Abstract
Oral strains of Actinomyces spp. express type 1 fimbriae, which are composed of major FimP subunits, and bind preferentially to salivary acidic proline-rich proteins (APRPs) or to statherin. We have mapped genetic differences in the fimP subunit genes and the peptide recognition motifs within the host proteins associated with these differential binding specificities. The fimP genes were amplified by PCR from Actinomyces viscosus ATCC 19246, with preferential binding to statherin, and from Actinomyces naeslundii LY7, P-1-K, and B-1-K, with preferential binding to APRPs. The fimP gene from the statherin-binding strain 19246 is novel and has about 80% nucleotide and amino acid sequence identity to the highly conserved fimP genes of the APRP-binding strains (about 98 to 99% sequence identity). The novel FimP protein contains an amino-terminal signal peptide, randomly distributed single-amino-acid substitutions, and structurally different segments and ends with a cell wall-anchoring and a membrane-spanning region. When agarose beads with CNBr-linked host determinant-specific decapeptides were used, A. viscosus 19246 bound to the Thr42Phe43 terminus of statherin and A. naeslundii LY7 bound to the Pro149Gln150 termini of APRPs. Furthermore, while the APRP-binding A. naeslundii strains originate from the human mouth, A. viscosus strains isolated from the oral cavity of rat and hamster hosts showed preferential binding to statherin and contained the novel fimP gene. Thus, A. viscosus and A. naeslundii display structurally variant fimP genes whose protein products are likely to interact with different peptide motifs and to determine animal host tropism.
Colonization of host surfaces by commensal and pathogenic bacteria depends on bacterial adhesive and metabolic activities. Actinomyces naeslundii and Actinomyces viscosus form considerable portions of the microflora on oral surfaces in humans and in animal species like the rat and hamster, respectively. Adhesion of A. naeslundii (A. naeslundii genospecies 1 and 2 and A. viscosus serotype II, according to current taxonomy [28]) involves two distinct fimbriae, type 1 and type 2, while less is known about adhesive properties in A. viscosus (A. viscosus serotype I [28]) (6, 7, 20, 49). Type 1 fimbriae promote a protein-protein interaction with tooth-adsorbed salivary acidic proline-rich proteins (APRPs) and statherin (13, 15), while type 2 fimbriae bind to β-linked galactose and galactosamine structures on epithelial and bacterial cell surfaces (18, 34, 52, 56). Type 1 fimbriae are consequently thought to participate in early plaque development, and type 2 fimbriae are thought to participate in late plaque development and colonization of the oral mucosa (6, 7, 12, 18, 49, 50, 56). Thus, expression of type 1 and type 2 fimbriae and their variant binding types (20, 49) enables A. naeslundii to establish distinct intraoral colonization niches (6, 11, 18).
The genes encoding the major subunits of A. naeslundii type 1 and type 2 fimbriae have been cloned and sequenced from genospecies 2 (strain T14V) and genospecies 1 (ATCC 12104T), respectively (58–60). The type 1 (fimP) and type 2 (fimA) fimbrial subunit genes encode structural FimP and FimA subunit proteins of 533 and 534 amino acids, respectively, that have 34% amino acid identity. Both subunit proteins contain an amino-terminal signal peptide, seven conserved proline-containing regions, and a carboxy-terminal membrane-spanning domain in close proximity to a cell wall-anchoring LPXTG sequence (60). Moreover, the fimP gene is present with a cluster of six additional genes that are collectively involved in the biogenesis and function of type 1 fimbriae (61). To date, the precise genetic determinant responsible for APRP-binding activity has not been determined. In addition, the degree of structural variation within the type 1 major fimbrial subunit remains unknown.
APRPs and statherin are polymorphic multifunctional salivary proteins with an amino-terminal region for interactions with calcium and tooth surfaces (21, 26, 31, 42). The variants of APRPs are divided into two classes: allelic large APRPs (PIF-s, Db-s, Pa, PRP-1, and PRP-2), encoded by the PRH1 and PRH2 loci on chromosome 12p13.2 (4), and posttranslational variants, or small APRPs (PRP-3, PRP-4, PIF-f, and Db-f) (21). Moreover, statherin displays four structural variants due to alternative RNA splicing and posttranslational proteolytic cleavage (statherin, SV3, SV2, and SV4) (26). APRPs and statherin seem to be present in monkeys (43, 44), rabbits (48), rats (29), and hamsters (35), as well as humans, although the information is limited.
Like that of A. naeslundii (13, 15), the adhesion of streptococci (14, 16, 23), Candida albicans (27), and Porphyromonas gingivalis (2) is promoted by APRPs and statherin. The binding sites for A. naeslundii LY7 (45) and Streptococcus gordonii Blackburn (16) reside in the Pro149Gln150 carboxy termini of APRPs. The patterns of binding to APRPs and statherin differ among bacterial species and individual strains of A. naeslundii (20, 49, 51). Thus, strains of A. naeslundii genospecies 1 and 2 from the human mouth show preferential binding to APRPs over statherin (18), while A. viscosus ATCC 19246, originating from a human with cervicofacial actinomycosis, displays preferential binding to statherin (51). DNA-DNA hybridization with specific fimP probes shows a genetic diversity among fimP genes encoding type 1 fimbriae (20, 49). Recently, recombinant P. gingivalis fimbrillin, which binds to statherin and to APRPs, was found to interact with Leu29Tyr30 and Tyr41Thr42Phe43 of statherin (1). However, no further information is available concerning bacterial binding sites in statherin.
The aim of the present study was to characterize the type 1 fimbrial fimP subunit genes and host peptide recognition motifs associated with the different statherin- and APRP-binding specificities and to investigate the biological significance of this variation in binding specificity. We show that human oral isolates of A. naeslundii genospecies 2, with preferential binding to APRPs, contain structurally conserved fimP subunit genes, while A. viscosus ATCC 19246 contains a novel fimP gene which is associated with a carboxy-terminal ThrPhe recognition motif in statherin. The novel fimP gene and statherin-binding specificity were demonstrated in A. viscosus strains originating from the oral cavities of rat and hamster hosts.
MATERIALS AND METHODS
Bacterial strains, culture conditions, and radiolabeling.
A. naeslundii genospecies 2 P-1-K and B-1-K (18, 19), LY7 (from R. J. Gibbons, Forsyth Dental Center, Boston, Mass.), and T14V (from P. Kolenbrander, National Institutes of Health, Bethesda, Md.) and A. viscosus R28 (from M. Yeung, University of Texas Health Science Center, San Antonio [57]), 14476, 35451, and 35452 (all three from the Culture Collection of the University of Göteborg [CCUG]), and ATCC 19246 (51) were used in this study (Table 1). All strains were cultured overnight at 37°C in a nitrogen atmosphere with 5% CO2 and 10% H2 on Columbia II agar base plates (Becton Dickinson and Company, Cockeysville, Md.) supplemented with 30 ml of a human erythrocyte suspension per liter. For hydroxyapatite binding experiments, the bacteria were metabolically labeled by adding [35S]methionine (200 μCi) (Tran 35S-Label; ICN Pharmaceuticals Inc., Irvine, Calif.) to bacteria suspended in 100 μl of 10 mM phosphate-buffered saline, pH 7.2, prior to growth. For peptide binding experiments, strains LY7 and ATCC 19246 were metabolically labeled with 4 μCi of [3H]thymidine (ICN Pharmaceuticals) per ml, grown in Todd-Hewitt broth supplemented with 0.2% yeast extract (Difco Laboratories, Detroit, Mich.), and incubated in an atmosphere of 80% N2, 10% CO2, and 10% H2 (BBL Gaspack; Becton Dickinson) at 37°C for 18 h (LY7) or 26 h (ATCC 19246).
