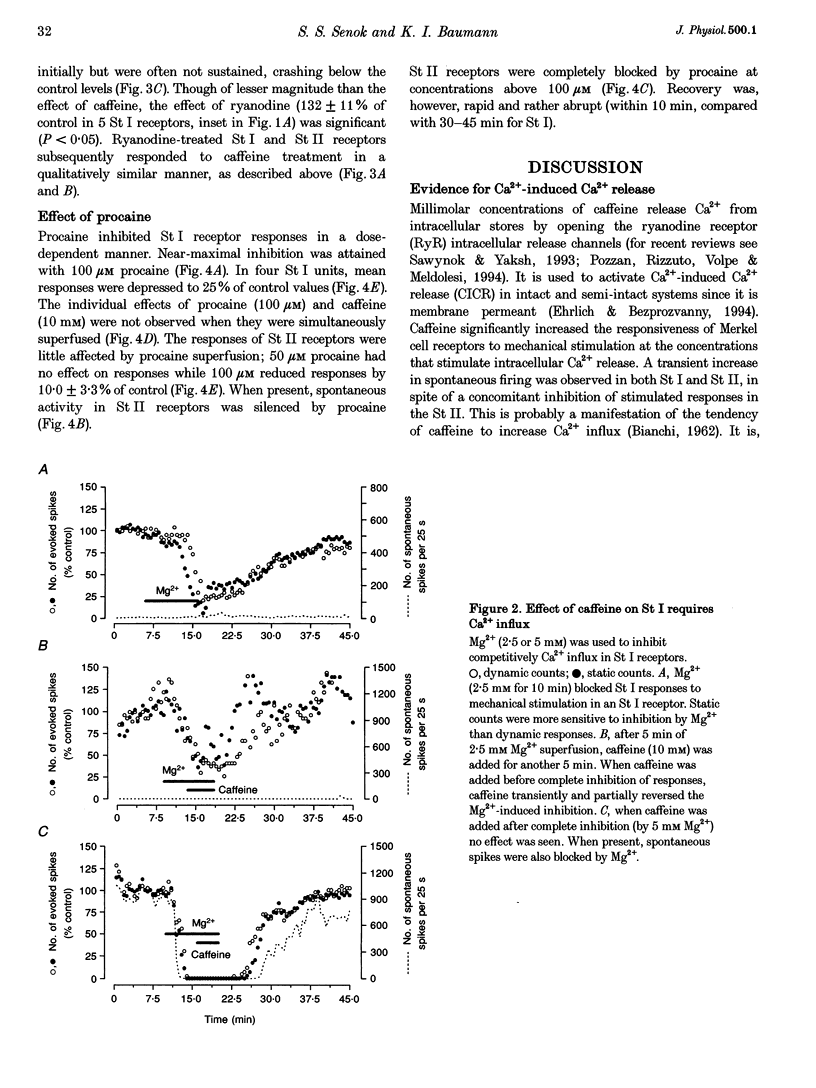
PDF

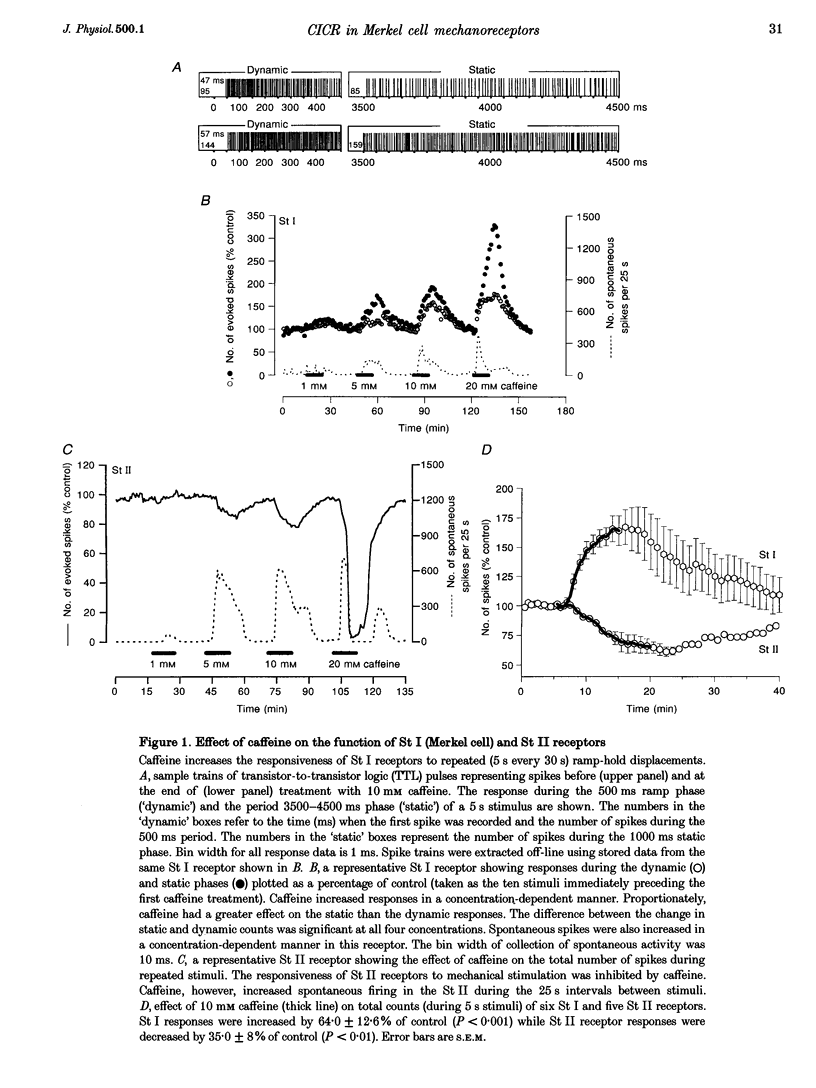
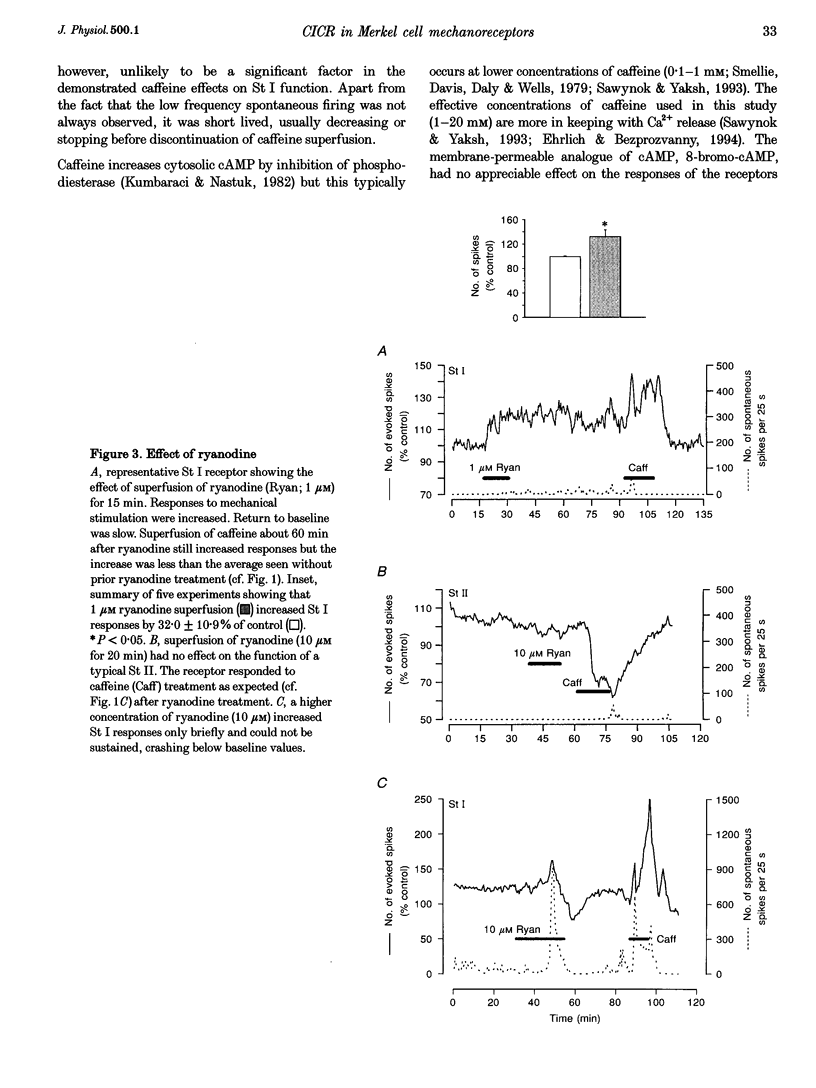



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BIANCHI C. P. Kinetics of radiocaffeine uptake and release in frog sartorius. J Pharmacol Exp Ther. 1962 Oct;138:41–47. [PubMed] [Google Scholar]
- Baumann K. I., Chan E., Halata Z., Senok S. S., Yung W. H. An isolated rat vibrissal preparation with stable responses of slowly adapting mechanoreceptors. Neurosci Lett. 1996 Jul 26;213(1):1–4. doi: 10.1016/0304-3940(96)12813-5. [DOI] [PubMed] [Google Scholar]
- Bezprozvanny I., Bezprozvannaya S., Ehrlich B. E. Caffeine-induced inhibition of inositol(1,4,5)-trisphosphate-gated calcium channels from cerebellum. Mol Biol Cell. 1994 Jan;5(1):97–103. doi: 10.1091/mbc.5.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretag A. H. Synthetic interstitial fluid for isolated mammalian tissue. Life Sci. 1969 Mar 1;8(5):319–329. doi: 10.1016/0024-3205(69)90283-5. [DOI] [PubMed] [Google Scholar]
- Brown G. R., Sayers L. G., Kirk C. J., Michell R. H., Michelangeli F. The opening of the inositol 1,4,5-trisphosphate-sensitive Ca2+ channel in rat cerebellum is inhibited by caffeine. Biochem J. 1992 Mar 1;282(Pt 2):309–312. doi: 10.1042/bj2820309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan E., Yung W. H., Baumann K. I. Cytoplasmic Ca2+ concentrations in intact Merkel cells of an isolated, functioning rat sinus hair preparation. Exp Brain Res. 1996 Mar;108(3):357–366. doi: 10.1007/BF00227259. [DOI] [PubMed] [Google Scholar]
- Ehrlich B. E., Bezprozvanny I. Intracellular calcium release channels. Chin J Physiol. 1994;37(1):1–7. [PubMed] [Google Scholar]
- Galione A., Lee H. C., Busa W. B. Ca(2+)-induced Ca2+ release in sea urchin egg homogenates: modulation by cyclic ADP-ribose. Science. 1991 Sep 6;253(5024):1143–1146. doi: 10.1126/science.1909457. [DOI] [PubMed] [Google Scholar]
