Abstract
Motility of Helicobacter species has been shown to be essential for successful colonization of the host. We have investigated the organization of a flagellar export locus in Helicobacter pylori. A 7-kb fragment of the H. pylori CCUG 17874 genome was cloned and sequenced, revealing an operon comprising an open reading frame of unknown function (ORF03), essential housekeeping genes (ileS and murB), flagellar export genes (fliI and fliQ), and a homolog to a gene implicated in virulence factor transport in other pathogens (virB11). A promoter for this operon, showing similarity to the Escherichia coli ς70 consensus, was identified by primer extension. Cotranscription of the genes in the operon was demonstrated by reverse transcription-PCR, and transcription of virB11, fliI, fliQ, and murB was detected in human or mouse biopsies obtained from infected hosts. The genetic organization of this locus was conserved in a panel of H. pylori clinical isolates. Engineered fliI and fliQ mutant strains were completely aflagellate and nonmotile, whereas a virB11 mutant still produced flagella. The fliI and fliQ mutant strains produced reduced levels of flagellin and the hook protein FlgE. Production of OMP4, a member of the outer membrane protein family identified in H. pylori 26695, was reduced in both the virB11 mutant and the fliI mutant, suggesting related functions of the virulence factor export protein (VirB11) and the flagellar export component (FliI).
Helicobacter pylori is a spiral, gram-negative, microaerophilic, slow-growing bacterium (5). It is a very common human gastrointestinal pathogen, with estimated prevalences of up to 50 and 90% in developed and developing countries, respectively (11). Colonization of the gastric epithelium is linked to chronic superficial gastritis that may subsequently lead to peptic ulceration (5). In addition, gastric adenocarcinoma and mucosa-associated lymphoid tissue lymphoma are recognized as possible outcomes of an H. pylori infection (27, 59).
Motility of H. pylori cells is one of the few traits experimentally proven as necessary for successful colonization in an animal model (12, 13). The bacterium moves by virtue of a bundle of lophotrichate sheathed flagella (18). Investigation of the structural flagellar compounds revealed a number of differences from the gram-negative bacterium Salmonella typhimurium, which can be regarded as the model organism for flagellar biosynthesis (36). In H. pylori, the flagellar filament consists of two separate flagellins, the more abundant FlaA (34) and a second, slightly larger protein, FlaB (30). The flagellin genes are under the control of different promoters (ς28 for flaA; ς54 for flaB), they are not genetically linked, and they share 58% identical amino acids (51). Expression of both flagellins appears to be necessary for full motility (29) and is required for the establishment of persistent H. pylori infection in gnotobiotic piglets (13).
A third structural gene of the H. pylori flagellum, which encodes the hook protein FlgE, has been studied. Isogenic mutants lacking this gene are aflagellate and nonmotile and were not compromised in flagellin production, indicating a lack of the key negative regulatory element FlgM in H. pylori (43). In S. typhimurium, FlgM inhibits flagellin transcription in response to a defective hook-basal body complex (26). In H. pylori, the only regulatory element shown to be indispensable for flagellin and hook protein production is FlbA, a member of the FlbF/LcrD protein family (47).
The annotated genome sequence of H. pylori 26695 contains at least 40 genes likely to be needed for flagellar function and/or assembly (53). Genetic organization of these elements in H. pylori differs dramatically from that found in other bacterial genomes (1, 6, 16, 31). Whereas many flagellar genes appear to be clustered in well-defined genomic regions in S. typhimurium, Escherichia coli, Bacillus subtilis, and Borrelia burgdorferi, they are scattered throughout the H. pylori genome. Furthermore, H. pylori exhibits a number of other atypical features in its flagellar genetics. Some well-established flagellar genes could not be identified in the H. pylori 26695 genome; these include the gene for the anti-sigma factor, flgM; the hook length controller, fliK; and the master regulators flhC and flhD. However, the H. pylori genome contains genes for several additional flagellar proteins, including second homologues for the flagellins and the hook protein and two putative flaG genes encoding polar flagellins (53). In addition to pecularities possibly specific to a specialized gastric pathogen, the altered complement of flagellar genes may be a feature of bacteria using unipolar flagella for motility, or they may help the bacterium to evade the mucosal immune response of the host.
Our understanding of the flagellum-specific transport process is still relatively limited. Evidence has accumulated for a number of proteins, including FliI, FliP, FliQ, FliR, and FlhB, being part of a flagellar export apparatus (37, 39, 57, 62). One of the candidates, FliI, is an ATPase necessary for flagellar assembly (14). The most interesting feature of all presumptive flagellum-specific export apparatus components is their striking similarity to type III export systems (38) in a variety of organisms, including proteins of the spa (surface presentation of invasion plasmid antigens) locus of Shigella flexneri (56), the ysc-encoded type III secretion system of Yops in Yersinia pseudotuberculosis (49), and the hrp (hypersensitive reaction and pathogenicity) gene clusters in Xanthomonas campestris (15) and Pseudomonas syringae (23) (for an overview of homologs, see reference 33). On the basis of this sequence identity and mechanistic similarities, export of flagellar components may thus be classified as a variety of type III secretion. However, despite striking identities of up to 50% (10, 21), functional complementation between members of the type III secretion and flagellar export systems has not yet been reported.