TABLE 1.
Sequence identity among fimP subunit genes of A. naeslundii and A. viscosus with statherin- or APRP-binding specificities
| Straina | Binding specificityb
|
DNA identityc | Site of isolationd | |
|---|---|---|---|---|
| APRPs | Statherin | |||
| A. naeslundii | ||||
| T14V | +++ (72) | + (6) | Reference | Human periodontal pocket (7) |
| LY7 | ++ (56) | + (1) | 98.1 | Human dental plaque (13) |
| P-1-K | ++ (26) | + (1) | 98.6 | Human dental plaque (18) |
| B-1-K | +++ (62) | + (3) | 98.6 | Human buccal mucosa (18) |
| A. viscosus ATCC 19246 | ++ (35) | +++ (72) | 83.7 | Human cervicofacial actinomycosis (51) |
The strains were identified by multivariate statistical analyses of phenotypic characteristics, serological reactions, and protein banding patterns of cell extracts analyzed by SDS-PAGE (18).
Binding to APRPs or statherin was scored as strong (+++), moderate (++), or weak (+) based on adhesion of radiolabeled bacteria to protein-coated hydroxyapatite beads. The figures within parentheses give the proportion (percentage of added cells) of bacteria attaching to protein-coated hydroxyapatite beads.
Nucleotide sequence identity (%) of the fimP genes (GenBank accession numbers are given in Materials and Methods) compared to the fimP gene of T14V reference strain (60). Ten regions of comparably high identity (83 to 97%; nucleotide sequences 1 to 210, 226 to 303, 361 to 501, 517 to 576, 595 to 741, 823 to 870, 949 to 1200, 1225 to 1266, 1297 to 1431, and 1456 to 1599) are interspersed with 10 regions of lower identity (38 to 78%; nucleotide sequences 211 to 225, 304 to 360, 502 to 516, 577 to 594, 742 to 822, 871 to 948, 1201 to 1224, 1267 to 1296, 1432 to 1455, and 1600 to 1617). The numbering starts from the bp 1 to 3 ATG start codon after alignment to the fimP gene of strain T14V.
Site of isolation, with a literature reference (in parentheses). The P-1-K and B-1-K strains were from different sites in the same individual but belonged to different ribotypes (19).
Typing of Actinomyces.
The strains originating from the human oral cavity (B-1-K, P-1-K, LY7, and T14V) and strain ATCC 19246 were characterized by the API products rapid ID 32 strep and ID coryne and by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) of the whole-cell soluble proteins (18). The strains originating from the oral cavities of rat and hamster hosts (35452, R28, 35451, and 14476) were characterized by SDS-PAGE of whole-cell soluble proteins. All strains were then analyzed by whole-cell agglutination by specific rabbit antisera 1 to 7 (40) (kindly provided by G. H. Bowden, University of Manitoba, Winnipeg, Canada).
Chromosomal DNA isolation.
Chromosomal DNA was isolated according to procedures described earlier (20).
PCR.
PCRs were performed in 50-μl reaction volumes containing 1 μg of chromosomal DNA, 0.8 μM (each) primer, 1.6 U of Taq DNA polymerase (MBI Fermentas Ltd., Vilnius, Lithuania), 200 μM (each) dATP, dGTP, dCTP, and dTTP (deoxynucleoside triphosphate [dNTP] mixture; Boehringer Mannheim, Mannheim, Germany), 0.75 mM MgCl2, and 0.02 μg of bovine serum albumin in PCR buffer (10 mM Tris-HCl, 50 mM KCl, 0.08% Nonidet P-40, pH 8.8). For strain ATCC 19246, the MgCl2 concentration was increased to 1.5 mM. To digoxigenin label the DNA fragments, the dNTP mixture was replaced with DIGdNTP labeling mixture (Boehringer Mannheim) in the second PCR amplification.
Three PCR programs were used. PCR program 1 involved 1 cycle of denaturing at 94°C for 5 min, annealing at 59°C for 150 s, and elongation at 72°C for 150 s, followed by 33 cycles of denaturing at 94°C for 75 s, annealing at 59°C for 150 s, and elongation at 72°C for 150 s and a final extension at 72°C for 7 min. PCR program 2 involved denaturing at 94°C for 4 min followed by three cycles of denaturing at 94°C for 60 s, annealing at 60°C for 60 s, and elongation at 72°C for 60 s. This was then followed by additional cycles differing only in the annealing temperatures: 57°C (3 cycles), 54°C (3 cycles), 53°C (3 cycles), and 51°C (24 cycles). The final extension was at 72°C for 7 min. PCR program 3 involved 30 cycles of denaturing at 94°C for 60 s, annealing at 55°C for 90 s, and elongation at 72°C for 180 s.
PCR primer pairs.
To amplify and sequence fimP genes and to generate DNA probes, the following primers were designed and used (the gene, strain origin, and primer locations are given within parentheses): no. 1, 5′-ACCCTCTCCGGTGTGGACAA-3′ (fimP; strain T14V [60]; forward primer, bp 550 to 569 counting from the bp 1 to 3 ATG start codon for all fimP genes); no. 5, 5′-ACAGCAATGCACTCCCTCAA-3′ (fimP; strain T14V; forward primer, bp −6 to 14); no. 27, 5′-TACGAGTGCACCAAGACCGC-3′ (fimP; strain T14V; forward primer, bp 1147 to 1166); no. 36, 5′-TGGTAAGCAGACCTTCACGACTGAG-3′ (fimP; strain 19246; forward primer, bp 1239 to 1263); no. 50, 5′-ACCTCGTTCTGACCGACGAT-3′ (fimP; strain T14V; reverse primer, bp 758 to 739); no. 52, 5′-IGGIGCYTTIGTYTCIAC-3′ (fimP; strain T14V; reverse primer, bp 1368 to 1351); no. 54, 5′-TGCTTGGCAACGTGACGGC-3′ (fimP; strain T14V; reverse primer, bp 1598 to 1580); no. 61, 5′-GCGGTCTTGGTGCACTCGTA-3′ (fimP; strain T14V; reverse primer, bp 1166 to 1147); no. 22, 5′-CATCCCAACAACACAGGAG-3′ (upstream fimP; strain T14V; forward primer, bp 66 to 84 according to reference 60); no. 76, 5′-TCACCTCAGTGGCTGCCAGT-3′ (downstream fimP; strain T14V; reverse primer, bp 1730 to 1711 according to reference 60); and no. 86, 5′-GGTCACGAAGTGATGGGAGTAG-3′ (orf4; strain T14V; reverse primer, bp 6672 to 6651 according to reference 61).
