Abstract
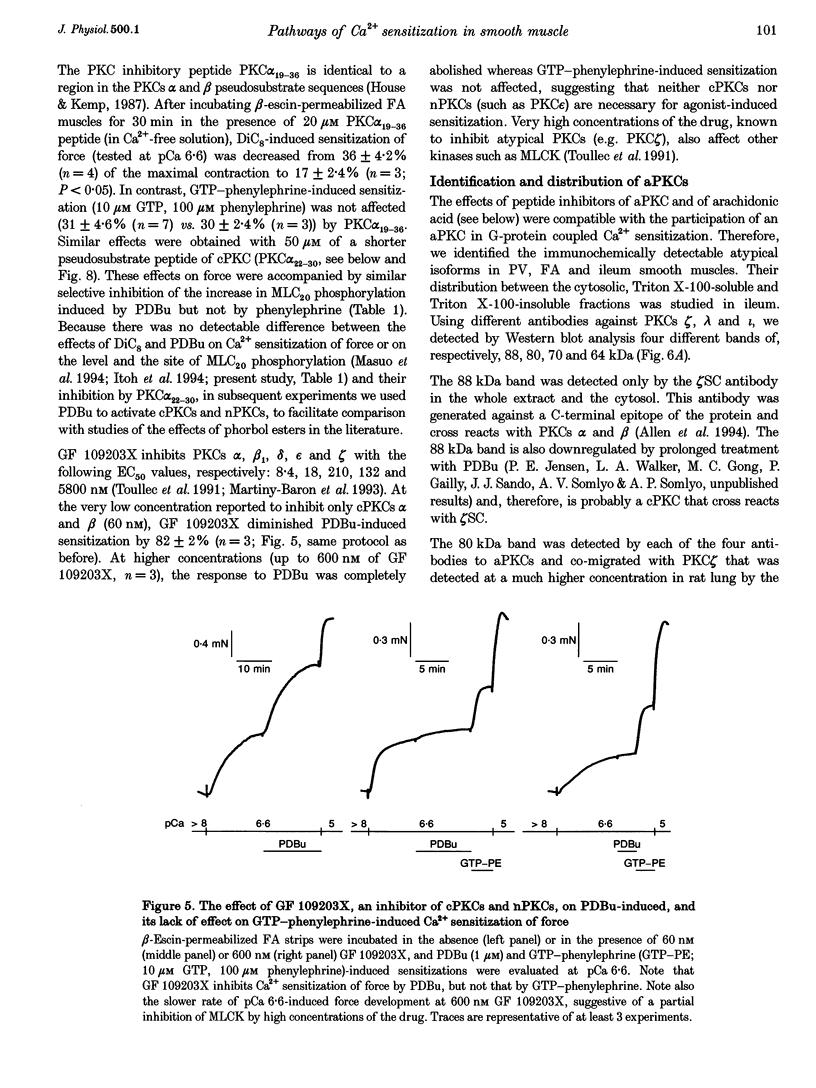
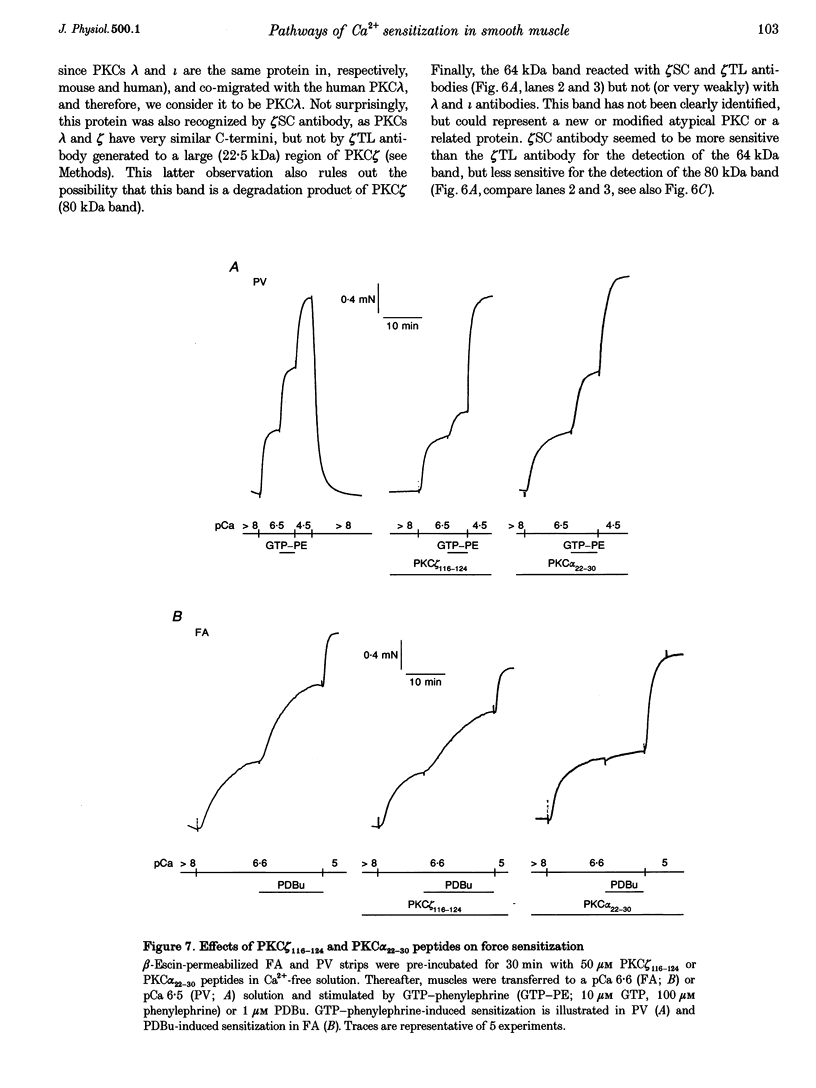
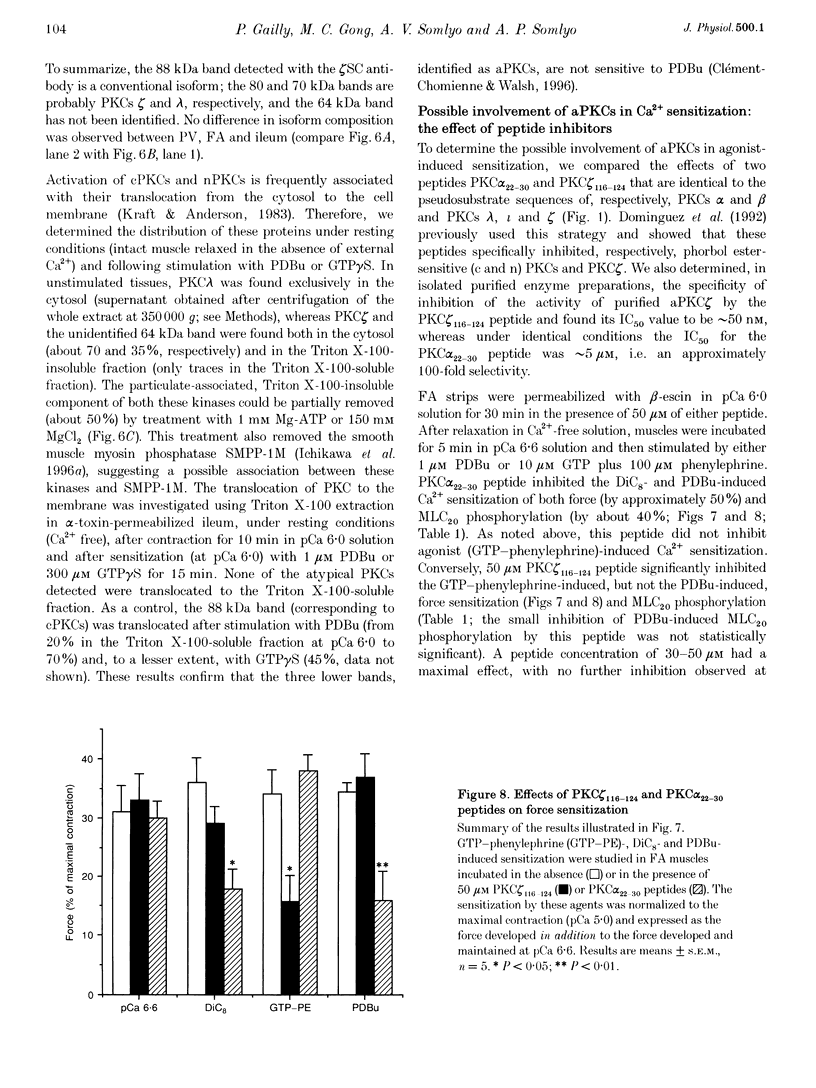
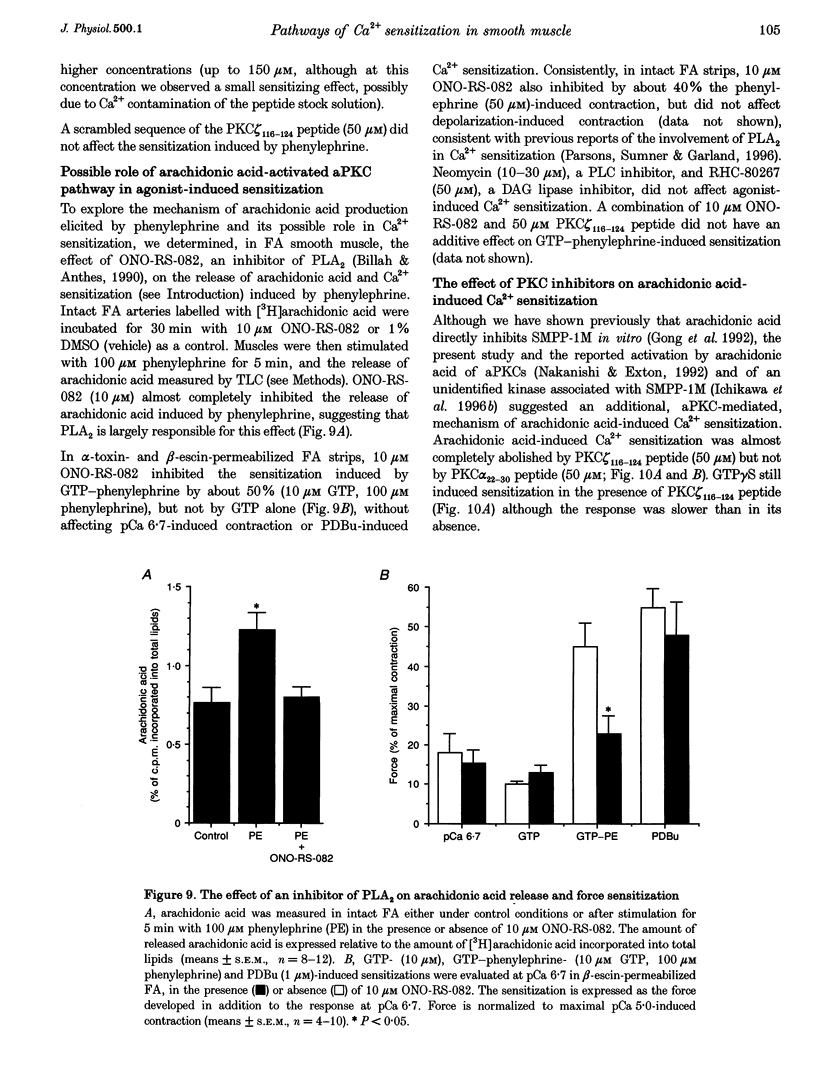
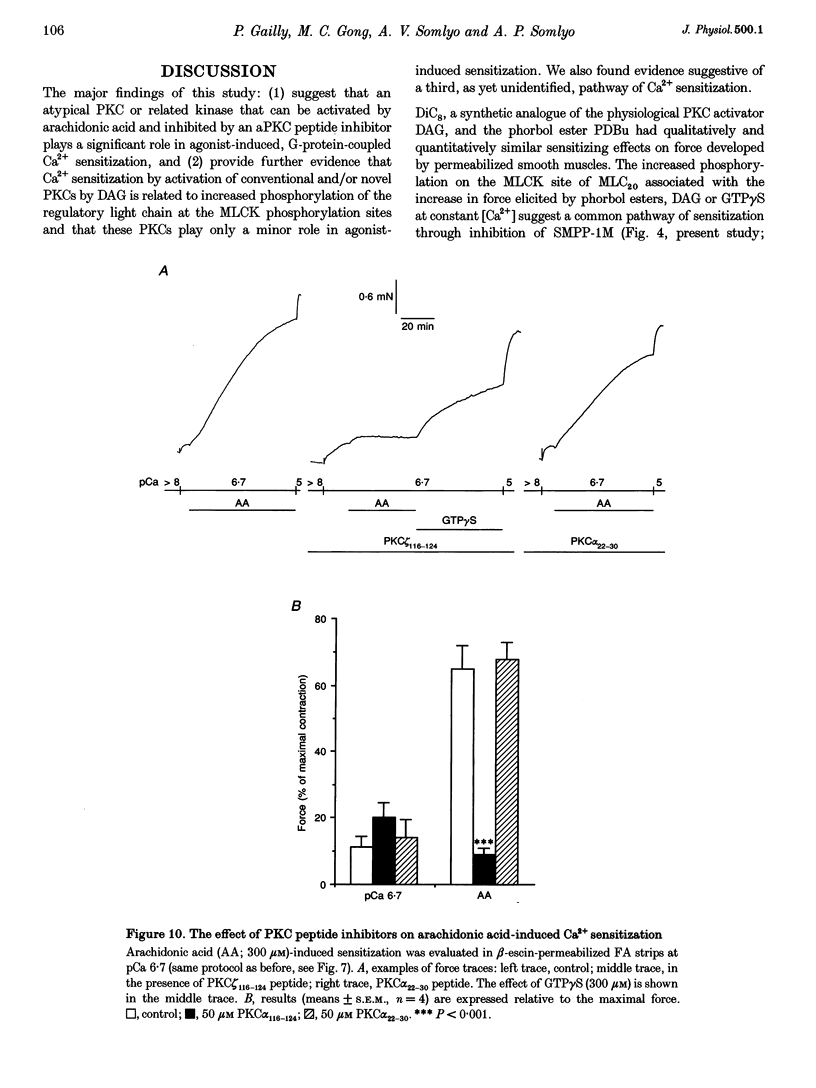
1. Diacylglycerol (DAG; 10 microM), an activator of conventional and novel protein kinases C (cPKCs and nPKCs), induced Ca2+ sensitization of force in isolated intact and alpha-toxin-permeabilized femoral artery (FA) and portal vein (PV), and increased the phosphorylation of myosin light chain (MLC20) at the same peptides phosphorylated by myosin light chain kinase. 