The main objective of this study was to investigate a locus of the H. pylori genome which encodes proteins likely to be involved in flagellum-specific export. We report on the unique linkage of genes for a tRNA synthetase (ileS), a protein with a predicted function in virulence factor transfer (virB11), two flagellar export apparatus candidates (fliI and fliQ), and an enzyme presumably involved in cell wall metabolism (murB). We extend the results of a characterization of fliI published during the course of this study (28) and examine the effect of knockout mutations of virB11, fliI, and fliQ on expression and localization of both flagellar and nonflagellar components to assess gene functions and involvement in export processes.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
H. pylori strains used in this study were CCUG 17874 (identical to NCTC 11637, the type strain of H. pylori); CCUG 915 (Culture Collection of the University of Göteborg [Göteborg, Sweden] isolate), a type II strain (25); SS1, the so-called Sydney strain, a clinical isolate from a gastroenterology clinic in Sydney, Australia, that has been adapted to mice (32); and New Zealand isolates from the P. W. O’Toole laboratory strain collection (32). E. coli ER2206 [endA1 thi1 supE44 mcr67 (mcrA) (mcrBC-hsdRMS-mrr)114::IS10 (lac)U169/F′ proAB lacIq Z M15 Tn10], a kind gift from New England Biolabs (Beverly, Mass.), was used as host strain for plasmid cloning experiments. The H. mustelae type strain 4298 was from J. G. Fox (Division of Comparative Medicine, Massachusetts Institute of Technology, Cambridge, Mass.).
Bacteria were cultured as described previously (41), using chocolate blood agar plates at 37°C in an atmosphere containing 5% CO2 for H. pylori. Alternatively, H. pylori liquid cultures were grown in tryptone soya broth under agitation in microaerobic conditions generated by CampyGen sachets (Oxoid Ltd., Basingstoke, Hampshire, United Kingdom). Antibiotics were added to growth media as required, using the following levels for E. coli: ampicillin, 100 μg/ml; kanamycin, 50 μg/ml; and chloramphenicol, 10 μg/ml. H. pylori transformants were selected on chocolate blood agar plates containing chloramphenicol at 10 μg/ml.
Molecular techniques.
Standard procedures and plasmids were used for plasmid cloning in E. coli (46). Vectors and recombinant plasmids used in this study are listed in Table 1. Helicobacter DNA was isolated as previously described (43). A genomic library of H. pylori CCUG 17874 was constructed in λZAP EXPRESS (Stratagene, La Jolla, Calif.) by ligating genomic DNA partially digested with Sau3A to BamHI-digested, calf intestinal phosphatase-treated phage arms as previously described (43). Gel-excised restriction fragments or PCR products were purified with Qiaex resin (Qiagen Inc., Valencia, Calif.). Alternatively, Hi-Pure columns (Boehringer Mannheim, Mannheim, Germany) were used to deproteinize modification reactions. Oligonucleotides used in this study are described in Table 2. PCRs were performed with custom primers (Life Technologies, Inc., Gaithersburg, Md.) and 0.5 U of Pwo polymerase (Boehringer Mannheim) or Taq polymerase (Life Technologies) per reaction. DNA probes were labeled directly by incorporation of digoxigenin-dUTP (Boehringer Mannheim) during PCR or by using an ECL labeling/detection kit from Amersham Life Science Ltd., Buckinghamshire, United Kingdom. Southern hybridization was performed under conditions of high stringency (46), and detection was carried out as recommended by the manufacturer of the probe label.
TABLE 1.
Plasmids used in this study
| Plasmid | Characteristicsa | Reference or source |
|---|---|---|
| pUC18 | Apr; ColE1 ori; blue/white; MCS | 60 |
| pUC19 | Apr; ColE1 ori; blue/white; MCS | 60 |
| pBK-CMV | Neor; SV40, f1− and ColE1 ori; blue/white; MCS | Stratagene |
| pHP042 | Neor; 3.5-kb genomic fragment of H. pylori 17874 in pBK-CMV | This work |
| pRY109 | Apr; Cmr; cat gene in BamHI site of pUC18 | 61 |
| pSP101 | Apr; 6.3-kb HindIII/BglII genomic fragment of H. pylori 17874 in HindIII/BamHI-cut pUC18 | This work |
| pSP102 | Apr; 7-kb HindIII/BglII genomic fragment of H. pylori 17874 in HindIII/BamHI-cut pUC18 | This work |
| pSP105 | Apr; composite fliQ PCR fragment in SmaI-cut pUC19 | This work |
| pSP106 | Apr; composite fliI PCR fragment in SmaI/HincII-cut pUC19 | This work |
| pSP107 | Apr; Cmr; cat gene from pRY109 in primer introduced BglII site of pSP105 | This work |
| pSP110 | Apr; Cmr; cat gene from pRY109 in primer introduced BamHI site of pSP106 | This work |
| pSP116 | Apr; composite virB11 PCR fragment in SmaI-cut pUC19 | This work |
| pSP118 | Apr; Cmr; cat gene from pRY109 in primer introduced BamHI site of pSP116 | This work |
ColE1, colicin E1 origin of replication; MCS, multiple cloning site; SV40, simian virus 40.
TABLE 2.