The fimP genes of strains B-1-K, P-1-K, and ATCC 19246 were generated from chromosomal DNA by first amplifying an internal fimP gene fragment, followed by a gene fragment upstream and one downstream from the internal fragment. The fimP genes of strains P-1-K and B-1-K were first amplified with primers 5 and 54 (PCR program 1), followed by primers 22 and 50 and primers 27 and 76 (both in PCR program 2). The fimP gene of strain ATCC 19246 was first amplified by primers 1 and 52 (PCR program 2), followed by primers 22 and 61 (PCR program 2) and 36 and 86 (PCR program 3). The fimP gene of strain LY7 was generated by amplifying two overlapping gene fragments with primers 22 and 50 and primers 1 and 76 (both in PCR program 2).
DNA probes corresponding to either the full-length fimP gene or a central portion of the gene, encoding preferential binding to APRPs (type 1:1 fimP gene) or to statherin (type 1:2 fimP gene), were generated for use in slot blot or Southern blot hybridization assays. A full-length and a central fimP DNA probe were generated from the fimP gene of strain T14V chromosomal DNA as previously described (20). A central type 1:2 fimP probe was amplified from the fimP gene of strain ATCC 19246 chromosomal DNA with primers 1 and 52 (PCR program 2).
Nucleotide sequencing.
The PCR product was electrophoretically analyzed on 1% agarose gels with 0.3 μg of ethidium bromide/ml in 1× Tris-borate-EDTA (TBE) buffer. The PCR fragment was then purified with phenol chloroform and ligated to the pGEM-T vector with T4 DNA ligase (Promega Corp., Madison, Wis.) according to the manufacturer’s instructions. The ligation mixture was transformed into Escherichia coli JM 109 competent cells (Promega), and transformants containing the appropriate clone were identified by growth on Luria-Bertani agar plates containing carbenicillin (50 μg/ml), 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal; 40 μg/ml), and isopropyl-β-d-thiogalactopyranoside (IPTG; 0.1 mM). Plasmid DNA was isolated with the plasmid Maxi kit (Qiagen GmbH, Hilden, Germany), and the sizes of DNA inserts were subsequently confirmed by SalI and NcoI (Promega) cleavage.
Sequencing reactions, employing the Thermo Sequenase radiolabeled terminator cycle sequencing kit (Amersham-Life Science, Cleveland, Ohio), were performed according to the manufacturer’s instructions. The T7 and SP6 sequencing primers (Pharmacia Biotech, Uppsala, Sweden) and additional internal synthetic primers specific for cloned DNA were used.
Southern blot analysis.
Chromosomal DNA (8 μg) from strain ATCC 19246 was digested with BssHII and BamHI, and DNAs from B-1-K, P-1-K, LY7, and T14V were digested with BamHI prior to separation on 0.7% agarose gels. Southern blotting was performed with nylon membranes (Hybond-N+; Amersham International plc, Amersham, United Kingdom). Nylon membranes containing bacterial DNA were probed with fimP type 1:1 full-length (LY7, B-1-K, and P-1-K) and fimP central type 1:2 (ATCC 19246) DNA probes. Prehybridization was performed at 70°C for 4 h in a solution of 5× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate), 0.02% N-lauroylsarcosine, 0.1% SDS, and 1% blocking reagent (Boehringer Mannheim), and hybridization was done at 70°C overnight in prehybridization solution containing the probe (20). The membranes were then washed twice in 2× SSC–0.1% SDS buffer at room temperature for 5 min and twice in 0.5× SSC–0.1% SDS buffer at 70°C for 15 min. DNA hybridized to probe DNA was detected with a digoxigenin DNA detection kit (Boehringer Mannheim) as described by the manufacturer.
Slot blot hybridization assay.
Chromosomal DNA (3 μg) was loaded on nylon membranes (Hybond N+) with a slot blot manifold filter system (Milliblot-S; Millipore, Västra Frölunda, Sweden) as described previously (3). Essentially, hybridization with fimP type 1:1 full-length DNA probe and central type 1:1 and type 1:2 DNA probes and washing and detection conditions were as described for Southern blot hybridization except that hybridization and the second stringent washing were done at 80°C. The degree of hybridization was scored from 0 to 6 by comparison with a standardized scale based on densitometric measures (20) (GS-700 imaging densitometer and Molecular Analyst software; Bio-Rad, Hercules, Calif.): 0, <0.01; 1, 0.01 to <0.04; 2, 0.04 to <0.10; 3, 0.10 to <0.16; 4, 0.16 to <0.22; 5, 0.22 to <0.27; and 6, ≥0.27.
Computer analysis.
Complete open reading frames of fimP nucleotide sequences, deduced amino acid sequences, and hydrophobicity profiles (30) were analyzed with the Wisconsin Package (version 9.0) from the Genetics Computer Group (University of Wisconsin, Madison).
Isolation of APRPs and statherin.
Freshly collected parotid saliva from one individual homozygous for allelic APRP variants (PRP-1 and PIF-s) was separated on a DEAE-Sephacel column (15 by 1.6 cm; Pharmacia) with a linear gradient of 25 mM to 1.0 M NaCl in 50 mM Tris-HCl, pH 8.0. The peak containing APRPs and statherin was concentrated by ultrafiltration on a Centriprep 10 concentrator (Amicon Inc., Beverly, Mass.) and separated by gel filtration (HiLoad 26/60 Superdex S-200 Prepgrade; Pharmacia) in 20 mM Tris-HCl–0.5 M NaCl, pH 8.0. Each of the resolved PRP-1–PIF-s, PRP-3–PIF-f, and statherin protein peaks was finally purified on a Macroprep high Q column (15 by 1.6 cm; Bio-Rad) with a linear gradient of 25 mM to 1.0 M NaCl in 50 mM Tris-HCl, pH 8.0. The identities and purities of APRPs and statherin were confirmed by SDS-PAGE, native alkaline electrophoresis, NH2-terminal amino acid sequencing, and bacterial binding properties.
Peptide synthesis.
Synthetic decapeptides mimicking the carboxy-terminal portion of statherin and with a core of six Asp and various Glu residues were synthesized (Quality Controlled Biochemicals Inc., Hopkinton, Mass.). The amino and carboxy termini were unmodified. High-performance liquid chromatography with reversed-phase and anion-exchange columns confirmed that the purity of the peptides exceeded 95%. The synthetic decapeptides mimicking the carboxy-terminal portions of APRPs were characterized as previously described (16). A Glu6 hexapeptide was used as a control (Sigma Chemical Co., St. Louis, Mo.).
Linking of peptides to CNBr-agarose beads.
Linkage of peptides to CNBr-activated Sepharose 6MB agarose beads (Pharmacia) was performed as described by the manufacturer. Essentially, the activated beads were incubated for at least 20 min in 1 mM HCl and washed with HCl on a sintered glass filter. The peptides, dissolved in coupling buffer (0.1 M NaHCO3–0.5 M NaCl, pH 8.3), were added to the beads on a molarity basis and rotated end over end for 2 h at room temperature. The beads were sedimented by low-speed centrifugation (300 × g for 15 min) and washed with coupling buffer. Reversed-phase high-performance liquid chromatography on the supernatants confirmed linking of peptides. Any remaining CNBr groups were blocked with glycine (0.2 M) followed by five washes in coupling buffer alternated with five washes in acetate buffer (0.1 M sodium acetate–0.5 M NaCl, pH 4.0) on a sintered glass filter. The prepared beads were stable for at least 1 week in coupling buffer at 4°C.