- Gottschaldt K. M., Iggo A., Young D. W. Functional characteristics of mechanoreceptors in sinus hair follicles of the cat. J Physiol. 1973 Dec;235(2):287–315. doi: 10.1113/jphysiol.1973.sp010388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., McBride D. W., Jr Rapid adaptation of single mechanosensitive channels in Xenopus oocytes. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7462–7466. doi: 10.1073/pnas.89.16.7462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose K., Iino M., Endo M. Caffeine inhibits Ca(2+)-mediated potentiation of inositol 1,4,5-trisphosphate-induced Ca2+ release in permeabilized vascular smooth muscle cells. Biochem Biophys Res Commun. 1993 Jul 30;194(2):726–732. doi: 10.1006/bbrc.1993.1882. [DOI] [PubMed] [Google Scholar]
- Iggo A., Muir A. R. The structure and function of a slowly adapting touch corpuscle in hairy skin. J Physiol. 1969 Feb;200(3):763–796. doi: 10.1113/jphysiol.1969.sp008721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda I., Yamashita Y., Ono T., Ogawa H. Selective phototoxic destruction of rat Merkel cells abolishes responses of slowly adapting type I mechanoreceptor units. J Physiol. 1994 Sep 1;479(Pt 2):247–256. doi: 10.1113/jphysiol.1994.sp020292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoyi M. A., Dalziel H. H., Zhang L., Bjur R. A., Gerthoffer W. T., Buxton I. L., Westfall D. P. [Ca2+]i-sensitive, IP3-independent Ca2+ influx in smooth muscle of rat vas deferens revealed by procaine. Br J Pharmacol. 1993 Dec;110(4):1353–1358. doi: 10.1111/j.1476-5381.1993.tb13968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumbaraci N. M., Nastuk W. L. Action of caffeine in excitation-contraction coupling of frog skeletal muscle fibres. J Physiol. 