Oligonucleotide primers
| Primer | Sequence 5′-3′ | Comments; priming orientation | Function |
|---|---|---|---|
| FP | GTT TTC CCA GTC ACG AC | pUC18/19; ←a | fliI and fliQ mutagenesis |
| SP002 | CAA ATC ATC GCC CTC TAC TAG C | Internal fliI; ← | fliI probe preparation; fliI transcript detection |
| SP003 | ATC CGC ACT TTC CTT ATT GCC | Internal fliI; → | fliI probe preparation; fidelity test pSP102 |
| SP009 | CCC ATC AAT GCT CTT CAC C | Internal fliI; ← | virB11 mutagenesis; conservation studies |
| SP010 | GTG CCG ATT TTC ACA CTG C | Near murB 5′ end; ← | Fidelity test pSP102; transcript analysis |
| SP013 | GTG CTT AAC CCT TTA GGG C | Internal fliI; → | Operon conservation studies; characterization of H. pylori fliI mutants; fliI transcript detection |
| SP014 | GCA CGA TCA CAT CAA TCG C | Near virB11 3′ end; ← | Characterization of H. pylori virB11 mutants; virB11 transcript detection |
| SP015 | GTG TGC CTA GGC TTA AGC | Internal virB11; → | fliQ mutagenesis; transcript analysis; virB11 transcript detection |
| SP016 | GCA CAC GGA TGT TGA GCG | Internal virB11; ← | Transcript analysis |
| SP019 | GAA ACG CCA GCA TTC ACG | Near ileS 3′ end; → | fliI mutagenesis; genotype characterization of H. pylori virB11 mutants |
| SP020 | CCA AAC CAG AAT GAT GGC C | Near ORF02 5′ end; → | Transcript analysis |
| SP021 | CCA TCG TGG ATC CGG GCT GCA TGA CAG GCG CTA ATC GC | Internal fliI; ←; 5′ tail sticky to SP022, BamHI site | fliI mutagenesis |
| SP022 | GCA GCC CGG ATC CAC GAT GGG ACA TCA TCA GCG AGT CTC | Internal fliI; →; 5′ tail sticky to SP021, BamHI site | fliI mutagenesis |
| SP023 | CCA TCG TAG ATC TGG GCT GCG CGA GTT TCA TGA GTT GTG | Near fliQ 5′ end; ←; 5′ tail sticky to SP024, BglII site | fliQ mutagenesis; characterization of H. pylori flil mutants |
| SP024 | GCA GCC CAG ATC TAC GAT GGA CCG GTA TTA CTA GCG GG | Near fliQ 5′ end; →; 5′ tail sticky to SP023, BglII site | fliQ mutagenesis |
| SP027 | GGA ATG AGC TTG ATT AAG G | Near fliQ 3′ end; ← | Transcript analysis; characterization of H. pylori fliQ mutants; fliQ transcript detection |
| SP028 | CAC AAC TCA TGA AAC TCG C | Near fliQ 5′ end; → | Transcript analysis; characterization of H. pylori fliQ mutants; fliQ transcript detection |
| SP032 | CAT CGT AGA TCT GGG CTG CGT TGC TCG CAA AAC CTC AGC | Internal virB11; ←; 5′ tail sticky to SP033, BglII site | virB11 mutagenesis |
| SP033 | GCA GCC CAG ATC TAC GAT GGA TGA AAG GGT GGT GAG CG | Internal virB11; →; 5′ tail sticky to SP032, BglII site | virB11 mutagenesis |
| SP034 | AGG GAG CGA TCA GCA TAG G | Internal ileS; → | virB11 mutagenesis; transcript analysis; conservation studies |
| SP035 | GCA GCC CAG ATC TAC GAT GGG GCG AAT GAT TTA GAG GG | Internal murB; →; 5′ BglII site | murB transcript detection |
| SP036 | TGC TCT TTG GCA AAG CCC | Internal murB; ← | Transcript analysis; murB transcript detection; conservation studies |
| SP039 | GTG GGT TTA GTG AAG CGG | Near ORF03 5′ end; → | Transcript analysis |
| SP040 | TCT TCG CAT GAT CTC GGC | Near ileS 5′ end; ← | Transcript analysis |
| SP043 | AGC AAC CCT ACT TAT GGG | Near TIGR HP1417 3′ end; ← | Conservation studies |
| SP048 | AAC GCG CCG CTT CAC TAA ACC | Near ORF03 5′ end; ← | Primer extension |
| SP050 | ACT AGC CTT AGC GCA ACT CCC | Internal ORF03; ← | Primer extension |
| SP051 | TGG TTG TGT TTA AGT TTA GGG TGT C | Near ileS 5′ end; ← | Primer extension |
Priming orientation (relative to Fig. 1).
DNA sequence determination.
Sequence data were obtained by using custom oligonucleotide primers on double-stranded templates, Taq polymerase (Life Technologies), dye-labeled terminators, and a Perkin-Elmer 9600 thermal cycler, for analysis with an ABI Prism 377 automated sequencer. The data were assembled with the Geneworks package (IntelliGenetics, El Camino, Calif.) and analyzed with MacVector (IBI, New Haven, Conn.) and PCGene version 6.85 (IntelliGenetics) software. Databases were scanned by using the BLAST algorithm (4). Multiple sequence alignment was produced with the Clustal V program (24), using the default parameters.
Mutagenesis of H. pylori.
For the construction of mutants, a two-step PCR-based procedure followed by allelic exchange mutagenesis was used as described previously (42). PCRs were performed with plasmid pSP102 as the template.