2. Ca2+ sensitization by DAG was specifically inhibited by a pseudosubstrate peptide inhibitor of cPKCs (PKC alpha(22-30) peptide; 50 microM). Similarly, GF 109203X (600 nM), an inhibitor of cPKCs and nPKCs, completely abolished Ca2+ sensitization by phorbol 12,13-dibutyrate (PDBu; 1 microM). In contrast, Ca2+ sensitization induced by the alpha1-adrenergic agonist phenylephrine (100 microM) was not inhibited by these inhibitors of cPKCs and nPKCs. 3. A pseudosubstrate peptide inhibitor of the atypical PKCs (aPKCs) PKC zeta(116-124) (50 microM) significantly (about 50%) inhibited the Ca2+ sensitization of force and MLC20 phosphorylation induced by 100 microM phenylephrine and by 300 microM arachidonic acid, but not that by DAG (10 microM) or PDBu (1 microM). 4. A phospholipase A2 (PLA2) inhibitor, ONO-RS-082 (10 microM), abolished the release of arachidonic acid and partially (by 40%) inhibited the Ca2+ sensitization induced by phenylephrine in FA smooth muscle. This effect was not additive to the inhibition observed with the aPKC inhibitor peptide, suggesting that arachidonic acid and aPKCs exert their effects via the same pathway, probably through activation of aPKC(s) by arachidonic acid. 5. Western blot analysis with antibodies to aPKCs revealed aPKCs zeta, lambda (or iota) and an unidentified 64 kDa protein. The distribution (cytosolic and particulate) of these proteins was not affected by PDBu (1 microM). 6. Our results are consistent with a significant role for atypical (or related) PKCs through a PLA2-arachidonic acid-aPKC pathway in agonist-induced Ca2+ sensitization, in parallel with a similar, but minor role of the DAG-cPKC cascade. The inability of the combination of the two (aPKC and cPKC) inhibitors to completely eliminate Ca2+ sensitization also suggests the presence of a third, still unidentified, pathway of this mechanism.
