Abstract
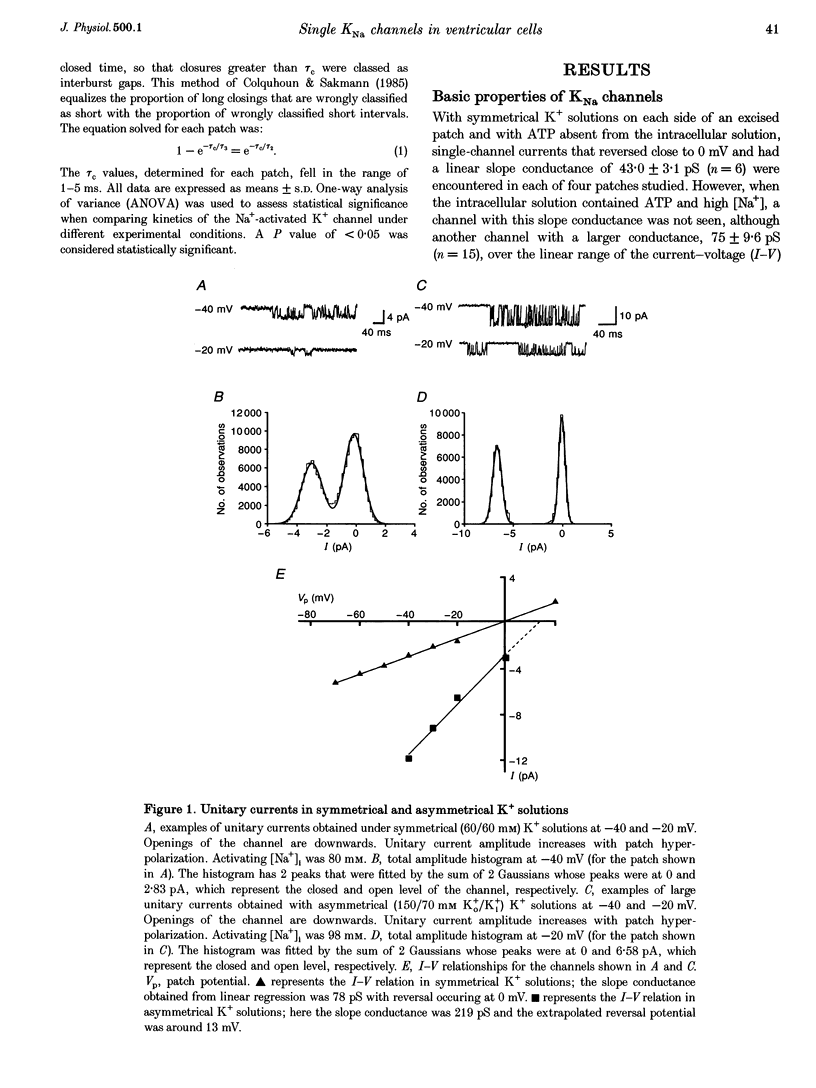
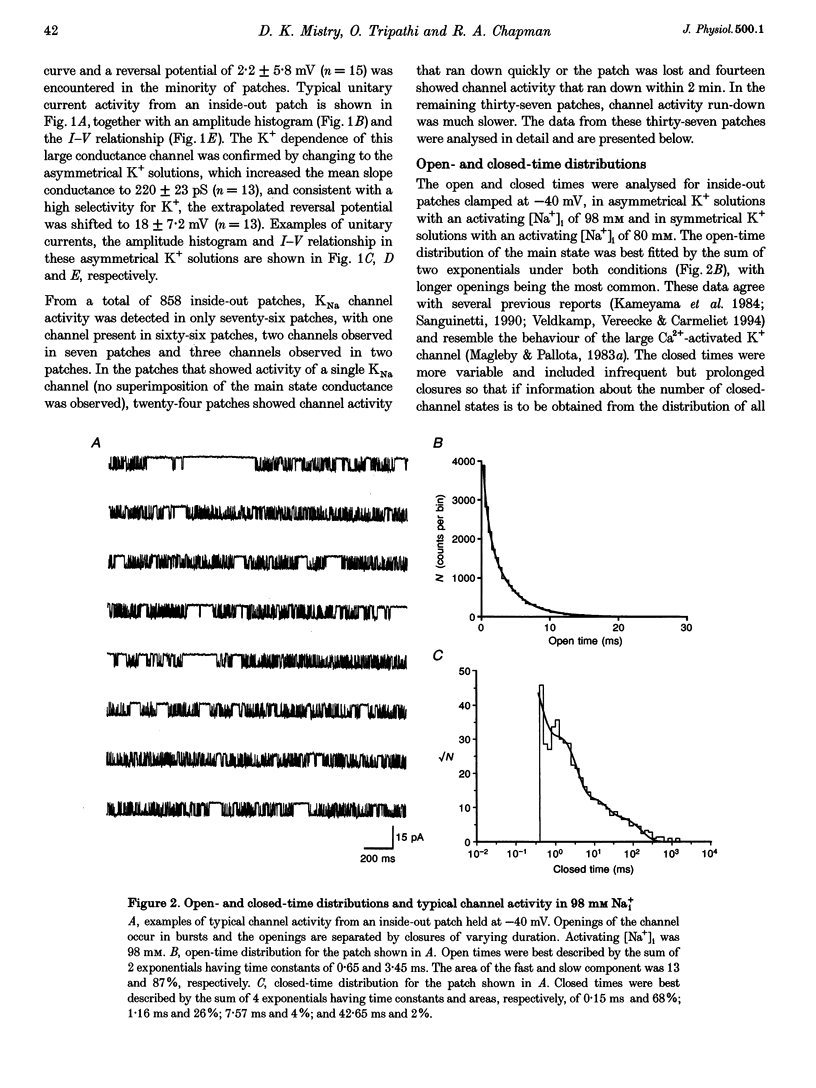
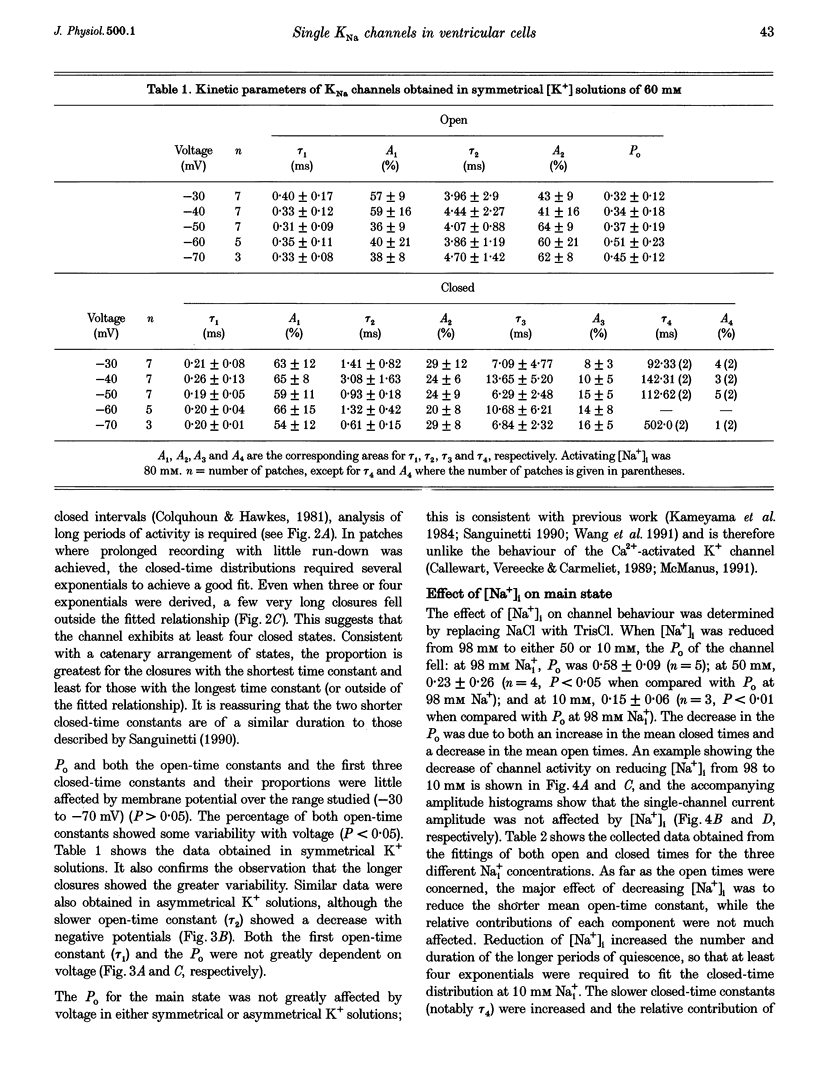
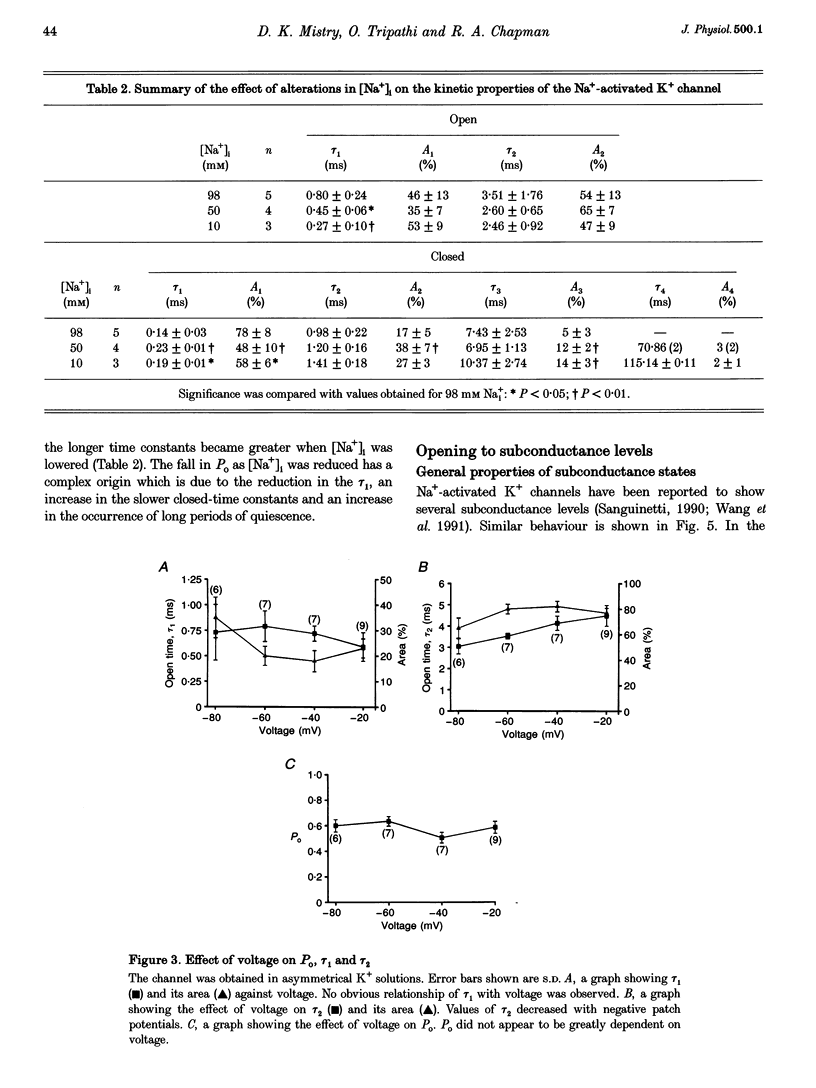
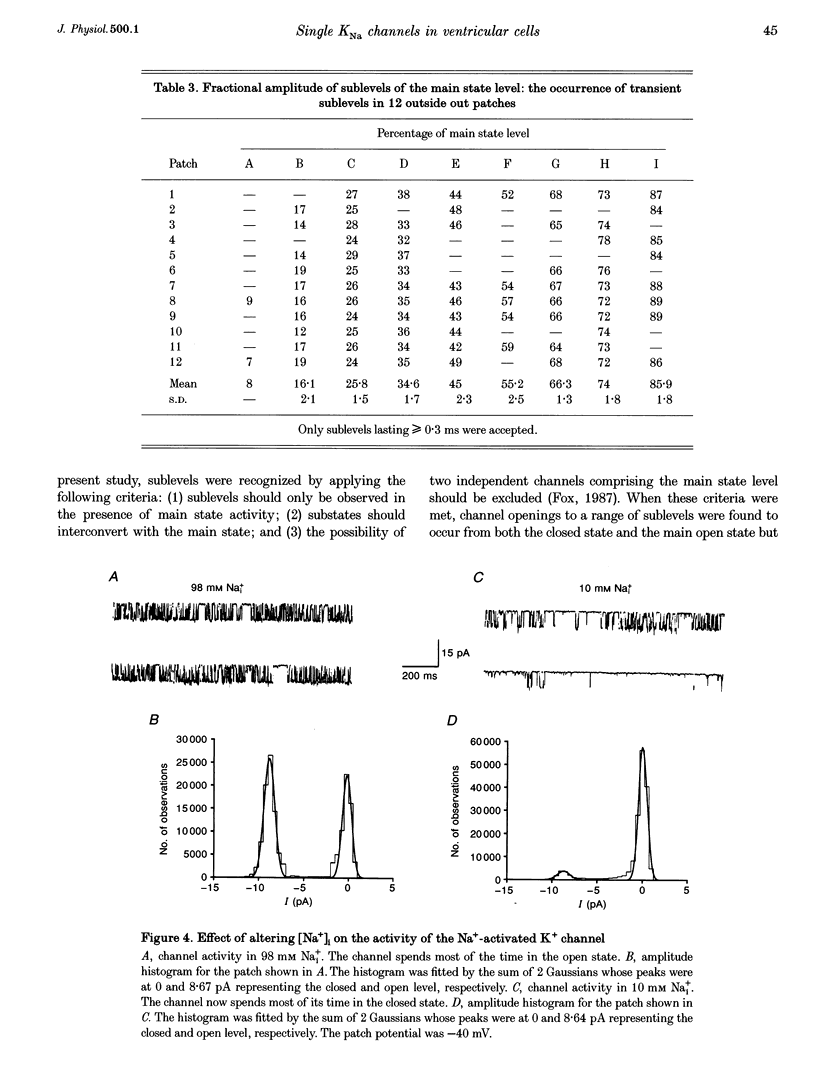
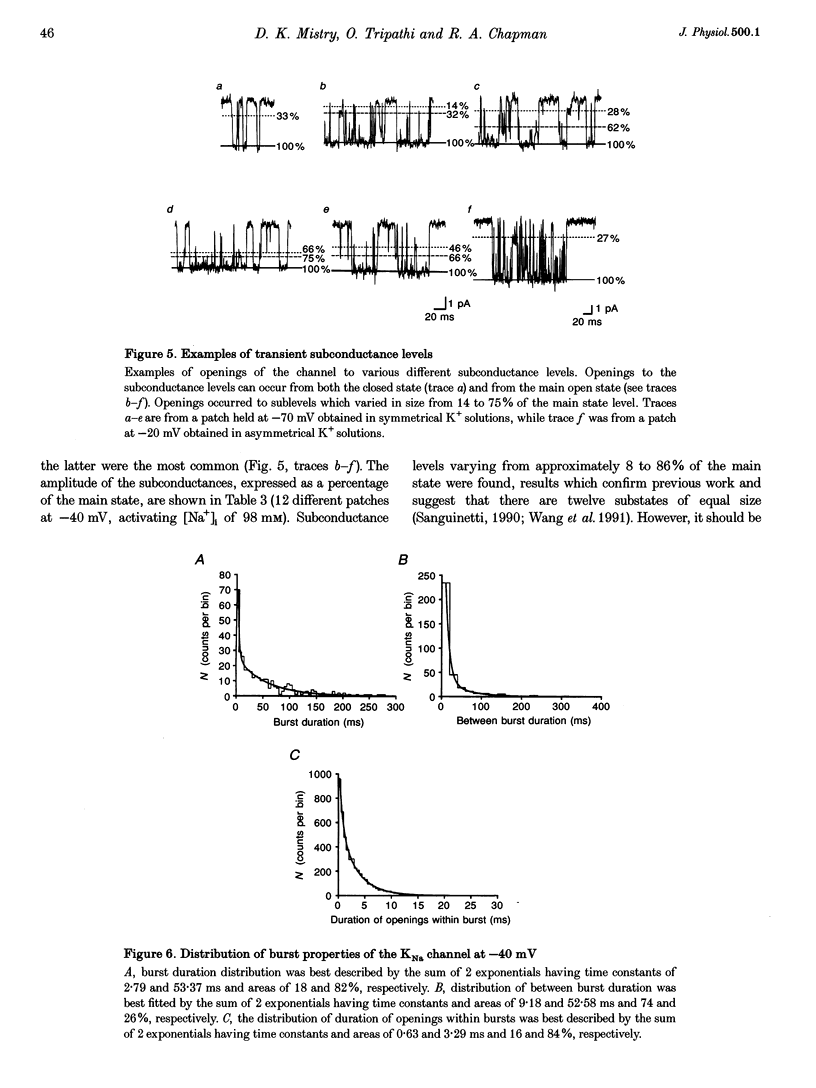
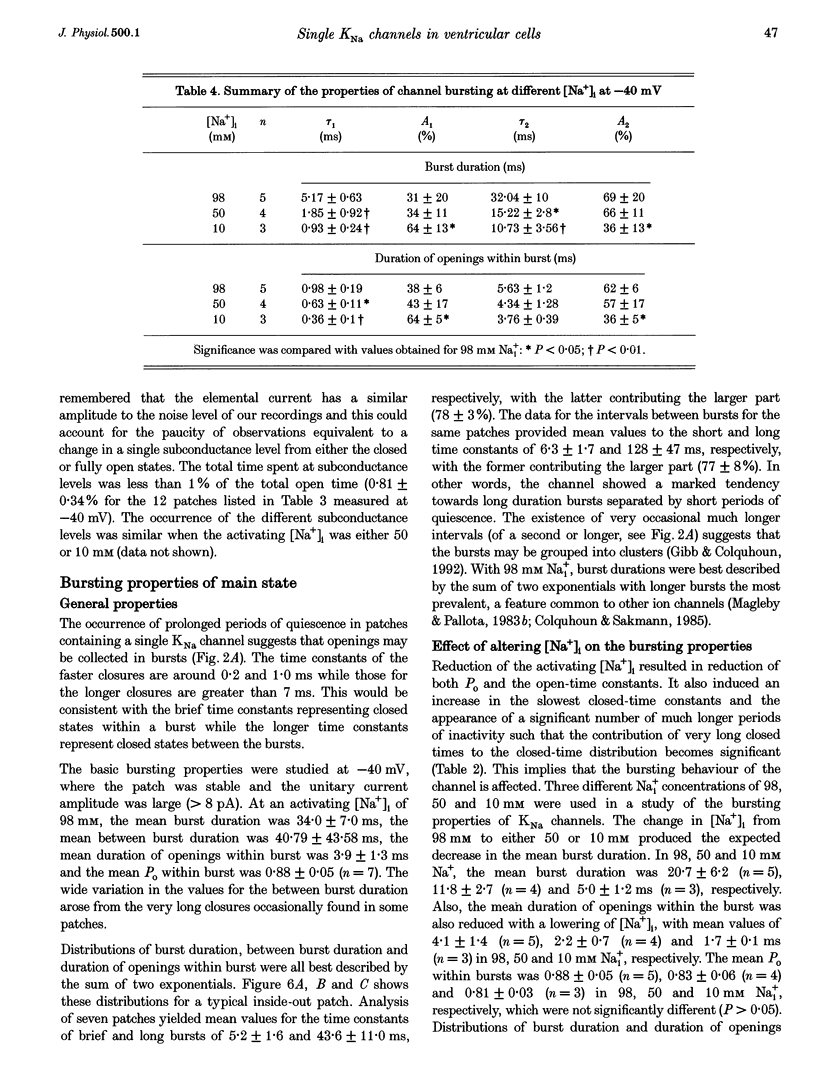
1. Single Na+-activated K+ channels (K(Na)) were investigated by means of the inside-out patch clamp technique in ventricular myocytes isolated from the guinea-pig heart. 2. Na+-activated K+ channels were observed at very low density (< 9% of patches). In symmetrical (60/60 mM) K+ solutions, K(Na) channels had a mean slope conductance of 75 pS and in asymmetrical (150/70 mM; outside/inside) K+ solutions, they had a mean slope conductance of 220 pS. The reversal potentials obtained under these two ionic conditions were close to the equilibrium potential for K+, suggesting K+ selectivity. 3. In high (98 mM) [Na+]i, the channel showed two open states and up to four closed states, and K(Na) channels also displayed long closures (of the order of seconds). The opening probability (Po) was not voltage dependent. Transient sublevels between 8 and 86% of the main state were identified and appeared to be a common feature of K(Na) channels. 