For mutagenesis of the virB11 gene, primer pairs SP034-SP032 and SP033-SP009 were used in the first PCR step, amplifying flanking fragments of 1,300 and 900 bp, respectively. The composite fragment, amplified with SP034-SP009 in the second step, lacked 300 bp of internal virB11 sequence including the predicted ATP/GTP binding site and contained a primer-derived BglII restriction site. This fragment was cloned into SmaI-digested pUC19 to generate plasmid pSP116. The chloramphenicol resistance (cat) cassette, lacking a transcription terminator, of plasmid pRY109 (61) was cloned into the BglII site of pSP116 to result in the virB11 mutagenic plasmid pSP118.
For mutagenesis of fliI, primer pairs SP019-SP021 and SP022-FP amplified arms of 1,450 and 1,150 bp, respectively. The composite fragment, amplified with SP019-FP, contained the primer-derived BamHI site and lacked 700 bp internal fliI sequence including the sequence motifs of the Walker boxes A and B (58). The fragment was cloned into SmaI/HincII-digested pUC19. The resulting construct, pSP106, was digested with BamHI, and the cat cassette was introduced to generate the fliI mutagenic plasmid pSP110.
fliQ mutagenesis was performed as follows. Primer pairs SP015-SP023 and SP024-FP amplified left and right arms (1,950 and 650 bp, respectively); the composite fragment was obtained with SP024-FP, lacking 36 bp of internal fliQ sequence and containing a primer-derived BglII site instead. This composite fragment was cloned into SmaI-digested pUC19 to create plasmid pSP105, and the cat cassette was cloned into the BglII site to generate the fliQ mutagenic construct pSP107.
Electroporation-competent H. pylori CCUG 17874 cells were prepared (48) and transformed with the mutagenic plasmids by electroporation as described by Ge and Taylor (17).
Sample preparation for Western analysis.
H. pylori liquid cultures were centrifuged at 20,800 × g for 1 min, and the supernatant was collected and frozen at −70°C. Residual medium was removed from the cell pellet after an additional spin of 1 min at 20,800 × g. Pellets were resuspended in sterile water, boiled for 10 min, and stored at −70°C.
Alternatively, subcellular fractions were prepared as described previously (43). Briefly, cells grown on solid medium were harvested by elution in phosphate-buffered saline and collected by centrifugation at 10,000 × g for 15 min. The supernatant was removed, concentrated by ultrafiltration, and stored at −70°C as the culture supernatant fraction. The cell pellet was resuspended in Tris-saline, and the cells were lysed by sonication. The sonicate was separated into crude soluble and envelope fractions by centrifugation at 45,000 × g for 30 min. The envelope pellet was resuspended in Tris-saline and stored at −70°C until required. Partially assembled flagellar filaments and aggregated flagellin subunits were removed from the crude soluble fraction by centrifugation at 100,000 × g for 2 h, and the pelleted material also resuspended in Tris-saline.
Electrophoresis and Western blotting.
Protein samples were electrophoresed in polyacrylamide gels containing sodium dodecyl sulfate in a minigel format (8- by 5-cm separating gel). Sample quantities were standardized by using the Bio-Rad Laboratories (Hercules, Calif.) protein assay. When required, separated proteins were transferred from a polyacrylamide slab gel to nitrocellulose paper by using the methanol-Tris-glycine system described by Towbin et al. (54). Electroblotting and subsequent steps were carried out by standard methods (44). The reactive bands were visualized by using 5-bromo-4-chloro-3-indolylphosphate (Boehringer Mannheim) as the alkaline phosphatase substrate and nitroblue tetrazolium (Sigma, St. Louis, Mo.) as the color development reagent. Alternatively, 30% hydrogen peroxide solution and 4-chloro-1-naphthol (Sigma) were used as horseradish peroxidase substrate and chromogenic reagents. Primary antibodies used in this study were rabbit polyclonal anti-CagA and anti-VacA, kind gifts from T. Cover, Vanderbilt University, Nashville, Tenn.; rabbit polyclonal anti-Fla, previously described by O’Toole et al. (43); and rabbit polyclonal anti-HopB, mouse monoclonal anti-UreB, and anti-OMP4 (TIGR HP0127), kind gifts from P. Doig, Astra Research Center, Boston, Mass. (9).
Microscopy.
Motility was examined by phase-contrast microscopy at a magnification of ×600, using a Nikon Diaphot inverted microscope and a hanging drop preparation from a liquid culture of H. pylori. Cells to be examined by electron microscopy were subjected to negative staining as follows. A 200-mesh grid covered with a Formvar film was floated on a drop of the sample suspension for approximately 1 min. The excess sample was withdrawn by touching the edge of the grid to a cut edge of Whatman no. 1 filter paper. The grids were then floated onto a drop of 1% (wt/vol) phosphotungstic acid, adjusted to pH 7.0 with potassium hydroxide. The grids were examined in a Philips 201C transmission electron microscope operated at an accelerating voltage of 60 kV under conventional bright-field illumination conditions. Images were recorded on Agfa Copex Positive PET 10 film.
RNA methods.
RNA was extracted from liquid cultures of H. pylori cells, using the Trizol reagent (Life Technologies) as instructed by the manufacturers. RNA electrophoresis was performed by standard methods (46).
Transcript analyses were performed with either the Titan One Tube reverse transcriptase (RT)-mediated PCR (RT-PCR) system (Boehringer Mannheim) or the Superscript One-Step RT-PCR system (Life Technologies). Total RNA preparations were subjected to RNase-free DNase I (Boehringer Mannheim) treatment at 37°C for up to 60 min, using approximately 5 U of DNase per μg of RNA followed by 10 min of incubation at 75°C (heat inactivation). In subsequent RT-PCR trials, negative controls were performed where the RT of the RT-PCR kit used was either heat-killed prior to the reaction or replaced by Taq polymerase.