Full text
PDF





Images in this article
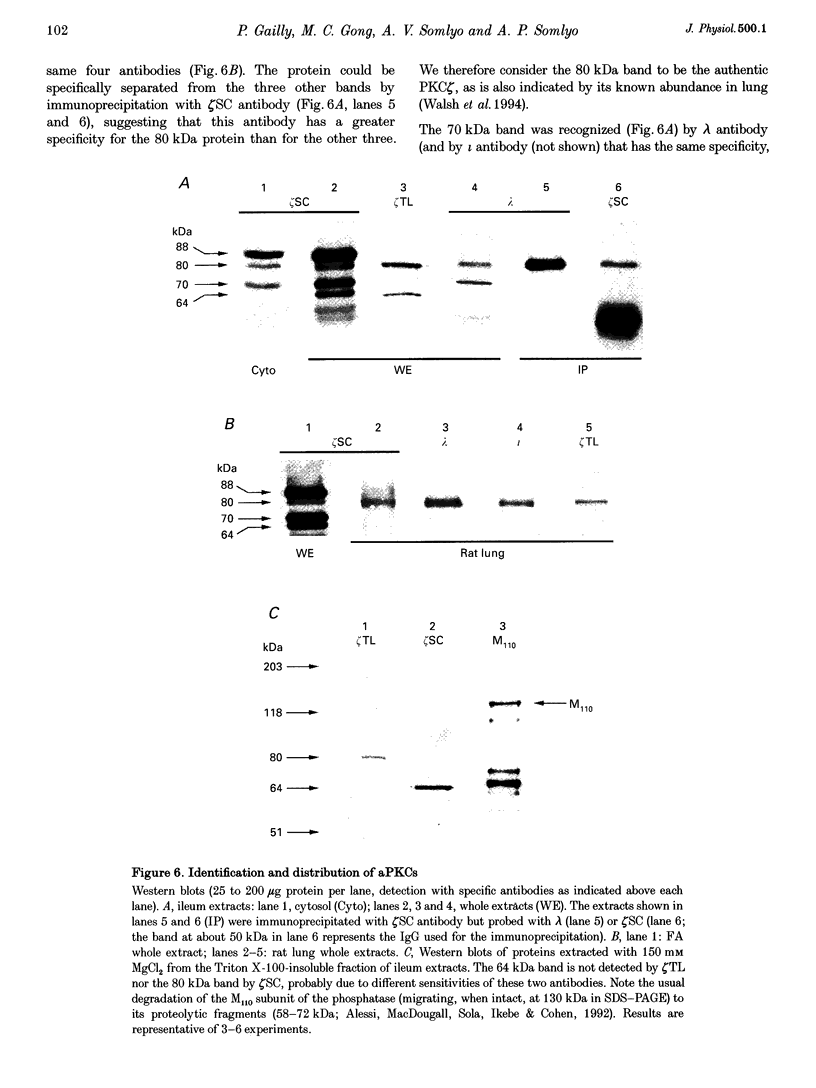
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akimoto K., Mizuno K., Osada S., Hirai S., Tanuma S., Suzuki K., Ohno S. A new member of the third class in the protein kinase C family, PKC lambda, expressed dominantly in an undifferentiated mouse embryonal carcinoma cell line and also in many tissues and cells. J Biol Chem. 1994 Apr 29;269(17):12677–12683. [PubMed] [Google Scholar]
- Alessi D., MacDougall L. K., Sola M. M., Ikebe M., Cohen P. The control of protein phosphatase-1 by targetting subunits. The major myosin phosphatase in avian smooth muscle is a novel form of protein phosphatase-1. Eur J Biochem. 1992 Dec 15;210(3):1023–1035. doi: 10.1111/j.1432-1033.1992.tb17508.x. [DOI] [PubMed] [Google Scholar]
- Allen B. G., Andrea J. E., Walsh M. P. Identification and characterization of protein kinase C zeta-immunoreactive proteins. J Biol Chem. 1994 Nov 18;269(46):29288–29298. [PubMed] [Google Scholar]
- Billah M. M., Anthes J. C. The regulation and cellular functions of phosphatidylcholine hydrolysis. Biochem J. 1990 Jul 15;269(2):281–291. doi: 10.1042/bj2690281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brozovich F. V. PKC regulates agonist-induced force enhancement in single alpha-toxin-permeabilized vascular smooth muscle cells. Am J Physiol. 1995 May;268(5 Pt 1):C1202–C1206. doi: 10.1152/ajpcell.1995.268.5.C1202. [DOI] [PubMed] [Google Scholar]
- Clément-Chomienne O., Walsh M. P. Identification of protein kinase C isoenzymes in smooth muscle: partial purification and characterization of chicken gizzard PKC zeta. Biochem Cell Biol. 1996;74(1):51–65. doi: 10.1139/o96-006. [DOI] [PubMed] [Google Scholar]
- Dominguez I., Diaz-Meco M. T., Municio M. M., Berra E., García de Herreros A., Cornet M. E., Sanz L., Moscat J. Evidence for a role of protein kinase C zeta subspecies in maturation of Xenopus laevis oocytes. Mol Cell Biol. 1992 Sep;12(9):3776–3783. doi: 10.1128/mcb.12.9.3776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly R., Yang K., Omary M. B., Azhar S., Black J. L. Expression of multiple isoenzymes of protein kinase C in airway smooth muscle. Am J Respir Cell Mol Biol. 1995 Sep;13(3):253–256. doi: 10.1165/ajrcmb.13.3.7654381. [DOI] [PubMed] [Google Scholar]
- Fujita A., Takeuchi T., Nakajima H., Nishio H., Hata F. Involvement of heterotrimeric GTP-binding protein and rho protein, but not protein kinase C, in agonist-induced Ca2+ sensitization of skinned muscle of guinea pig vas deferens. J Pharmacol Exp Ther. 1995 Jul;274(1):555–561. [PubMed] [Google Scholar]