Binding of bacteria to peptides linked to agarose beads.
Samples containing 7 mg of the peptide-linked agarose beads were aliquoted into each well of a microtiter plate and washed three times in KCl buffer supplemented with 0.5% albumin. Following washings in buffered KCl and resuspension in buffered KCl supplemented with albumin, aliquots of 125 μl of a suspension (16 × 104 cpm/ml; 6 × 108 bacteria/ml) of strains ATCC 19246 and LY7 were added to each well, and the mixture was rotated end over end for 1 h at room temperature. Triplicate samples were done. After the samples were washed with KCl-albumin buffer, the number of bacteria remaining attached to the beads was estimated by scintillation counting.
Hydroxyapatite assay.
Adherence of [35S]methionine-labeled bacteria (5 × 104 to 15 × 104 cpm/ml; 5 × 108 bacteria/ml) to purified proteins (6 μg/ml) adsorbed onto hydroxyapatite beads (Fluka, Chemie AG, Buchs, Switzerland) was measured as described previously (13).
Nucleotide sequence accession numbers.
The GenBank accession numbers for the fimP gene sequences are AF106035 (strain B-1-K), AF107020 (strain LY7), AF107019 (strain P-1-K), AF106034 (strain ATCC 19246), and M32067 (strain T14V) (60).
RESULTS
Highly conserved fimP subunit genes in A. naeslundii isolates with preferential binding to APRPs.
To investigate the degree of structural diversity in fimP genes among A. naeslundii genospecies 2 strains, fimP genes were amplified by PCR and sequenced from strains LY7, P-1-K, and B-1-K (Table 1). Each fimP gene had an open reading frame of 1,602 nucleotides and a putative ribosome binding site with similarity to the E. coli consensus sequence (33) 9 nucleotides upstream of the ATG start codon. No internal repeat sequences were observed, and each fimP gene contained a high G/C ratio (67 mol%). The respective fimP gene had between 98.1 and 98.6% nucleotide sequence identity to the fimP gene of A. naeslundii T14V (60) (Table 1). Nucleotide substitutions were randomly distributed throughout the individual fimP genes. Thus, the fimP genes from isolates with the same APRP-binding specificity were highly conserved.
A novel fimP subunit gene in A. viscosus ATCC 19246 with preferential binding to statherin.
To investigate the genetic basis for preferential binding of A. viscosus ATCC 19246 to statherin, the fimP gene was amplified by PCR and sequenced from strain ATCC 19246. The fimP gene contained 1,608 nucleotides and a putative ribosome binding site similar to the E. coli consensus sequence 9 nucleotides upstream of the ATG start codon. This fimP gene had a G+C content of 63.5 mol%, and no internal repeat sequences were identified. The fimP gene from strain ATCC 19246 had 83.7% nucleotide sequence identity to the fimP gene of A. naeslundii T14V (60) (Table 1). Interspersed with 10 regions of comparably high sequence identity (ranging from 83 to 97%) were regions of lower sequence identity (ranging from 38 to 77%). In addition, evenly distributed single-nucleotide substitutions were present. Thus, A. viscosus ATCC 19246 exhibits a genetically variant fimP gene conferring preferential binding to statherin.
In Southern blot hybridization of restriction enzyme-digested chromosomal DNA from strains ATCC 19246 (BssHII and BamHI digestion), LY7, P-1-K, and B-1-K (BamHI digestion) with fimP DNA probes, hybridization to single DNA fragments occurred (data not shown). Thus, the fimP genes were present as single gene copies.
Predicted amino acid sequence for the novel FimP subunit protein associated with statherin-binding specificity.
The fimP gene of A. viscosus ATCC 19246 encodes a protein product with 535 amino acid residues. The N terminus contains a putative signal peptide containing a 22-amino-acid hydrophobic segment (residues 9 to 30) preceded by an 8-amino-acid stretch harboring three basic amino acids (Fig. 1). The structural arrangement of amino acids at the C terminus of the signal peptide suggests a probable cleavage site between the two alanine residues at positions 30 and 31 (55). Cleavage would generate a mature fimbrial subunit protein of 505 residues (53.56 kDa). The major structural portion of FimP is dominated by hydrophilic amino acid residues consistent with an isoelectric point of 4.7, contains four cysteine residues, and is rich in Thr (13.4%). The FimP protein ends with a cell wall-anchoring LeuProLeuThrGly sequence followed by a membrane-spanning domain composed of 17 hydrophobic amino acids and a short charged tail of 5 basic amino acids (Fig. 1).
FIG. 1.
Amino acid sequence alignment of the FimP major subunit proteins of A. naeslundii T14V (upper sequence) and A. viscosus ATCC 19246 (lower sequence). Identical (|), conserved (:), and similar (.) amino acids are indicated. Boldface indicates a putative N-terminal signal peptide with a cleavage site (↓) and a C-terminal LPXTG sequence. The seven shaded boxes indicate domains, each incorporating a proline residue and reported to be conserved between the FimP and FimA subunit proteins (60).
Comparison of the variant FimP subunit proteins.
The novel FimP protein of A. viscosus ATCC 19246 had 80.6% amino acid sequence identity to the 533-amino-acid-residue FimP protein of A. naeslundii T14V. The FimP proteins from A. naeslundii LY7, P-1-K, and B-1-K showed 97.4 to 98.7% amino acid sequence identity to FimP of A. naeslundii T14V. The A. viscosus ATCC 19246 FimP protein showed 10 regions of high sequence identity (≥85%) to the FimP protein of A. naeslundii T14V, and 7 of those regions contained proline residues (Fig. 1), which are considered to be conserved in FimP and FimA proteins (60). Four cysteine residues were present in both FimP proteins. Interspersed between the high-sequence-identity regions were regions of lower amino acid sequence identity (≤52%), including a 19-amino-acid region (residues 102 to 120) with only 21% identity.
The variant FimP subunit proteins are associated with ThrPhe and ProGln recognition motifs in statherin and APRPs.
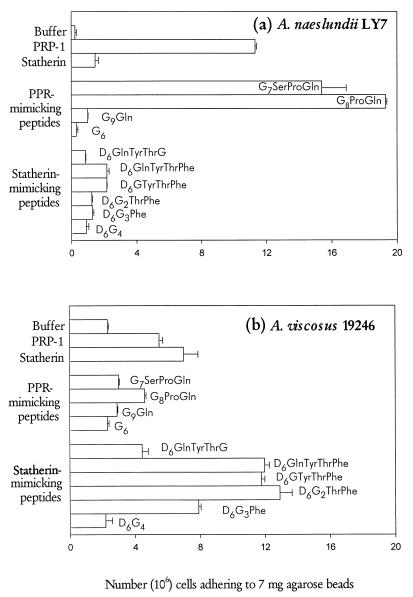
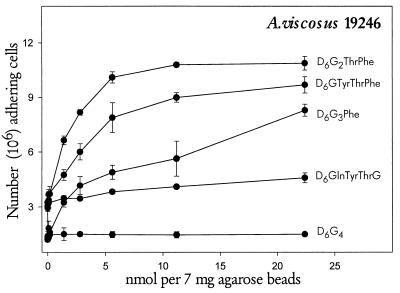
To investigate the peptide recognition motif in statherin for A. viscosus ATCC 19246, radiolabeled bacteria were bound to agarose beads with covalently linked pure PRP-1, statherin, or synthetic decapeptides mimicking the carboxy termini of the two proteins (Fig. 2 and 3). A. viscosus ATCC 19246 and A. naeslundii LY7 bound preferentially to statherin and PRP-1, respectively, although binding to the reciprocal protein was observed (Fig. 2). Binding to fixed and varying amounts of synthetic peptides (Figs. 2 and 3) distinguished similar characteristics for the recognition motifs in statherin and PRP-1. First, A. viscosus ATCC 19246 and A. naeslundii LY7 bound most abundantly to the C-terminal segments of statherin (Thr42Phe43) and PRP-1 (Pro149Gln150), respectively. Second, addition to the synthetic peptides of residue 41 (Tyr) and 148 (Ser), respectively, reduced, rather than enhanced, binding by bacteria. In addition, substitution of Gly for Phe at residue 43 of statherin dramatically diminished binding by strain ATCC 19246, which displayed partial binding activity to peptides harboring only the Phe43 residue of statherin.