Primer extension reactions on 30 μg of total RNA were performed with a primer extension kit from Promega Inc. (Madison, Wis.) as instructed by the vendor. Sequencing reactions were generated by using 32P-end-labeled primers, 100 ng of pSP102 as the template, and an AmpliCycle sequencing kit (Perkin Elmer-Applied Biosystems, Foster City, Calif.). Reactions were separated on conventional sequencing gels (46) and visualized by autoradiography.
RT-PCR on biopsy samples.
Human biopsy samples were obtained from confirmed H. pylori culture-positive patients and were kindly supplied by Marianne Quiding-Järbrink and Ann-Mari Svennerholm, University of Göteborg. For RT-PCR from infected human and mouse tissues, the following procedure was applied. Upon retrieval, human biopsy material was placed in 500 μl of 5.0 M guanidine thiocyanate (pH 7.0)–15% (wt/vol) glycerol–0.2 M β-mercaptoethanol and flash frozen in liquid nitrogen for storage at −80°C. The β-mercaptoethanol was added just prior to use. For RNA extraction, the biopsy material was thawed, removed from the storage buffer, and resuspended in sterile water to osmotically lyse the eukaryotic cells. Bacterial cells were then spun down for 2 min at 16,000 × g in a microcentrifuge and washed once with 500 μl of sterile water. H. pylori SS1 cells (32) from experimentally infected mouse stomachs were obtained from sacrificed animals. Stomachs were surgically removed, and the inner surface was scraped to collect the bacterium-containing material. The material obtained was resuspended in sterile water to osmotically lyse the eukaryotic cells.
RNA was isolated from the human or mouse H. pylori-containing material by using a SNAP total RNA isolation kit (Invitrogen, Carlsbad, Calif.), with the following modifications. Total nucleic acids were resuspended in 175 μl of sterile H2O; 50 μl of this material was kept for positive control PCRs, and the remaining 125 μl was treated with DNase I. All RNA-containing samples were stored at −80°C.
RT-PCR was performed with a Superscript RT-PCR kit (Life Technologies). For each reaction, 1 μl of total nucleic acid was amplified with Taq DNA polymerase (positive control), 5 μl of RNA was amplified with Taq DNA polymerase (negative control), and 5 μl of RNA was amplified with RT-Taq DNA polymerase.
Nucleotide sequence accession number.
The nucleotide sequences for the H. pylori CCUG 17874 virB11, fliI, and fliQ genes reported in this article appear in the GenBank database under accession no. U75584.
RESULTS
Cloning and sequencing of an H. pylori export operon.
Predating the release of the H. pylori 26695 genome sequence, we performed random sequencing of excisant plasmids (pBK-CMV) derived from a λZAP library of partially Sau3A-digested H. pylori CCUG 17874 chromosomal DNA. One of the excisants, plasmid pHP042, contained a 3.5-kb fragment, one end of which exhibited significant similarity to fliI, the gene coding for an ATPase involved in flagellar biosynthesis (14). Primer pair SP002-SP003 was designed based on the fliI sequence present on pHP042, allowing amplification of a 96-bp fragment of the H. pylori fliI gene. This fragment was used as a specific probe against restriction-digested H. pylori 17874 genomic DNA. A 7.0-kb HindIII/BglII fragment was thus cloned into pUC18 to generate pSP102.
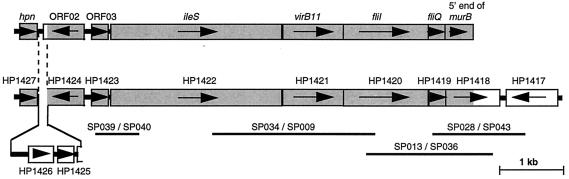
Sequencing of the pSP102 insert revealed the presence of seven complete open reading frames (ORFs) and one incomplete ORF. The genetic organization on the pSP102 insert is shown in Fig. 1. In addition, the genetic map of the corresponding region found in the H. pylori 26695 genome (53) is illustrated, including the assigned TIGR ORF numbers. Excluding a stretch of 85 bp downstream of the first ORF hpn that has been replaced by 1,387 bp encoding two new genetic elements and a changed ORF02 3′ end in H. pylori 26695, the regions are identical. However, the displayed arrangement of the pSP102 genes from hpn through murB in the pSP102 insert was found to be completely identical in the genome sequence of H. pylori J99 (3). Predicted sizes of putative gene products found in pSP102, results of similarity searches, and annotated putative gene names are summarized in Table 3.
FIG. 1.
Schematic representation of the genetic organization of the pSP102 insert cloned from H. pylori 17874 (top) and the corresponding region in the H. pylori 26695 genome (bottom). Identical predicted coding sequences are shaded. Gene annotations and TIGR ORF numbers are shown. The black bars represent regions amplified during investigation of the conservation of this locus (see also Fig. 2), and the respective primer pairs are indicated.
TABLE 3.
