Abstract
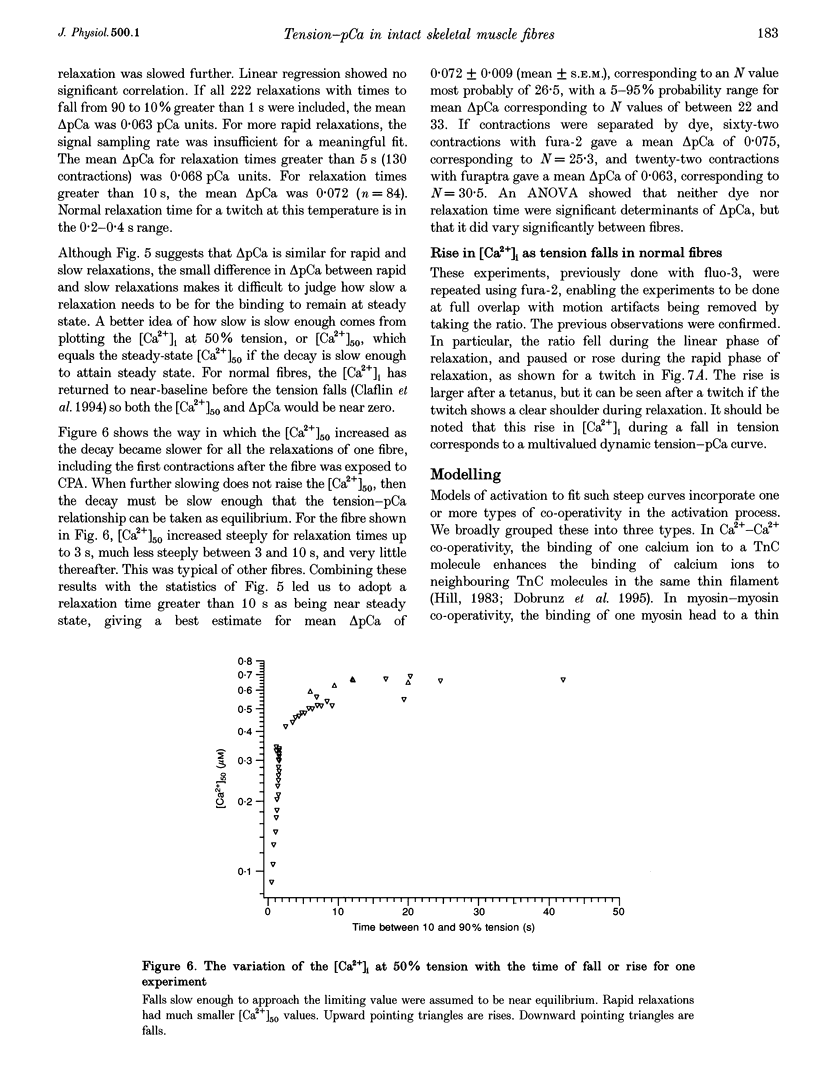
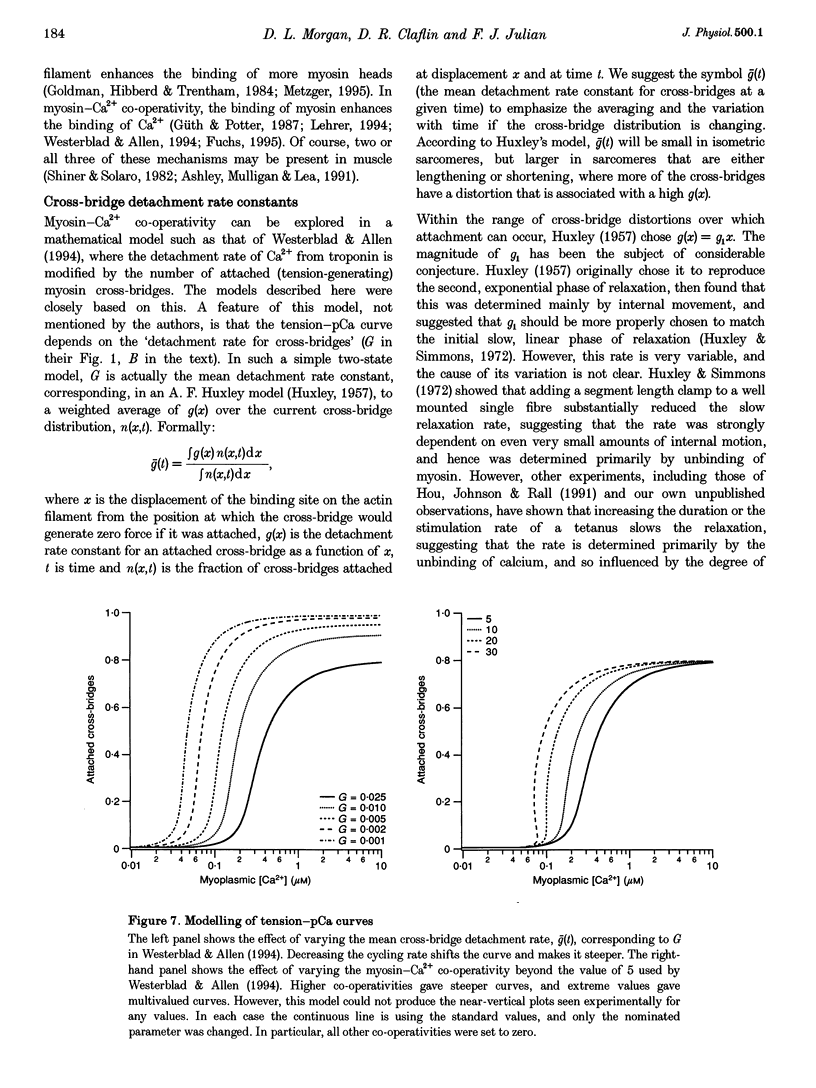
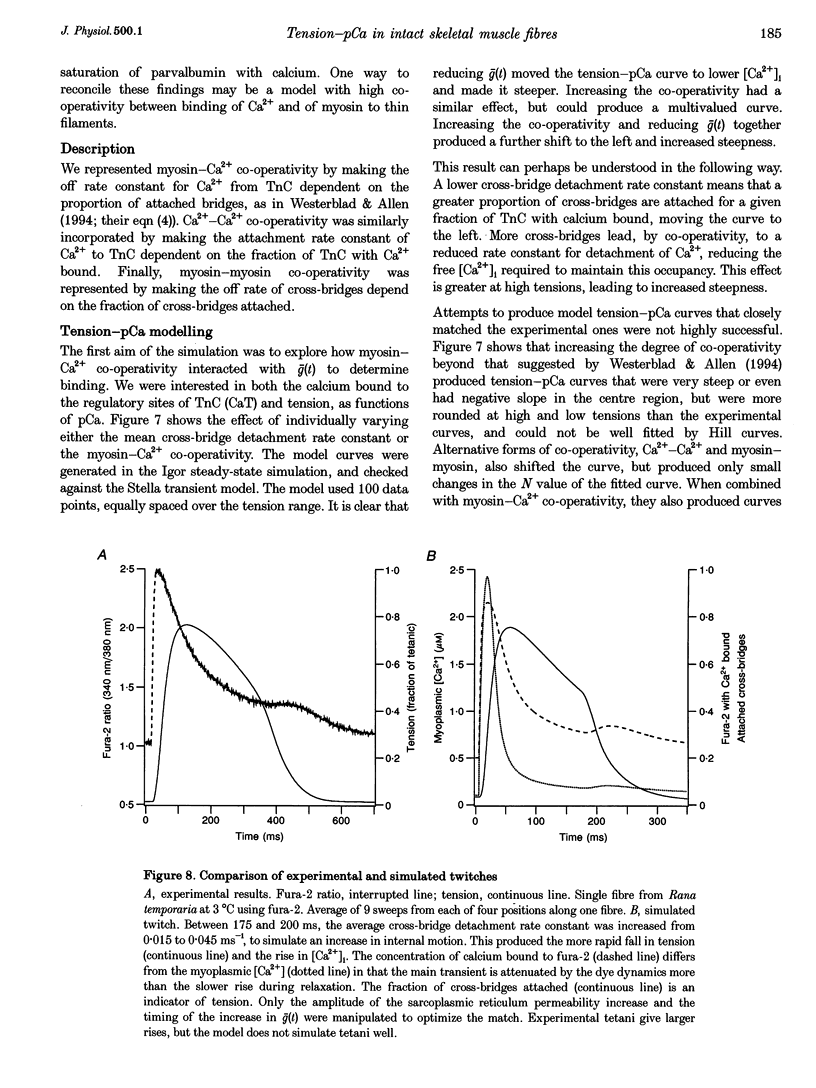
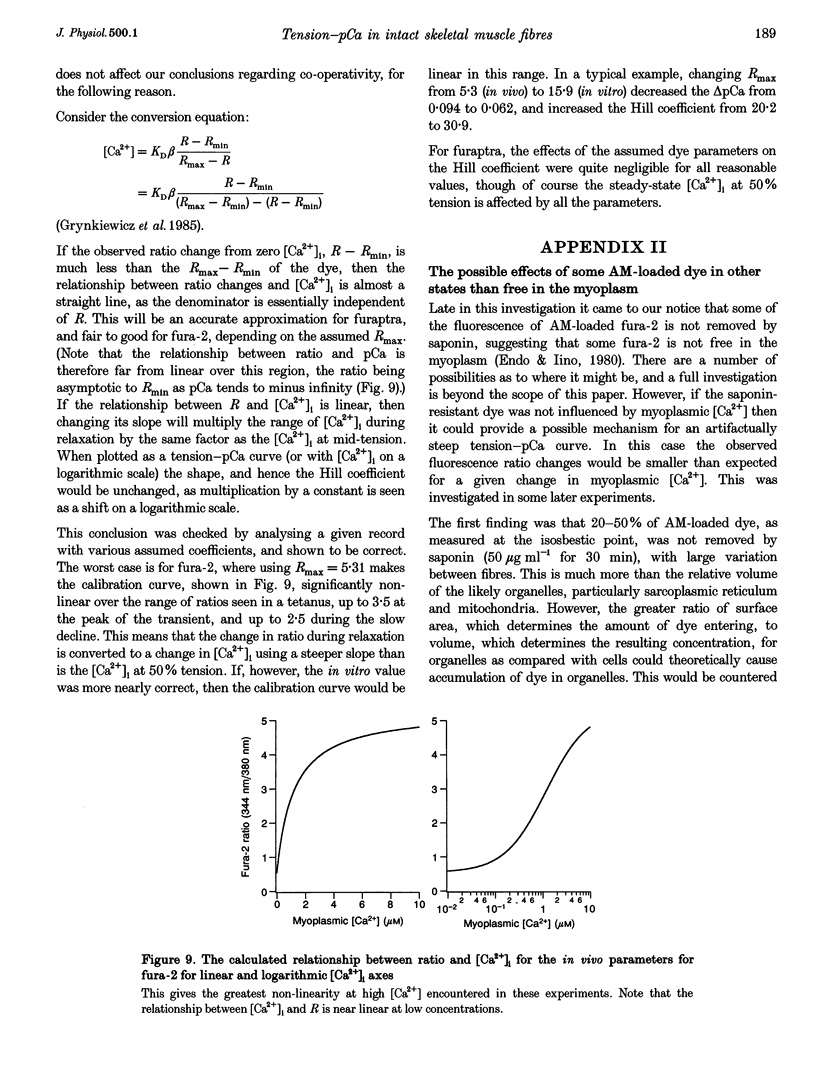
1. The relationship between intracellular calcium concentration, [Ca2+]i, and fixed-end tension was investigated in intact single muscle fibres from frogs. A slow decline of tension was produced by cyclopiazonic acid (CPA), a sarcoplasmic reticulum Ca2+ uptake pump inhibitor. The fluorescent dyes fura-2 and furaptra (mag-fura-2) were used to estimate [Ca2+]i. 2. Neither the steepness nor the position of the curve changed consistently over a wide range of tension decay times from a few seconds to over 100 s. For these near-steady-state curves, the 10-90% tension change occurred, on average, in 0.07 pCa units, corresponding to a Hill coefficient > 25, much steeper than previously reported. Possible artifacts could reduce that to 15. 3. Methoxyverapamil (D600) reduces the calcium released in response to an action potential. Contractions with D600 and CPA had a slow rise composed of many small steps, and a slow fall. Comparing rise and fall showed little or no hysteresis in the tension-[Ca2+]i relationship. 4. A model involving co-operativity between the binding of Ca2+ and myosin to thin filaments is shown to produce a tension-pCa relationship that is substantially altered by the mean rate constant for detachment of myosin cross-bridges, which in turn is likely to be affected by sarcomere movements. 5. Such a model is shown to be capable of reproducing the small rise in [Ca2+]i previously reported during the late phase of fixed-end relaxation of intact fibres.