FIG. 2.
Adhesion of A. naeslundii LY7 (a) and A. viscosus ATCC 19246 (b) to statherin or PRP-1 (0.6 nmol/7 mg of beads) and to statherin- or PRP-1-mimicking decapeptides (17.4 nmol/7 mg of beads) covalently linked to agarose beads. The bars represent the means (plus standard errors) of two independent experiments in which triplicate samples were assayed.
FIG. 3.
Adhesion of A. viscosus ATCC 19246 to varying amounts of statherin-mimicking decapeptides covalently linked to agarose beads. Triplicate determinations were used in two independent experiments. The data are presented as means ± standard errors.
The variant FimP proteins are typical of strains of Actinomyces originating from the oral cavity of human versus rat and hamster hosts.
To investigate the biological significance of the variant FimP proteins, Actinomyces strains of different animal origins were tested for binding specificity and the presence of variant fimP genes (Table 2). Oral isolates of A. viscosus from rats and hamsters displayed preferential binding to statherin, and chromosomal DNA from these strains hybridized with DNA probes specific to the fimP gene encoding statherin-binding fimbriae. In contrast, chromosomal DNA from isolates of A. naeslundii from the human mouth with preferential binding to APRPs hybridized exclusively with a DNA probe specific to the fimP gene encoding APRP-binding fimbriae. Thus, the variant FimP proteins may determine the animal tropism for A. naeslundii and A. viscosus.
TABLE 2.
Presence of variant FimP subunit proteins in A. naeslundii and A. viscosus strains of different animal host origins
| Straina | Binding specificityb
|
Hybridization with fimP DNA probesc
|
FimP proteind
|
Origine | ||||
|---|---|---|---|---|---|---|---|---|
| APRPs | Statherin | Type 1 full-length probe | Type 1:1 central probe | Type 1:2 central probe | Type 1:1 | Type 1:2 | ||
| A. naeslundii T14V | +++ (72) | + (6) | 5 | 4 | 0 | Yes | No | Human periodontal pocket (7) |
| A. naeslundii LY7 | ++ (56) | + (1) | 4 | 3 | 0 | Yes | No | Human dental plaque (13) |
| A. naeslundii P-1-K | ++ (26) | + (1) | 4 | 4 | 0 | Yes | No | Human dental plaque (18) |
| A. naeslundii B-1-K | +++ (62) | + (3) | 5 | 4 | 0 | Yes | No | Human buccal mucosa (18) |
| A. viscosus ATCC 19246 | ++ (35) | +++ (72) | 3 | 0 | 5 | No | Yes | Human actinomycosis (51) |
| A. viscosus 35452 | ++ (44) | +++ (73) | 4 | 0 | 5 | No | Yes | Rat mouth (CCUG) |
| A. viscosus R28 | ++ (56) | +++ (81) | 4 | 0 | 5 | No | Yes | Rat mouth (57) |
| A. viscosus 35451 | ++ (38) | +++ (70) | 4 | 0 | 4 | No | Yes | Hamster mouth (CCUG) |
| A. viscosus 14476 | ++ (37) | +++ (80) | 4 | 0 | 4 | No | Yes | Hamster mouth (CCUG) |
The isolates were identified by multivariate statistical analyses of phenotypic characteristics, serological reactions, and protein banding patterns of cell extracts analyzed by SDS-PAGE (18).
Binding to APRPs or statherin was scored as strong (+++), moderate (++), or weak (+) based on adhesion of radiolabeled bacteria to protein-coated hydroxyapatite beads. The figures within parentheses give the proportion (percentage of added cells) of bacteria attaching to protein-coated hydroxyapatite beads.
The fimP type 1:1 full-length DNA probe was generated from the entire fimP type 1 fimbrial gene of A. naeslundii T14V, and the fimP central type 1:1 (APRP-binding specificity) and type 1:2 (statherin-binding specificity) DNA probes were generated from the central segments of the fimP type 1 fimbrial genes of A. naeslundii T14V and A. viscosus ATCC 19246, respectively (see reference 20 and Materials and Methods). The DNA probes were labeled with digoxigenin and used in slot blot hybridization to probe chromosomal DNA from Actinomyces spp. under high-stringency conditions. The degree of hybridization was scored from 0 to 6 by comparison with a scale based on densitometric measures: 0, 0.01; 1, 0.01 to <0.04; 2, 0.04 to <0.10; 3, 0.10 to <0.16; 4, 0.16 to <0.22; 5, 0.22 to <0.27; and 6, ≥0.27.
Presence of the different FimP subunit proteins based on binding specificity and fimP DNA probe hybridization pattern.
Host and tissue origins of the strains, with references (parentheses).
DISCUSSION
In the present study, we show genetically variant fimP subunit genes in A. viscosus and A. naeslundii that correlate to different ThrPhe and ProGln recognition motifs in statherin and APRPs and to different animal host tropisms. Similar to other surface proteins of gram-positive bacteria, the novel statherin-binding FimP protein contains a signal peptide and ends with cell wall-anchoring and membrane-spanning domains. Our findings extend the present knowledge of surface-associated proteins for bacterial adherence and colonization, and Actinomyces spp. forming distinct fimbriae may serve as a model to study the assembly and function of adhesive organelles in gram-positive bacteria.
The statherin-binding FimP protein of A. viscosus ATCC 19246 contains features typical of surface-associated proteins of gram-positive bacteria (9, 37), including (i) an amino-terminal signal peptide and (ii) a carboxy-terminal portion with a cell wall-anchoring (LPXTG motif) and a putative membrane-anchoring domain. The FimP protein lacks both tandem repeats and a distinct Pro-rich wall-spanning domain, which are typical features of the M and Sfb proteins of Streptococcus pyogenes (22, 53) and the collagen binding adhesin of Staphylococcus aureus (39). Similar to FimA (60), the FimP proteins contain seven proline-containing regions and four cysteine residues. The proline residues may maintain extended peptide conformations (36) or mediate noncovalent protein-protein interactions (10, 38, 41), while the cysteine residues may form disulfide bridges stabilizing functional domains for subunit-subunit interactions or adhesion activity (24).