Genetic elements in the pSP102 insert
| ORF | Size (bp) | Predicted molecular mass of gene product (kDa) | Similarity to: | % Identity/ % similarity | Annotated gene name |
|---|---|---|---|---|---|
| 01 | 180 | 7.1 | H. pylori Hpn | 100/100 | hpn |
| 02 | 624 | 23.7 | ORF02 | ||
| 03 | 251 | 9.4 | B. subtilis 9.7-kDa hyp. protein | 35/61 | ORF03 |
| 04 | 2,760b | 106.1b | C. crescentus IleS | 49b/66b | ileS |
| E. coli IleS | 39b/56b | ||||
| 05 | 912 | 34.6 | A. tumefaciens TrbB | 28/47 | virB11 |
| E. coli TraG | 28/46 | ||||
| A. tumefaciens VirB11 | 21/39 | ||||
| H. pylori 26695 TIGR HP0525 | 29/53 | ||||
| 06 | 1,302 | 47.6 | S. typhimurium Flil | 44/60 | flil |
| A. tumefaciens Flil | 40/56 | ||||
| Y. enterocolitica YscN | 43/62 | ||||
| X. campestris HrpB6 | 43/62 | ||||
| E. amylovora HrcN | 41/62 | ||||
| 07 | 264 | 9.8 | B. subtilis FliQ | 45/65 | fliQ |
| E. coli FliQ | 51/73 | ||||
| S. typhimurium SpaQ | 30/47 | ||||
| Y. pseudotuberculosis YscS | 34/58 | ||||
| 08a | 780b | 28.6b | B. subtilis MurB | 31b/49b | murB |
First 393 nt present on pSP102.
Based on the complete gene sequences in the corresponding H. pylori 26695 region.
ORF01, the H. pylori hpn gene, encodes an extremely histidine-rich Ni2+- and Zn2+-binding polypeptide that has been studied by Gilbert et al (19). Following ORF02 and ORF03 (Table 3), ORF04 is the isoleucyl-tRNA synthetase gene of H. pylori, only a single copy of which was found in the H. pylori 26695 genome (53). The three elements which follow, ORF05, ORF06, and ORF07, are all similar to genes with predicted functions in protein export, and we refer to them collectively as a protein export locus.
We annotated ORF05, the first gene of this locus, as virB11. It displays significant identity to several genes encoding presumptive ATP-binding proteins known to be involved in nucleoprotein transport (Table 3). In the H. pylori 26695 genome, this element was annotated as trbB. However, sequence identity of 28.6% and 52.6% similarity to a virB11 homolog present on the cag pathogenicity island of H. pylori (ORF11 in cagII [2]), as well as the fact that ORF05 is as similar to the Agrobacterium tumefaciens virB11 as the cagII ORF11, motivated us to adapt our virB11 annotation.
ORF06 is the H. pylori 17874 fliI gene. An H. pylori N6 fliI gene has been studied previously (28). Our almost identical fliI likewise exhibits high similarity to a number of fliI genes in other bacteria as well as to certain type III export system components (Table 3). The presumptive gene product contains the two Walker boxes thought to function in ATP/GTP binding (58). ORF07 is the H. pylori fliQ homolog. It closely resembles fliQ genes of other bacteria as well as elements suspected to encode parts of the membrane channel for protein export type III systems (Table 3).
ORF08, the gene immediately downstream of this export locus, was annotated murB. It is moderately similar to the B. subtilis UDP-N-acetylenolpyruvoylglucosamine reductase gene, the product of which is essential for cell wall biosynthesis (45).
The spacing of the described genes on pSP102 was noteworthy and strongly suggested ORF03, ileS, virB11, fliI, fliQ, and murB to be transcribed on a single mRNA. Whereas intragenic regions between hpn and ORF02 and between ORF02 and ORF03 are 103 and 101 bp, respectively, the following ORFs are all positioned very close to each other (3 to 27 intergenic nucleotides [nt]). A transcription terminator structure could not be detected between ORF03 and murB.
The export locus is conserved in H. pylori.
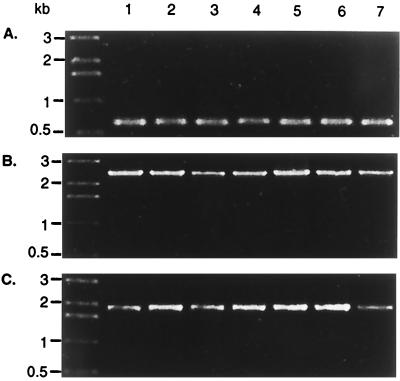
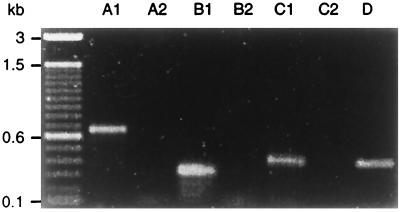
Conservation of the described locus was investigated by PCR amplification of fragments indicated in Fig. 1. The genetic linkage of ileS, virB11, fliI, fliQ, and murB was tested in 18 clinical H. pylori isolates from Auckland, New Zealand (8). Of these isolates, 13 were type I H. pylori strains (cagA+ VacA+) and 2 were type II (cagA VacA). The remaining strains were either cagA+ VacA− (two strains) or cagA VacA+ (one isolate). Amplifications linking ORF03 to ileS, ileS to fliI, and fliI to murB resulted in exactly the same expected product patterns in all strains examined, consistent with the type I lab strain H. pylori 17874 (Fig. 2). Identical products were also amplified from the mouse-adapted clinical isolate H. pylori SS1 and the type II strain H. pylori 915 (not shown). Within the panel of strains tested, the physical arrangement of the described locus is therefore highly conserved.
FIG. 2.