Full text
PDF

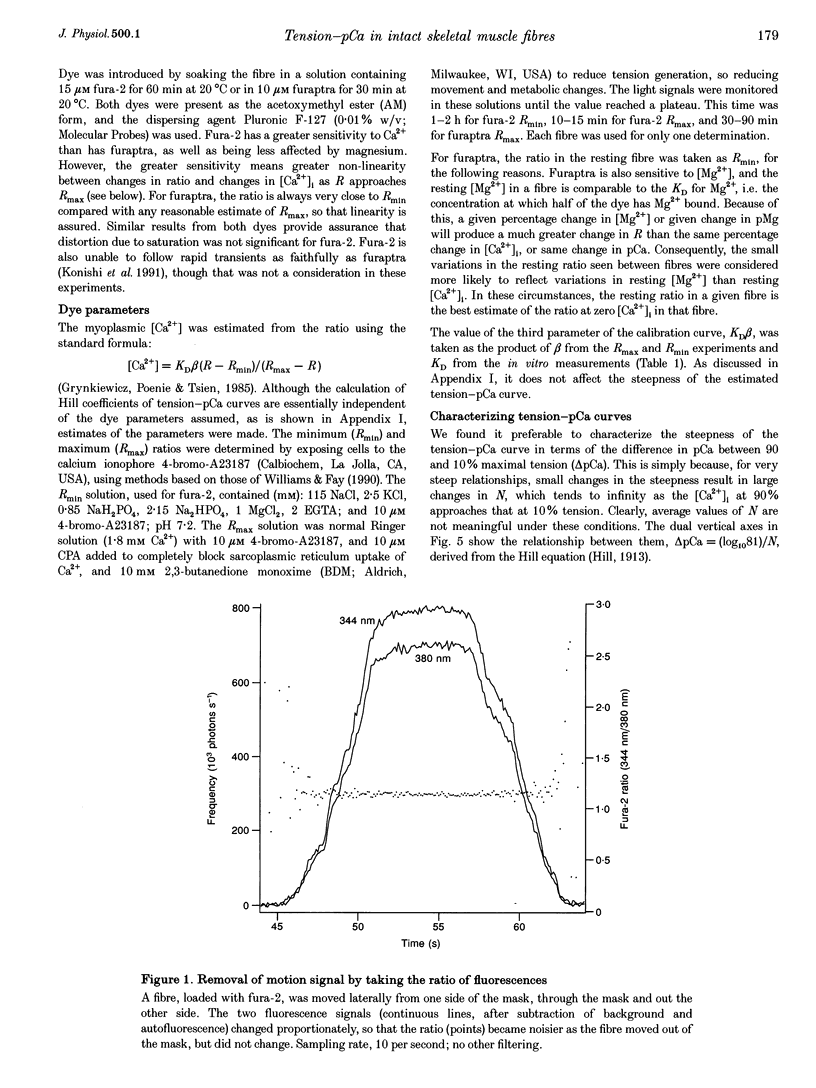
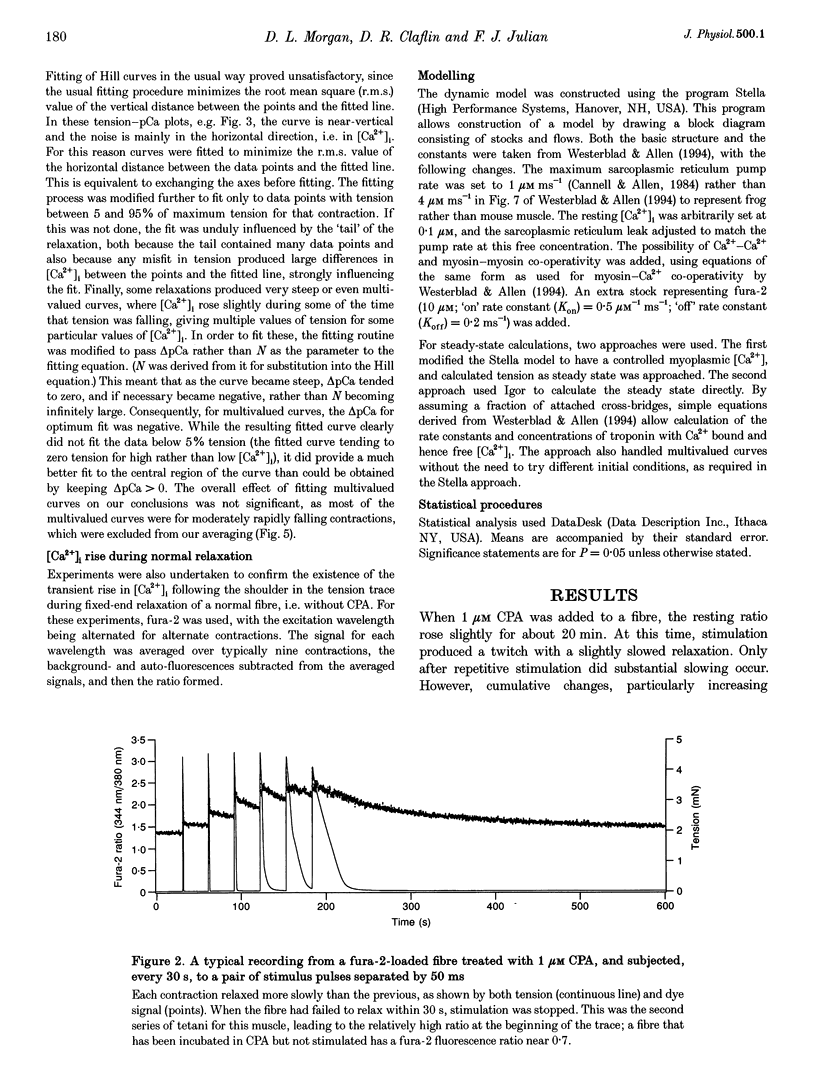
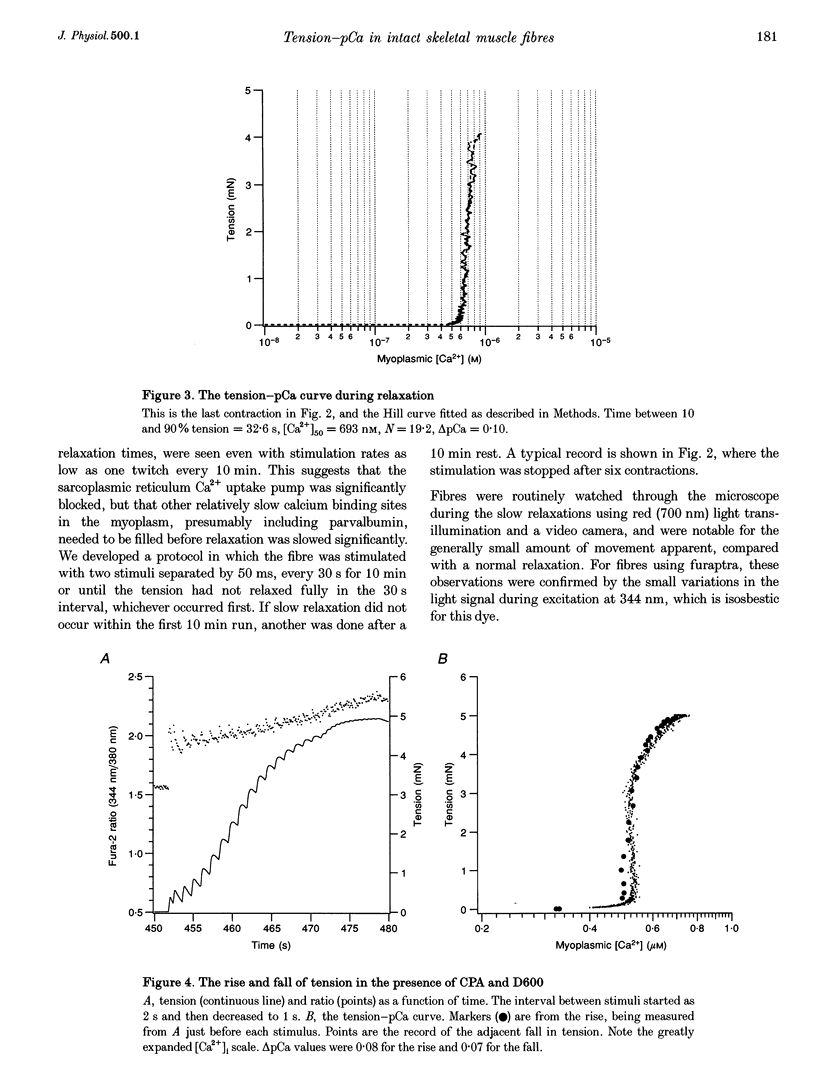
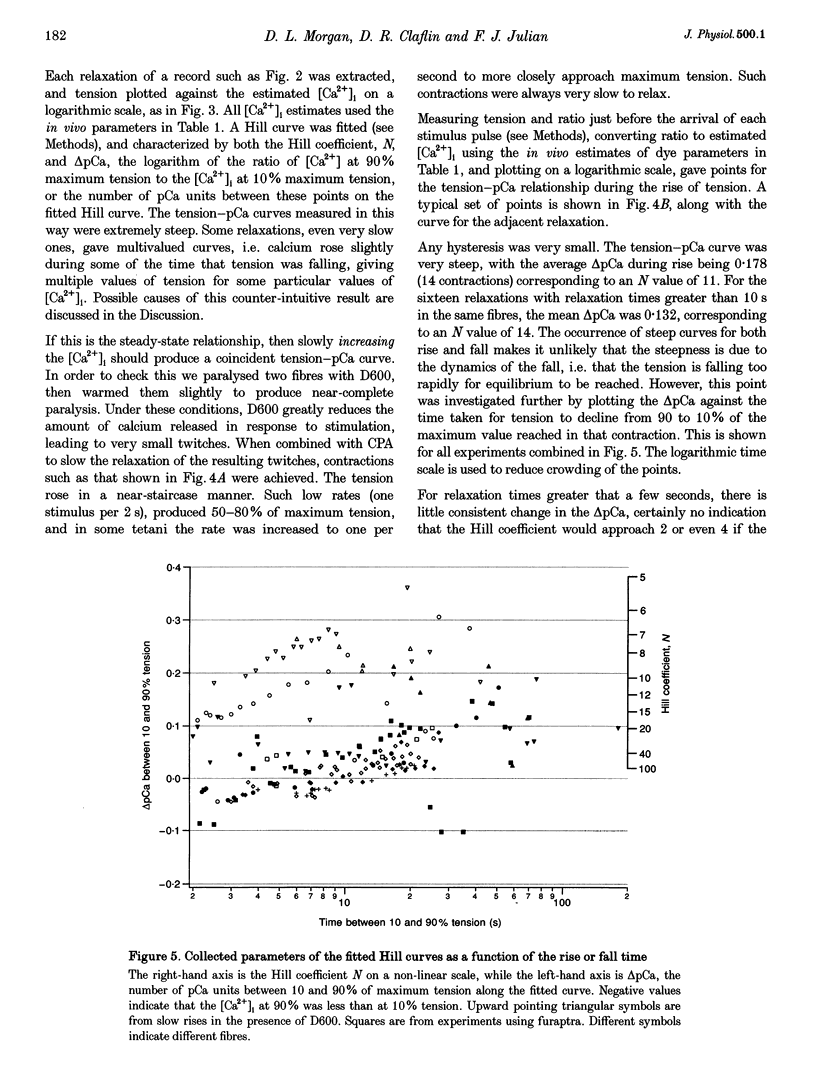





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashley C. C., Mulligan I. P., Lea T. J. Ca2+ and activation mechanisms in skeletal muscle. Q Rev Biophys. 1991 Feb;24(1):1–73. doi: 10.1017/s0033583500003267. [DOI] [PubMed] [Google Scholar]
- Baker A. J., Brandes R., Weiner M. W. Effects of intracellular acidosis on Ca2+ activation, contraction, and relaxation of frog skeletal muscle. Am J Physiol. 1995 Jan;268(1 Pt 1):C55–C63. doi: 10.1152/ajpcell.1995.268.1.C55. [DOI] [PubMed] [Google Scholar]
- Berwe D., Gottschalk G., Lüttgau H. C. Effects of the calcium antagonist gallopamil (D600) upon excitation-contraction coupling in toe muscle fibres of the frog. J Physiol. 1987 Apr;385:693–707. doi: 10.1113/jphysiol.1987.sp016515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt P. W., Diamond M. S., Schachat F. H. The thin filament of vertebrate skeletal muscle co-operatively activates as a unit. J Mol Biol. 1984 Dec 5;180(2):379–384. doi: 10.1016/s0022-2836(84)80010-8. [DOI] [PubMed] [Google Scholar]
- Brandt P. W., Gluck B., Mini M., Cerri C. Hysteresis of the mammalian pCa/tension relation is small and muscle specific. J Muscle Res Cell Motil. 1985 Apr;6(2):197–205. doi: 10.1007/BF00713061. [DOI] [PubMed] [Google Scholar]