4. Decreasing the activating [Na+]i, reduced Po and this was associated with both an increase in mean closed times and a decrease in mean open times. Lowering [Na+]i also increased the longer closed-time constants and their relative proportions. The first open-time constant was more sensitive to alterations in [Na+]i. 5. Distributions of burst duration, between burst duration and openings within bursts were best described by the sum of two exponentials. Lowering [Na+]i decreased the burst duration and the duration of openings within burst. 6. These observations show that the Na+-activated K+ channel from guinea-pig ventricular myocytes has complex gating and bursting behaviour.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bader C. R., Bernheim L., Bertrand D. Sodium-activated potassium current in cultured avian neurones. Nature. 1985 Oct 10;317(6037):540–542. doi: 10.1038/317540a0. [DOI] [PubMed] [Google Scholar]
- Callewaert G., Vereecke J., Carmeliet E. Existence of a calcium-dependent potassium channel in the membrane of cow cardiac Purkinje cells. Pflugers Arch. 1986 Apr;406(4):424–426. doi: 10.1007/BF00590947. [DOI] [PubMed] [Google Scholar]
- Carmeliet E. A fuzzy subsarcolemmal space for intracellular Na+ in cardiac cells? Cardiovasc Res. 1992 May;26(5):433–442. doi: 10.1093/cvr/26.5.433. [DOI] [PubMed] [Google Scholar]