The protein export locus of H. pylori is conserved. Total genomic DNA of H. pylori strains was subjected to PCR using primers to test the linkage of ORF03-ileS (A, primers SP039 and SP040), ileS-fliI (B, primers SP034 and SP009), and fliI-murB (C, primers SP013 and SP036). Lanes 1 and 2, H. pylori type II isolates (cagA VacA−); lanes 3 and 4, H. pylori type I isolates (cagA+ VacA+); lane 5, intermediate H. pylori isolate (cagA VacA+); lane 6, intermediate H. pylori isolate (cagA+ VacA−); lane 7, H. pylori 17874.
In contrast, when conservation of the next genetic element downstream of murB, TIGR HP1417, was tested, amplifications demonstrated less consistency. Five of eleven strains tested (including H. pylori CCUG 17874) displayed a major PCR product which was approximately 700 bp larger than expected (not shown).
Neither of these PCRs resulted in any amplification product with H. mustelae 4298 genomic DNA. Moreover, we were unable to amplify the individual genes virB11, fliI, fliQ, and murB when using primer pairs designed on H. pylori 17874 sequence, suggesting a low (if any) degree of residue identity of those genes in H. mustelae (data not shown).
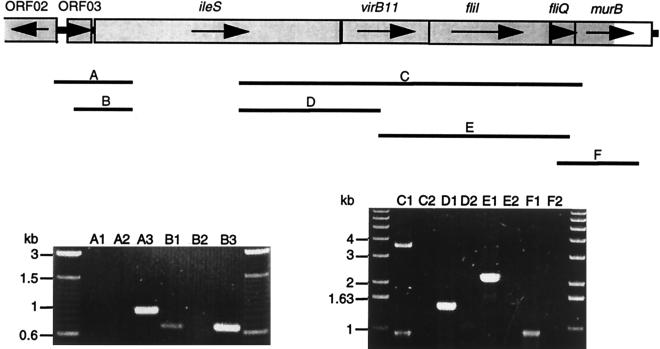
Transcript analysis of the protein export locus.
Transcription analysis of the putative operon was performed by RT-PCR, and the results are summarized in Fig. 3. Successful amplification of transcript sequence from ileS to murB (primer pair SP034-SP010) indicated the presence of a single continuous transcript including the five genetic elements ileS, virB11, fliI, fliQ, and murB. However, presumptive RNA degradation during DNase treatment led to inconsistencies during repeated amplification trials. To confirm the existence, and define the ends, of the large transcript, we performed a series of overlapping RT-PCRs of smaller fragments as indicated in Fig. 3. The results confirmed that the six genetic elements ORF03, ileS, virB11, fliI, fliQ, and murB are indeed cotranscribed.
FIG. 3.
Cotranscription of ORF03, ileS, virB11, fliI, fliQ, and murB. Lanes A to F correspond to reactions using primer pairs amplifying the indicated regions A to F. Lanes: 1, RT-PCR on H. pylori 17874 total RNA; 2, DNA contamination controls on H. pylori 17874 total RNA; 3, PCR on genomic H. pylori 17874 DNA using Taq polymerase. For negative controls, the supplier’s enzyme mixes containing RT were either replaced by Taq polymerase (lanes A2 and B2) or heat treated prior to reverse transcription (lanes C2, D2, E2, and F2).
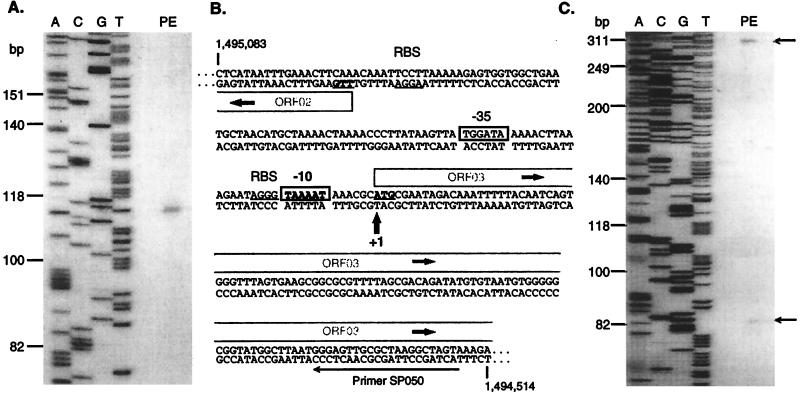
Failure of RT-PCRs using primer pair SP020-SP040 (Fig. 3, lane A1) suggested the start of the transcript to be within a region of approximately 170 bp upstream of ORF03. Primer extension analysis was performed to identify the start of the transcript. As shown in Fig. 4A, a single primer extension product was obtained with primer SP050, and the transcript start site was thus mapped to a nucleotide corresponding to the A residue of the presumptive ATG initiation codon of ORF03. Analysis of the nucleotide sequence revealed a close match (TAAAAT) to the E. coli −10 motif (TATAAT) and a reasonable −35 sequence (TGGATA), with an 18-nt spacing from the −10 box (Fig. 4B). This start site was verified by using primers SP048 (not shown) and SP051 (Fig. 4C). The latter also gave rise to a minor product of 83 nt, corresponding to position −43 with respect to the ileS start codon. Examination of the relevant sequence revealed the weak potential promoter element GTGCCAN16TTTATAT, preceding the transcription start site by only 3 nt.
FIG. 4.