- Gailly P., Wu X., Haystead T. A., Somlyo A. P., Cohen P. T., Cohen P., Somlyo A. V. Regions of the 110-kDa regulatory subunit M110 required for regulation of myosin-light-chain-phosphatase activity in smooth muscle. Eur J Biochem. 1996 Jul 15;239(2):326–332. doi: 10.1111/j.1432-1033.1996.0326u.x. [DOI] [PubMed] [Google Scholar]
- Gong M. C., Fuglsang A., Alessi D., Kobayashi S., Cohen P., Somlyo A. V., Somlyo A. P. Arachidonic acid inhibits myosin light chain phosphatase and sensitizes smooth muscle to calcium. J Biol Chem. 1992 Oct 25;267(30):21492–21498. [PubMed] [Google Scholar]
- Hori M., Sato K., Miyamoto S., Ozaki H., Karaki H. Different pathways of calcium sensitization activated by receptor agonists and phorbol esters in vascular smooth muscle. Br J Pharmacol. 1993 Dec;110(4):1527–1531. doi: 10.1111/j.1476-5381.1993.tb13996.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- House C., Kemp B. E. Protein kinase C contains a pseudosubstrate prototope in its regulatory domain. Science. 1987 Dec 18;238(4834):1726–1728. doi: 10.1126/science.3686012. [DOI] [PubMed] [Google Scholar]
- Ichikawa K., Hirano K., Ito M., Tanaka J., Nakano T., Hartshorne D. J. Interactions and properties of smooth muscle myosin phosphatase. Biochemistry. 1996 May 21;35(20):6313–6320. doi: 10.1021/bi960208q. [DOI] [PubMed] [Google Scholar]
- Ichikawa K., Ito M., Hartshorne D. J. Phosphorylation of the large subunit of myosin phosphatase and inhibition of phosphatase activity. J Biol Chem. 1996 Mar 1;271(9):4733–4740. doi: 10.1074/jbc.271.9.4733. [DOI] [PubMed] [Google Scholar]
- Ikebe M., Hartshorne D. J., Elzinga M. Phosphorylation of the 20,000-dalton light chain of smooth muscle myosin by the calcium-activated, phospholipid-dependent protein kinase. Phosphorylation sites and effects of phosphorylation. J Biol Chem. 1987 Jul 15;262(20):9569–9573. [PubMed] [Google Scholar]
- Itoh T., Suzuki A., Watanabe Y. Effect of a peptide inhibitor of protein kinase C on G-protein-mediated increase in myofilament Ca(2+)-sensitivity in rabbit arterial skinned muscle. Br J Pharmacol. 