- Cannell M. B., Allen D. G. Model of calcium movements during activation in the sarcomere of frog skeletal muscle. Biophys J. 1984 May;45(5):913–925. doi: 10.1016/S0006-3495(84)84238-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannell M. B. Effect of tetanus duration on the free calcium during the relaxation of frog skeletal muscle fibres. J Physiol. 1986 Jul;376:203–218. doi: 10.1113/jphysiol.1986.sp016149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caputo C., Edman K. A., Lou F., Sun Y. B. Variation in myoplasmic Ca2+ concentration during contraction and relaxation studied by the indicator fluo-3 in frog muscle fibres. J Physiol. 1994 Jul 1;478(Pt 1):137–148. doi: 10.1113/jphysiol.1994.sp020237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claflin D. R., Morgan D. L., Julian F. J. Earliest mechanical evidence of cross-bridge activity after stimulation of single skeletal muscle fibers. Biophys J. 1990 Mar;57(3):425–432. doi: 10.1016/S0006-3495(90)82559-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claflin D. R., Morgan D. L., Stephenson D. G., Julian F. J. The intracellular Ca2+ transient and tension in frog skeletal muscle fibres measured with high temporal resolution. J Physiol. 1994 Mar 1;475(2):319–325. doi: 10.1113/jphysiol.1994.sp020072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrunz L. E., Backx P. H., Yue D. T. Steady-state [Ca2+]i-force relationship in intact twitching cardiac muscle: direct evidence for modulation by