1982 Apr;325:195–211. doi: 10.1113/jphysiol.1982.sp014145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner G. Ryanodine receptor/Ca2+ release channels and their regulation by endogenous effectors. Annu Rev Physiol. 1994;56:485–508. doi: 10.1146/annurev.ph.56.030194.002413. [DOI] [PubMed] [Google Scholar]
- Okwuasaba F. K., Otubu J. A., Udoh F. V. Mode of inhibitory actions of acute and chronic chloroquine administration on the electrically stimulated mouse diaphragm in vitro. Br J Pharmacol. 1990 Sep;101(1):133–139. doi: 10.1111/j.1476-5381.1990.tb12102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacitti E. G., Findlater G. S. Calcium channel blockers and Merkel cells. Prog Brain Res. 1988;74:37–42. doi: 10.1016/s0079-6123(08)62995-7. [DOI] [PubMed] [Google Scholar]
- Parker I., Ivorra I. Caffeine inhibits inositol trisphosphate-mediated liberation of intracellular calcium in Xenopus oocytes. J Physiol. 1991 Feb;433:229–240. doi: 10.1113/jphysiol.1991.sp018423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzan T., Rizzuto R., Volpe P., Meldolesi J. Molecular and cellular physiology of intracellular calcium stores. Physiol Rev. 1994 Jul;74(3):595–636. doi: 10.1152/physrev.1994.74.3.595. [DOI] [PubMed] [Google Scholar]
- Sawynok J., Yaksh T. L. Caffeine as an analgesic adjuvant: a review of pharmacology and mechanisms of action. Pharmacol Rev. 1993 Mar;45(1):43–85. [PubMed] [Google Scholar]
- Senok S. S., Baumann K. I., Halata Z. Selective phototoxic destruction of quinacrine-loaded Merkel cells is neither selective nor complete. Exp Brain Res. 1996 Aug;110(3):325–334. doi: 10.1007/BF00229133. [DOI] [PubMed] [Google Scholar]
- Senok S. S., Halata Z., Baumann K. I. Chloroquine specifically impairs Merkel cell mechanoreceptor function in isolated rat sinus hairs. Neurosci Lett. 1996 Aug 23;214(2-3):167–170. doi: 10.1016/0304-3940(96)12906-2. [DOI] [PubMed] [Google Scholar]