- Chapman R. A., Tunstall J. The calcium paradox of the heart. Prog Biophys Mol Biol. 1987;50(2):67–96. doi: 10.1016/0079-6107(87)90004-6. [DOI] [PubMed] [Google Scholar]
- Cohen C. J., Fozzard H. A., Sheu S. S. Increase in intracellular sodium ion activity during stimulation in mammalian cardiac muscle. Circ Res. 1982 May;50(5):651–662. doi: 10.1161/01.res.50.5.651. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Hawkes A. G. On the stochastic properties of single ion channels. Proc R Soc Lond B Biol Sci. 1981 Mar 6;211(1183):205–235. doi: 10.1098/rspb.1981.0003. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Sakmann B. Fast events in single-channel currents activated by acetylcholine and its analogues at the frog muscle end-plate. J Physiol. 1985 Dec;369:501–557. doi: 10.1113/jphysiol.1985.sp015912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale N. A large, sustained Na(+)- and voltage-dependent K+ current in spinal neurons of the frog embryo. J Physiol. 1993 Mar;462:349–372. doi: 10.1113/jphysiol.1993.sp019559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dryer S. E., Fujii J. T., Martin A. R. A Na+-activated K+ current in cultured brain stem neurones from chicks. J Physiol. 1989 Mar;410:283–296. doi: 10.1113/jphysiol.1989.sp017533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dryer S. E. Na(+)-activated K+ channels and voltage-evoked ionic currents in brain stem and parasympathetic neurones of the chick. J Physiol. 1991 Apr;435:513–532. doi: 10.1113/jphysiol.1991.sp018522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J. A. Ion channel subconductance states. J Membr Biol. 1987;97(1):1–8. doi: 10.1007/BF01869609. [DOI] [PubMed] [Google Scholar]
- Haimann C., Bader C. R. Sodium-activated potassium channel in avian sensory neurons. Cell Biol Int Rep. 1989 Dec;13(12):1133–1139. doi: 10.1016/0309-1651(89)90027-1. [DOI] [PubMed] [Google Scholar]
- Haimann C., Bernheim L., Bertrand D., Bader C. R. Potassium current activated by intracellular sodium in quail trigeminal ganglion neurons. J Gen Physiol. 1990 May;95(5):961–979. doi: 10.1085/jgp.95.5.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- James A. F., Okada Y. Maxi K+ channels from the apical membranes of rabbit oviduct epithelial cells. J Membr Biol. 1994 Jan;137(2):109–118. doi: 10.1007/BF00233480. [DOI] [PubMed] [Google Scholar]
- Kameyama M., Kakei M., Sato R., Shibasaki T., Matsuda H., Irisawa H. Intracellular Na+ activates a K+ channel in mammalian cardiac cells. Nature. 1984 May 24;309(5966):354–356. doi: 10.1038/309354a0. [DOI] [PubMed] [Google Scholar]