1994 Jan;111(1):311–317. doi: 10.1111/j.1476-5381.1994.tb14061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen P. E., Gong M. C., Somlyo A. V., Somlyo A. P. Separate upstream and convergent downstream pathways of G-protein- and phorbol ester-mediated Ca2+ sensitization of myosin light chain phosphorylation in smooth muscle. Biochem J. 1996 Sep 1;318(Pt 2):469–475. doi: 10.1042/bj3180469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitazawa T., Gaylinn B. D., Denney G. H., Somlyo A. P. G-protein-mediated Ca2+ sensitization of smooth muscle contraction through myosin light chain phosphorylation. J Biol Chem. 1991 Jan 25;266(3):1708–1715. [PubMed] [Google Scholar]
- Kitazawa T., Kobayashi S., Horiuti K., Somlyo A. V., Somlyo A. P. Receptor-coupled, permeabilized smooth muscle. Role of the phosphatidylinositol cascade, G-proteins, and modulation of the contractile response to Ca2+. J Biol Chem. 1989 Apr 5;264(10):5339–5342. [PubMed] [Google Scholar]
- Kitazawa T., Masuo M., Somlyo A. P. G protein-mediated inhibition of myosin light-chain phosphatase in vascular smooth muscle. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):9307–9310. doi: 10.1073/pnas.88.20.9307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft A. S., Anderson W. B. Phorbol esters increase the amount of Ca2+, phospholipid-dependent protein kinase associated with plasma membrane. Nature. 1983 Feb 17;301(5901):621–623. doi: 10.1038/301621a0. [DOI] [PubMed] [Google Scholar]
- Lee M. W., Severson D. L. Signal transduction in vascular smooth muscle: diacylglycerol second messengers and PKC action. Am J Physiol. 1994 Sep;267(3 Pt 1):C659–C678. doi: 10.1152/ajpcell.1994.267.3.C659. [DOI] [PubMed] [Google Scholar]
- Leung T., Manser E., Tan L., Lim L. A novel serine/threonine kinase binding the Ras-related RhoA GTPase which translocates the kinase to peripheral membranes. J Biol Chem. 1995 Dec 8;270(49):29051–29054. doi: 10.1074/jbc.270.49.29051. [DOI] [PubMed] [Google Scholar]
- Martiny-Baron G., Kazanietz M. G., Mischak H., Blumberg P. M., Kochs G., Hug H., Marmé D., Schächtele C. Selective inhibition of protein kinase C isozymes by the indolocarbazole Gö 6976. J Biol Chem. 1993 May 5;268(13):9194–9197. [PubMed] [Google Scholar]
- Masuo M., Reardon S., Ikebe M., Kitazawa T. A novel mechanism for the Ca(2+)-sensitizing effect of protein kinase C on vascular smooth muscle: inhibition of myosin light chain phosphatase. J Gen Physiol. 1994 Aug;104(2):265–286. doi: 10.1085/jgp.104.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui T., Amano M., Yamamoto T., Chihara K., Nakafuku M., Ito M., Nakano T., Okawa K., Iwamatsu A., Kaibuchi K. Rho-associated kinase, a novel serine/threonine kinase, as a putative target for small GTP binding protein Rho. EMBO J. 1996 May 1;15(9):2208–2216. [PMC free article] [PubMed] [Google Scholar]