The present findings show that structurally variant FimP proteins on A. viscosus and A. naeslundii recognize statherin and APRPs differently. The structural variation resulting from both single-amino-acid substitutions and prominent structural changes reduces the amino acid identity between the two FimP proteins to about 80%. Both single-amino-acid substitutions, as shown for S fimbriae of E. coli (17), and prominent structurally different amino-terminal domains, as shown for the tip-localized minor G adhesin of P fimbriae of E. coli (24), are known to alter binding specificity. The single-amino-acid substitutions changing binding specificity of S fimbriae reside in the fimbrial subunit and not in the minor SfaS adhesin (17), and structural differences in the subunit of mannose-binding fimbriae of E. coli affect the minor FimH adhesin specificity (32). However, since only a limited number of genes are involved in type 1 fimbriae biogenesis and function (61), and since the E. coli K88 and K99 major fimbrial subunits contain a carbohydrate or protein binding domain (5, 25), we suspect the FimP proteins to contain the statherin- or APRP-binding domain.
Another finding of the present study is the highly conserved nature of fimP genes from isolates with preferential APRP binding. Higher-than-97% nucleotide and amino acid sequence identity was observed among Actinomyces isolates originating from the human mouth. This is a novel finding, since the major subunit genes of P fimbriae of E. coli isolates from urinary tract infections display extensive genetic diversity as a result of antigenic variation (8, 54). The conserved nature of the fimP fimbrial genes may be a unique feature of Actinomyces spp., which are commensal bacteria colonizing the human mouth from birth (11). Early colonization by bacteria could induce an immunological tolerance toward surface-associated bacterial proteins generally considered to be immunodominant. One may speculate that FimP proteins from A. viscosus strains with preferential binding to statherin would also have highly conserved sequences.
The present findings provide evidence for a carboxy-terminal Thr42Phe43 motif in statherin targeted by the novel FimP protein of A. viscosus ATCC 19246. Among peptides mimicking the carboxy terminus of statherin, those containing Thr42Phe43 promoted high bacterial binding activity while residues 41 (Tyr) and 40 (Gln) did not contribute to binding. Moreover, substitution of Phe43 markedly diminished binding activity, and peptides harboring only Phe43 showed partial binding activity. Interestingly, the carboxy-terminal Pro149Gln150 motif in APRPs targeted by A. naeslundii LY7 displays similar characteristics (45). The dual binding activity of statherin and APRPs could be explained either by mimicking recognition motifs or the actual presence of ProGln and Phe in statherin and APRPs, respectively. Furthermore, since a diversity of fimP gene structure seems to exist in Actinomyces (20) and since both A. naeslundii genospecies 1 and 2 seem to harbor variant APRP-binding specificities (18), a further diversity of recognition motifs may exist among Actinomyces strains.
Taken together, the present findings suggest that variation in binding specificities may determine the ecological niches for isolates of Actinomyces spp. Significantly, in this study we have observed a relationship between genetic variation of fimP genes, altered binding specificity for host proteins, and tissue origin of bacterial isolates. While the APRP-binding FimP protein is a typical characteristic of A. naeslundii strains originating from different sites in the human mouth, the statherin-binding FimP protein seemed to be present in A. viscosus strains from the oral cavities of the rat and the hamster. Although this implies variation in type 1 fimbrial binding specificity in animal tropism, it should be noted that A. viscosus ATCC 19246 originates from a human case of cervicofacial actinomycosis (51) and that A. naeslundii contains serologically defined subpopulations of possibly deviating adhesive phenotypes (20, 28, 49). Interestingly, recent studies with E. coli have suggested that single-amino-acid substitutions in the mannose-binding FimH adhesin may change commensal phenotypes to pathogenic types by shifting their ecological niches (46, 47). Thus, structural variations in type 1 fimbriae could affect the specificity of Actinomyces spp. for different animal hosts and ecological niches as well as being involved in various skin or soft tissue infections.
ACKNOWLEDGMENTS
This study was supported by grants 10906 and 11302 from the Swedish Medical Research Board, the Marabou Research Foundation, Sweden, and by grants DE8601 and DE7009 from the National Institutes of Health, Bethesda, Md.
REFERENCES
- 1.Amano A, Kataoka K, Raj P A, Genco R J, Shizukuishi S. Binding site of salivary statherin for Porphyromonas gingivalis recombinant fimbrillin. Infect Immun. 1996;64:4249–4254. doi: 10.1128/iai.64.10.4249-4254.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amano A, Sojar H T, Lee J-Y, Sharma A, Levine M J, Genco R J. Salivary receptors for recombinant fimbrillin of Porphyromonas gingivalis. Infect Immun. 1994;62:3372–3380. doi: 10.1128/iai.62.8.3372-3380.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Current protocols in molecular biology. Vol. 1. New York, N.Y: John Wiley & Sons, Inc.; 1993. pp. 2.9.16–2.9.20. [Google Scholar]
- 4.Azen E A. Genetics of salivary protein polymorphisms. Crit Rev Oral Biol Med. 1993;4:479–485. doi: 10.1177/10454411930040033201. [DOI] [PubMed] [Google Scholar]
- 5.Bakker D, Willemsen P T, Simons L H, van Zijderveld F G, de Graaf F K. Characterization of the antigenic and adhesive properties of FaeG, the major subunit of K88 fimbriae. Mol Microbiol. 1992;6:247–255. doi: 10.1111/j.1365-2958.1992.tb02006.x. [DOI] [PubMed] [Google Scholar]
- 6.Cisar J O, Sandberg A L, Mergenhagen S E. The function and distribution of different fimbriae on strains of Actinomyces viscosus and Actinomyces naeslundii. J Dent Res. 1984;63:393–396. doi: 10.1177/00220345840630030701. [DOI] [PubMed] [Google Scholar]
- 7.Cisar J O, Vatter A E, Clark W B, Curl S H, Hurst-Calderone S, Sandberg A L. Mutants of Actinomyces viscosus T14V lacking type 1, type 2 or both types of fimbriae. Infect Immun. 1988;56:2984–2989. doi: 10.1128/iai.56.11.2984-2989.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Denich K, Blyn L B, Craiu A, Braaten B A, Hardy J, Low D A, O’Hanley P D. DNA sequences of three papA genes from uropathogenic Escherichia coli strains: evidence of structural and serological conservation. Infect Immun. 1991;59:3849–3858. doi: 10.1128/iai.59.11.3849-3858.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dramsi S, Dehoux P, Cossart P. Common features of Gram-positive bacterial proteins involved in cell recognition. Mol Microbiol. 1993;9:1119–1122. doi: 10.1111/j.1365-2958.1993.tb01241.x. [DOI] [PubMed] [Google Scholar]