isoproterenol and EMD 53998. Biophys J. 1995 Jul;69(1):189–201. doi: 10.1016/S0006-3495(95)79889-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo M., Iino M. Specific perforation of muscle cell membranes with preserved SR functions by saponin treatment. J Muscle Res Cell Motil. 1980 Mar;1(1):89–100. doi: 10.1007/BF00711927. [DOI] [PubMed] [Google Scholar]
- Gillis J. M. Relaxation of vertebrate skeletal muscle. A synthesis of the biochemical and physiological approaches. Biochim Biophys Acta. 1985 Jun 3;811(2):97–145. doi: 10.1016/0304-4173(85)90016-3. [DOI] [PubMed] [Google Scholar]
- Goldman Y. E., Hibberd M. G., Trentham D. R. Relaxation of rabbit psoas muscle fibres from rigor by photochemical generation of adenosine-5'-triphosphate. J Physiol. 1984 Sep;354:577–604. doi: 10.1113/jphysiol.1984.sp015394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Gunter T. E., Gunter K. K., Sheu S. S., Gavin C. E. Mitochondrial calcium transport: physiological and pathological relevance. Am J Physiol. 1994 Aug;267(2 Pt 1):C313–C339. doi: 10.1152/ajpcell.1994.267.2.C313. [DOI] [PubMed] [Google Scholar]
- Güth K., Potter J. D. Effect of rigor and cycling cross-bridges on the structure of troponin C and on the Ca2+ affinity of the Ca2+-specific regulatory sites in skinned rabbit psoas fibers. J Biol Chem. 1987 Oct 5;262(28):13627–13635. [PubMed] [Google Scholar]
- HUXLEY A. F. Muscle structure and theories of contraction. Prog Biophys Biophys Chem. 1957;7:255–318. [PubMed] [Google Scholar]