- Mukai H., Ono Y. A novel protein kinase with leucine zipper-like sequences: its catalytic domain is highly homologous to that of protein kinase C. Biochem Biophys Res Commun. 1994 Mar 15;199(2):897–904. doi: 10.1006/bbrc.1994.1313. [DOI] [PubMed] [Google Scholar]
- Nakanishi H., Exton J. H. Purification and characterization of the zeta isoform of protein kinase C from bovine kidney. J Biol Chem. 1992 Aug 15;267(23):16347–16354. [PubMed] [Google Scholar]
- Newton A. C. Protein kinase C: structure, function, and regulation. J Biol Chem. 1995 Dec 1;270(48):28495–28498. doi: 10.1074/jbc.270.48.28495. [DOI] [PubMed] [Google Scholar]
- Ohanian V., Ohanian J., Shaw L., Scarth S., Parker P. J., Heagerty A. M. Identification of protein kinase C isoforms in rat mesenteric small arteries and their possible role in agonist-induced contraction. Circ Res. 1996 May;78(5):806–812. doi: 10.1161/01.res.78.5.806. [DOI] [PubMed] [Google Scholar]
- Parsons S. J., Sumner M. J., Garland C. J. Phospholipase A2 and protein kinase C contribute to myofilament sensitization to 5-HT in the rabbit mesenteric artery. J Physiol. 1996 Mar 1;491(Pt 2):447–453. doi: 10.1113/jphysiol.1996.sp021228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persechini A., Hartshorne D. J. Ordered phosphorylation of the two 20 000 molecular weight light chains of smooth muscle myosin. Biochemistry. 1983 Jan 18;22(2):470–476. doi: 10.1021/bi00271a033. [DOI] [PubMed] [Google Scholar]
- Somlyo A. P., Somlyo A. V. Signal transduction and regulation in smooth muscle. Nature. 1994 Nov 17;372(6503):231–236. doi: 10.1038/372231a0. [DOI] [PubMed] [Google Scholar]
- Somlyo A. V., Somlyo A. P. Electromechanical and pharmacomechanical coupling in vascular smooth muscle. J Pharmacol Exp Ther. 1968 Jan;159(1):129–145. [PubMed] [Google Scholar]
- Toullec D., Pianetti P., Coste H., Bellevergue P., Grand-Perret T., Ajakane M., Baudet V., Boissin P., Boursier E., Loriolle F. The bisindolylmaleimide GF 109203X is a potent and selective inhibitor of protein kinase C. J Biol Chem. 1991 Aug 25;266(24):15771–15781. [PubMed] [Google Scholar]
- Walsh M. P., Andrea J. E., Allen B. G., Clément-Chomienne O., Collins E. M., Morgan K. G. Smooth muscle protein kinase C. Can J Physiol Pharmacol. 1994 Nov;72(11):1392–1399. doi: 10.1139/y94-201. [DOI] [PubMed] [Google Scholar]