- 10.Einbond A, Sudol M. Towards prediction of cognate complexes between the WW domain and proline-rich ligands. FEBS Lett. 1996;384:1–8. doi: 10.1016/0014-5793(96)00263-3. [DOI] [PubMed] [Google Scholar]
- 11.Ellen R P. Establishment and distribution of Actinomyces viscosus and Actinomyces naeslundii in the human oral cavity. Infect Immun. 1976;14:1119–1124. doi: 10.1128/iai.14.5.1119-1124.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gibbons R J. Bacterial adhesion to oral tissues: a model for infectious diseases. J Dent Res. 1989;56:439–445. doi: 10.1177/00220345890680050101. [DOI] [PubMed] [Google Scholar]
- 13.Gibbons R J, Hay D I. Human salivary acidic proline-rich proteins and statherin promote the attachment of Actinomyces viscosus LY7 to apatitic surfaces. Infect Immun. 1988;56:439–445. doi: 10.1128/iai.56.2.439-445.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gibbons R J, Hay D I. Adsorbed salivary acidic proline-rich proteins contribute to the adhesion of Streptococcus mutans JBP to apatitic surfaces. J Dent Res. 1989;68:1303–1307. doi: 10.1177/00220345890680090201. [DOI] [PubMed] [Google Scholar]
- 15.Gibbons R J, Hay D I, Cisar J O, Clark W B. Adsorbed salivary proline-rich protein 1 and statherin: receptors for type 1 fimbriae of Actinomyces viscosus T14V-J1 on apatitic surfaces. Infect Immun. 1988;56:2990–2993. doi: 10.1128/iai.56.11.2990-2993.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gibbons R J, Hay D I, Schlesinger D H. Delineation of a segment of adsorbed salivary acidic proline-rich proteins which promotes adhesion of Streptococcus gordonii to apatitic surfaces. Infect Immun. 1991;59:2948–2954. doi: 10.1128/iai.59.9.2948-2954.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hacker J, Kestler H, Hoschützky H, Jann K, Lottspeich F, Korhonen T K. Cloning and characterization of the S fimbrial adhesion II complex of an Escherichia coli O18:K1 meningitis isolate. Infect Immun. 1993;61:544–550. doi: 10.1128/iai.61.2.544-550.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hallberg K, Hammarström K-J, Falsen E, Dahlén G, Gibbons R J, Hay D I, Strömberg N. Actinomyces naeslundii genospecies 1 and 2 express different binding specificities to N-acetyl-β-d-galactosamine, whereas Actinomyces odontolyticus expresses a different binding specificity in colonizing the human mouth. Oral Microbiol Immunol. 1998;13:327–336. doi: 10.1111/j.1399-302x.1998.tb00687.x. [DOI] [PubMed] [Google Scholar]
- 19.Hallberg K, Holm C, Hammarström K-J, Kalfas S, Strömberg N. Ribotype diversity of Actinomyces with similar intraoral tropism but different types of N-acetyl-β-d-galactosamine binding specificity. Oral Microbiol Immunol. 1998;13:188–192. doi: 10.1111/j.1399-302x.1998.tb00731.x. [DOI] [PubMed] [Google Scholar]
- 20.Hallberg K, Holm C, Öhman U, Strömberg N. Actinomyces naeslundii displays variant fimP and fimA fimbrial subunit genes corresponding to different types of acidic proline-rich protein and beta-linked galactosamine binding specificity. Infect Immun. 1998;66:4403–4410. doi: 10.1128/iai.66.9.4403-4410.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hay D I, Bennick A, Schlesinger D H, Minaguchi K, Madapallimattam G, Schluckebier S K. The primary structures of six human salivary acidic proline-rich proteins (PRP-1, PRP-2, PRP-3, PRP-4, PIF-s and PIF-f) Biochem J. 1988;255:15–21. doi: 10.1042/bj2550015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hollingshead S K, Fischetti V A, Scott J R. Complete nucleotide sequence of type 6 M protein of the group A Streptococcus. Repetitive structure and membrane anchor. J Biol Chem. 1986;261:1677–1686. [PubMed] [Google Scholar]
- 23.Hsu S D, Cisar J O, Sandberg A L, Kilian M. Adhesive properties of viridans streptococcal species. Microb Ecol Health Dis. 1994;7:125–137. [Google Scholar]
- 24.Hultgren S J, Abraham S, Caparon M, Falk P, St. Geme III J W, Normark S. Pilus and nonpilus bacterial adhesins: assembly and function in cell recognition. Cell. 1993;73:887–901. doi: 10.1016/0092-8674(93)90269-v. [DOI] [PubMed] [Google Scholar]
- 25.Jacobs A A C, Simons B H, de Graaf F K. The role of lysine-132 and arginine-136 in the receptor-binding domain of the K99 fibrillar subunit. EMBO J. 1987;6:1805–1808. doi: 10.1002/j.1460-2075.1987.tb02434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jensen J L, Lamkin M S, Troxler R F, Oppenheim F G. Multiple forms of statherin in human salivary secretions. Arch Oral Biol. 1991;36:529–534. doi: 10.1016/0003-9969(91)90147-m. [DOI] [PubMed] [Google Scholar]
- 27.Johansson, I., P. Bratt, D. I. Hay, S. Schluckebier, and N. Strömberg. Unpublished data. [DOI] [PubMed]
- 28.Johnson J L, Moore L V, Kaneko B, Moore W E. Actinomyces georgiae sp. nov., Actinomyces gerencseriae sp. nov., designation of two genospecies of Actinomyces naeslundii, and inclusion of A. naeslundii serotypes II and III and Actinomyces viscosus serotype II in A. naeslundii genospecies 2. Int J Syst Bacteriol. 1990;40:273–286. doi: 10.1099/00207713-40-3-273. [DOI] [PubMed] [Google Scholar]
- 29.Kopec L K, Bowen W H. Adherence of microorganisms to rat salivary pellicles. Caries Res. 1995;29:507–512. doi: 10.1159/000262122. [DOI] [PubMed] [Google Scholar]
- 30.Kyte J, Doolittle R F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982;157:105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- 31.Lamkin M S, Oppenheim F G. Structural features of salivary function. Crit Rev Oral Biol Med. 1993;4:251–259. doi: 10.1177/10454411930040030101. [DOI] [PubMed] [Google Scholar]
- 32.Madison B, Ofek I, Clegg S, Abraham S N. Type 1 fimbrial shafts of Escherichia coli and Klebsiella pneumoniae influence sugar-binding specificities of their FimH adhesins. Infect Immun. 1994;62:843–848. doi: 10.1128/iai.62.3.843-848.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maizels N. E. coli lactose operon ribosome binding site. Nature. 1974;249:647–649. doi: 10.1038/249647b0. [DOI] [PubMed] [Google Scholar]
- 34.McIntire F C, Crosby L K, Barlow J J, Matta K L. Structural preferences of β-galactoside-reactive lectins on Actinomyces viscosus T14V, and Actinomyces naeslundii WVU45. Infect Immun. 1983;41:848–850. doi: 10.1128/iai.41.2.848-850.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mehansho H, Ann D K, Butler L G, Rogler J, Carlson M. Induction of proline-rich proteins in hamster salivary glands by isoproterenol treatment and an unusual growth inhibition by tannins. J Biol Chem. 1987;262:12344–12350. [PubMed] [Google Scholar]