- Hill A. V. The Combinations of Haemoglobin with Oxygen and with Carbon Monoxide. I. Biochem J. 1913 Oct;7(5):471–480. doi: 10.1042/bj0070471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang T., Acosta D., Jr Mitochondrial Ca2+ overload in primary cultures of rat renal cortical epithelial cells by cytotoxic concentrations of cyclosporine: a digitized fluorescence imaging study. Toxicology. 1995 Jan 6;95(1-3):155–166. doi: 10.1016/0300-483x(94)02901-6. [DOI] [PubMed] [Google Scholar]
- Julian F. J. The effect of calcium on the force-velocity relation of briefly glycerinated frog muscle fibres. J Physiol. 1971 Oct;218(1):117–145. doi: 10.1113/jphysiol.1971.sp009607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi M., Hollingworth S., Harkins A. B., Baylor S. M. Myoplasmic calcium transients in intact frog skeletal muscle fibers monitored with the fluorescent indicator furaptra. J Gen Physiol. 1991 Feb;97(2):271–301. doi: 10.1085/jgp.97.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer S. S. The regulatory switch of the muscle thin filament: Ca2+ or myosin heads? J Muscle Res Cell Motil. 1994 Jun;15(3):232–236. doi: 10.1007/BF00123476. [DOI] [PubMed] [Google Scholar]