- Koh D. S., Jonas P., Vogel W. Na(+)-activated K+ channels localized in the nodal region of myelinated axons of Xenopus. J Physiol. 1994 Sep 1;479(Pt 2):183–197. doi: 10.1113/jphysiol.1994.sp020287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk H. N., Carmeliet E. Na(+)-activated K+ current in cardiac cells: rectification, open probability, block and role in digitalis toxicity. Pflugers Arch. 1990 Aug;416(6):766–768. doi: 10.1007/BF00370627. [DOI] [PubMed] [Google Scholar]
- Magleby K. L., Pallotta B. S. Burst kinetics of single calcium-activated potassium channels in cultured rat muscle. J Physiol. 1983 Nov;344:605–623. doi: 10.1113/jphysiol.1983.sp014958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L., Pallotta B. S. Calcium dependence of open and shut interval distributions from calcium-activated potassium channels in cultured rat muscle. J Physiol. 1983 Nov;344:585–604. doi: 10.1113/jphysiol.1983.sp014957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManus O. B. Calcium-activated potassium channels: regulation by calcium. J Bioenerg Biomembr. 1991 Aug;23(4):537–560. doi: 10.1007/BF00785810. [DOI] [PubMed] [Google Scholar]
- McManus O. B., Magleby K. L. Kinetic time constants independent of previous single-channel activity suggest Markov gating for a large conductance Ca-activated K channel. J Gen Physiol. 1989 Dec;94(6):1037–1070. doi: 10.1085/jgp.94.6.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mistry D. K., Tripathi O., Chapman R. A. The occurrence of stable subconductance levels in Na(+)-activated K+ channels in excised membrane patches from guinea-pig ventricular myocytes. Exp Physiol. 1996 Nov;81(6):899–907. doi: 10.1113/expphysiol.1996.sp003991. [DOI] [PubMed] [Google Scholar]
- Mitani A., Shattock M. J. Role of Na-activated K channel, Na-K-Cl cotransport, and Na-K pump in [K]e changes during ischemia in rat heart. Am J Physiol. 1992 Aug;263(2 Pt 2):H333–H340. doi: 10.1152/ajpheart.1992.263.2.H333. [DOI] [PubMed] [Google Scholar]
- Patlak J. B. Sodium channel subconductance levels measured with a new variance-mean analysis. J Gen Physiol. 1988 Oct;92(4):413–430. doi: 10.1085/jgp.92.4.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigo G. C., Chapman R. A. A sodium-activated potassium current in intact ventricular myocytes isolated from the guinea-pig heart. Exp Physiol. 1990 Nov;75(6):839–842. doi: 10.1113/expphysiol.1990.sp003465. [DOI] [PubMed] [Google Scholar]
- Rodrigo G. C. The Na(+)-dependence of Na(+)-activated K(+)-channels (IK(Na)) in guinea pig ventricular myocytes, is different in excised inside/out patches and cell attached patches. Pflugers Arch. 1993 Feb;422(5):530–532. doi: 10.1007/BF00375082. [DOI] [PubMed] [Google Scholar]