- Smellie F. W., Davis C. W., Daly J. W., Wells J. N. Alkylxanthines: inhibition of adenosine-elicited accumulation of cyclic AMP in brain slices and of brain phosphodiesterase activity. Life Sci. 1979 Jun 25;24(26):2475–2482. doi: 10.1016/0024-3205(79)90458-2. [DOI] [PubMed] [Google Scholar]
- Sukharev S. I., Martinac B., Arshavsky V. Y., Kung C. Two types of mechanosensitive channels in the Escherichia coli cell envelope: solubilization and functional reconstitution. Biophys J. 1993 Jul;65(1):177–183. doi: 10.1016/S0006-3495(93)81044-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana T. The Merkel cell: recent findings and unresolved problems. Arch Histol Cytol. 1995 Oct;58(4):379–396. doi: 10.1679/aohc.58.379. [DOI] [PubMed] [Google Scholar]
- Torre V., Ashmore J. F., Lamb T. D., Menini A. Transduction and adaptation in sensory receptor cells. J Neurosci. 1995 Dec;15(12):7757–7768. doi: 10.1523/JNEUROSCI.15-12-07757.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita Y., Akaike N., Wakamori M., Ikeda I., Ogawa H. Voltage-dependent currents in isolated single Merkel cells of rats. J Physiol. 1992 May;450:143–162. doi: 10.1113/jphysiol.1992.sp019120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita Y., Ogawa H., Taniguchi K. Differential effects of manganese and magnesium on two types of slowly adapting cutaneous mechanoreceptor afferent units in frogs. Pflugers Arch. 1986 Feb;406(2):218–224. doi: 10.1007/BF00586686. [DOI] [PubMed] [Google Scholar]
- de Duve C., de Barsy T., Poole B., Trouet A., Tulkens P., Van Hoof F. Commentary. Lysosomotropic agents. Biochem Pharmacol. 1974 Sep 15;23(18):2495–2531. doi: 10.1016/0006-2952(74)90174-9. [DOI] [PubMed] [Google Scholar]