- 36.Naganagowda G A, Gururaja T L, Levine M J. Delineation of conformational preferences in human salivary statherin by 1H, 31P NMR and CD studies: sequential assignment and structure-function correlations. J Biomol Struct Dyn. 1998;16:91–107. doi: 10.1080/07391102.1998.10508230. [DOI] [PubMed] [Google Scholar]
- 37.Navarre W W, Schneewind O. Proteolytic cleavage and cell wall anchoring at the LPXTG motif of surface proteins in Gram-positive bacteria. Mol Microbiol. 1994;14:115–121. doi: 10.1111/j.1365-2958.1994.tb01271.x. [DOI] [PubMed] [Google Scholar]
- 38.Niebuhr K, Ebel F, Frank R, Reinhard M, Domann E, Carl U D, Walter U, Gertler F B, Wehland J, Chakraborty T. A novel proline-rich motif present in ActA of Listeria monocytogenes and cytoskeletal proteins is the ligand for the EVH1 domain, a protein module present in the Ena/VASP family. EMBO J. 1997;16:5433–5444. doi: 10.1093/emboj/16.17.5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Patti J M, Jonsson H, Guss B, Switalski L M, Wiberg K, Lindberg M, Höök M. Molecular characterization and expression of a gene encoding a Staphylococcus aureus collagen adhesin. J Biol Chem. 1992;267:4766–4772. [PubMed] [Google Scholar]
- 40.Putnins E E, Bowden G H. Antigenic relationships among oral Actinomyces isolates, Actinomyces naeslundii genospecies 1 and 2, Actinomyces howellii, Actinomyces denticolens, and Actinomyces slackii. J Dent Res. 1993;72:1374–1385. doi: 10.1177/00220345930720100601. [DOI] [PubMed] [Google Scholar]
- 41.Ren R, Mayer B J, Cicchetti P, Baltimore D. Identification of a ten-amino acid proline-rich SH3 binding site. Science. 1993;259:1157–1161. doi: 10.1126/science.8438166. [DOI] [PubMed] [Google Scholar]
- 42.Schlesinger D H, Hay D I. Complete covalent structure of statherin, a tyrosine-rich acidic peptide which inhibits calcium phosphate precipitation from human parotid saliva. J Biol Chem. 1977;252:1689–1695. [PubMed] [Google Scholar]
- 43.Schlesinger D H, Hay D I. Primary structure of the active tryptic fragments of human and monkey salivary anionic proline-rich proteins. Int J Pept Protein Res. 1981;17:34–41. doi: 10.1111/j.1399-3011.1981.tb01965.x. [DOI] [PubMed] [Google Scholar]
- 44.Schlesinger D H, Hay D I, Levine M J. Complete primary structure of statherin, a potent inhibitor of calcium phosphate precipitation, from the saliva of the monkey, Macaca arctoides. Int J Pept Protein Res. 1989;34:374–380. doi: 10.1111/j.1399-3011.1989.tb00705.x. [DOI] [PubMed] [Google Scholar]
- 45.Schluckebier S K, Hay D I, Gibbons R J, Schlesinger D H, Ferland M J. Effects of structural variations in carboxy-terminal residues of human salivary acidic proline-rich proteins. J Dent Res. 1992;71:762. . (Abstract.) [Google Scholar]
- 46.Sokurenko E V, Chesnokova V, Doyle R J, Hasty D L. Diversity of the Escherichia coli type 1 fimbrial lectin. Differential binding to mannosides and uroepithelial cells. J Biol Chem. 1997;272:17880–17886. doi: 10.1074/jbc.272.28.17880. [DOI] [PubMed] [Google Scholar]
- 47.Sokurenko E V, Chesnokova V, Dykhuizen D E, Ofek I, Xue-Ru W, Krogfelt K A, Struve C, Schembri M A, Hasty D L. Pathogenic adaptation of Escherichia coli by natural variation of the FimH adhesin. Proc Natl Acad Sci USA. 1997;95:8922–8926. doi: 10.1073/pnas.95.15.8922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Spielman A I, Bernstein A, Hay D I, Blum M, Bennick A. Purification and characterization of a rabbit salivary protein, a potent inhibitor of crystal growth of calcium phosphate salts. Arch Oral Biol. 1991;36:55–63. doi: 10.1016/0003-9969(91)90054-x. [DOI] [PubMed] [Google Scholar]
- 49.Strömberg N, Ahlfors S, Borén T, Bratt P, Hallberg K, Hammarström K-J, Holm C, Johansson I, Järvholm M, Kihlberg J, Li T, Ryberg M, Zand G. Anti-adhesion and diagnostic strategies for oro-intestinal bacterial pathogens. Adv Exp Med Biol. 1996;408:9–24. doi: 10.1007/978-1-4613-0415-9_2. [DOI] [PubMed] [Google Scholar]
- 50.Strömberg N, Borén T. Actinomyces tissue specificity may depend on differences in receptor specificity for GalNAc β-containing glycoconjugates. Infect Immun. 1992;60:3268–3277. doi: 10.1128/iai.60.8.3268-3277.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Strömberg N, Borén T, Carlén A, Olsson J. Salivary receptors for GalNAc β-sensitive adherence of Actinomyces spp.: evidence for heterogeneous GalNAc β and proline-rich protein receptor properties. Infect Immun. 1992;60:3278–3286. doi: 10.1128/iai.60.8.3278-3286.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Strömberg N, Karlsson K-A. Characterization of the binding of Actinomyces naeslundii (ATCC 12104) and Actinomyces viscosus (ATCC 19246) to glycosphingolipids, using a solid-phase overlay approach. J Biol Chem. 1990;265:11251–11258. [PubMed] [Google Scholar]
- 53.Talay S R, Valentin-Weigand P, Timmis K N, Chhatwal G S. Domain structure and conserved epitopes of Sfb protein, the fibronectin-binding adhesin of Streptococcus pyogenes. Mol Microbiol. 1994;13:531–539. doi: 10.1111/j.1365-2958.1994.tb00448.x. [DOI] [PubMed] [Google Scholar]
- 54.van Die I, Hoekstra W, Bergmans H. Analysis of the primary structure of P-fimbrillins of uropathogenic Escherichia coli. Microb Pathog. 1987;3:149–154. doi: 10.1016/0882-4010(87)90091-x. [DOI] [PubMed] [Google Scholar]
- 55.von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986;14:4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Whittaker C J, Klier C M, Kolenbrander P E. Mechanisms of adhesion by oral bacteria. Annu Rev Microbiol. 1996;50:513–552. doi: 10.1146/annurev.micro.50.1.513. [DOI] [PubMed] [Google Scholar]
- 57.Yeung M K. Conservation of an Actinomyces viscosus T14V type 1 fimbrial subunit homology among divergent groups of Actinomyces spp. Infect Immun. 1992;60:1047–1054. doi: 10.1128/iai.60.3.1047-1054.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yeung M K, Chassy B M, Cisar J O. Cloning and expression of a type 1 fimbrial subunit of Actinomyces viscosus T14V. J Bacteriol. 1987;169:1678–1683. doi: 10.1128/jb.169.4.1678-1683.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yeung M K, Cisar J O. Cloning and nucleotide sequence of a gene for Actinomyces naeslundii WVU45 type 2 fimbriae. J Bacteriol. 1988;170:3803–3809. doi: 10.1128/jb.170.9.3803-3809.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yeung M K, Cisar J O. Sequence homology between the subunits of two immunologically and functionally distinct types of fimbriae of Actinomyces spp. J Bacteriol. 1990;172:2462–2468. doi: 10.1128/jb.172.5.2462-2468.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yeung M K, Ragsdale P A. Synthesis and function of Actinomyces naeslundii T14V type 1 fimbriae require the expression of additional fimbria-associated genes. Infect Immun. 1997;65:2629–2639. doi: 10.1128/iai.65.7.2629-2639.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]