- Rothberg B. S., Bello R. A., Song L., Magleby K. L. High Ca2+ concentrations induce a low activity mode and reveal Ca2(+)-independent long shut intervals in BK channels from rat muscle. J Physiol. 1996 Jun 15;493(Pt 3):673–689. doi: 10.1113/jphysiol.1996.sp021414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanguinetti M. C. Na+1-activated and ATP-sensitive K+ channels in the heart. Prog Clin Biol Res. 1990;334:85–109. [PubMed] [Google Scholar]
- Sigworth F. J., Sine S. M. Data transformations for improved display and fitting of single-channel dwell time histograms. Biophys J. 1987 Dec;52(6):1047–1054. doi: 10.1016/S0006-3495(87)83298-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldkamp M. W., Vereecke J., Carmeliet E. Effects of intracellular sodium and hydrogen ion on the sodium activated potassium channel in isolated patches from guinea pig ventricular myocytes. Cardiovasc Res. 1994 Jul;28(7):1036–1041. doi: 10.1093/cvr/28.7.1036. [DOI] [PubMed] [Google Scholar]
- Wang Z., Kimitsuki T., Noma A. Conductance properties of the Na(+)-activated K+ channel in guinea-pig ventricular cells. J Physiol. 1991 Feb;433:241–257. doi: 10.1113/jphysiol.1991.sp018424. [DOI] [PMC free article] [PubMed] [Google Scholar]