- Metzger J. M. Myosin binding-induced cooperative activation of the thin filament in cardiac myocytes and skeletal muscle fibers. Biophys J. 1995 Apr;68(4):1430–1442. doi: 10.1016/S0006-3495(95)80316-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popp D., Maéda Y. Calcium ions and the structure of muscle actin filament. An X-ray diffraction study. J Mol Biol. 1993 Jan 20;229(2):279–285. doi: 10.1006/jmbi.1993.1032. [DOI] [PubMed] [Google Scholar]
- Shiner J. S., Solaro R. J. Activation of thin-filament-regulated muscle by calcium ion: considerations based on nearest-neighbor lattice statistics. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4637–4641. doi: 10.1073/pnas.79.15.4637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerblad H., Allen D. G. The influence of intracellular pH on contraction, relaxation and [Ca2+]i in intact single fibres from mouse muscle. J Physiol. 1993 Jul;466:611–628. [PMC free article] [PubMed] [Google Scholar]
- Westerblad H., Allen D. G. The role of sarcoplasmic reticulum in relaxation of mouse muscle; effects of 2,5-di(tert-butyl)-1,4-benzohydroquinone. J Physiol. 1994 Jan 15;474(2):291–301. doi: 10.1113/jphysiol.1994.sp020022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D. A., Fay F. S. Intracellular calibration of the fluorescent calcium indicator Fura-2. Cell Calcium. 1990 Feb-Mar;11(2-3):75–83. doi: 10.1016/0143-4160(90)90061-x. [DOI] [PubMed] [Google Scholar]
- Yue D. T., Marban E., Wier W. G. Relationship between force and intracellular [Ca2+] in tetanized mammalian heart muscle. J Gen Physiol. 1986 Feb;87(2):223–242. doi: 10.1085/jgp.87.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]