Primer extension analysis of the protein export locus of H. pylori. (A) The primer extension reaction (PE) was performed on H. pylori 17874 RNA with primer SP050. The DNA sequencing reaction products obtained by using primer SP050 on pSP102 are indicated, and the sequence is identical to the corresponding region of strain 26695. Sizes at the left are from the migration of end-labeled φX174 restriction fragments. (B) The relevant area of the DNA sequence of strain 26695 is indicated, with genome sequence coordinates indicated. The transcription start site identified by the primer extension reaction is arrowed, and the inferred −10 and −35 hexamers are boxed. Presumptive ribosome binding sites for ORF02 and ORF03 are indicated by RBS and underlining. Potential initiation codons are in bold and underlined. ORFs are indicated by open-ended grey boxes. (C) As for panel A, but with primer SP051 used for the primer extension reaction. The upper product arrowed corresponds to the same start site in panel A; the lower arrowed band is a weaker product discussed in Results.
The protein export locus is expressed during infection.
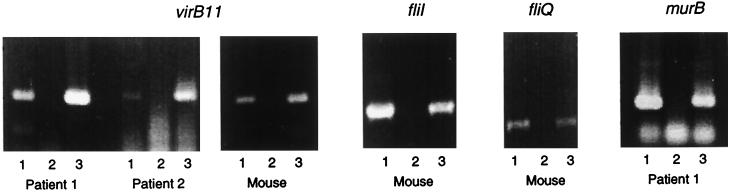
Total nucleic acid and RNA were each extracted from biopsy samples taken from mice following experimental infection with the H. pylori Sydney strain. The mRNAs corresponding to the virB11, fliI, and fliQ genes could be detected by RT-PCR (Fig. 5). Transcription of virB11 and murB was also demonstrated in RNA extracted from human gastritis patients (Fig. 5); limited availability of this material precluded investigation of other genes. Further RT-PCR experiments using primers extending from virB11 to fliI amplified the expected product from RNA of infected mouse biopsy (not shown), indicating that the cotranscription of these genes demonstrated in in vitro grown cells also occurs during infection.
FIG. 5.
The protein export locus is expressed during infection. Primers designed to amplify the indicated genes were used in PCR or RT-PCR on extracts from patient biopsy or infected mouse biopsy as follows: lanes 1, total nucleic acid, Taq polymerase; lane 2, RNA, Taq polymerase; lane 3, RNA, RT and Taq polymerase. Products and primers are SP014 and SP015 (527 nt, virB11), SP013 and SP002 (642 nt, fliI), SP028 and SP027 (244 nt, fliQ), and SP035 and SP036 (393 nt, murB).
Construction of virB11, fliI, and fliQ mutants.
For the construction of mutants, a two-step PCR-based procedure followed by allelic exchange mutagenesis was used (see Materials and Methods). Around 2.5 μg of the mutagenic constructs was electroporated into H. pylori 17874 cells. In chloramphenicol-resistant H. pylori transformants, the functional chromosomal gene was replaced with the disrupted copy of the mutagenic construct by a double-crossover event. Chloramphenicol-resistant mutant colonies were obtained at frequencies of 1.5 × 10−3, 2.2 × 10−3, and 4.7 × 10−4 for pSP118 (virB11 mutagenic construct), pSP110 (fliI mutagenic plasmid), and pSP107 (for fliQ mutagenesis), respectively. Mutant genotypes were verified by gene-specific PCR (not shown).
Nonpolarity of mutations.
Given that the genes of the protein export locus comprised an operon, it was important to show that individual cat insertion mutants retained transcription of downstream genes, by virtue of run-on transcription of the cat gene. RT-PCR was therefore performed on total RNA preparations of the H. pylori 17874 mutants. Primer pairs located upstream of the insertion sites of the cat cassette (but downstream of the mapped transcription start site) amplified the expected product in all examined mutant strains (not shown). More importantly, transcripts were also detected downstream of the insertion site of the cat cassette (Fig. 6), indicating that these genes were still transcribed. Further evidence of nonpolarity of cat insertion mutations was provided by phenotypic analysis (see below).
FIG. 6.
The cat insertion mutations are nonpolar. Lanes: A1, B1, and C1, RT-PCR on H. pylori 17874 mutant total RNA; A2, B2, and C2, DNA contamination controls (Taq polymerase only) on H. pylori 17874 mutant total RNA; A1 and A2, fliI internal primer pair SP013-SP002 on virB11 mutant RNA; B1 and B2, fliQ internal primer SP028-murB internal primer SP010 on fliI mutant RNA; C1 and C2, murB internal primer pair SP035-SP036 on fliQ mutant RNA; D, Taq polymerase control PCR using primer pair SP035-SP036 on genomic H. pylori 17874 DNA.
Motility and flagellum production.
Motility of the mutants was determined by phase-contrast microscopy with an inverted microscope, using 24- and 48-h liquid cultures. Evaluations were consistent and unambiguous. Whereas the H. pylori 17874 wild-type and virB11 mutant strains were clearly motile, indicated by characteristic rotation of cells, both the fliI and the fliQ mutant strains were completely nonmotile. Judged by a higher frequency of rotating cells and their higher spin velocity, the H. pylori virB11 mutant was consistently evaluated to be more motile than the wild-type strain.
Cells from 24-h liquid cultures were examined by negative staining and transmission electron microscopy (Fig. 7). Cell shapes of the three mutant strains and the wild type appeared to be identical. Sheathed unipolar flagella, usually single and occasionally a pair, were visible in both the wild-type strain and the virB11 mutant strains. The flagella observed in the mutant exhibited no detectable difference in shape or length compared to those of the wild-type cells.
FIG. 7.
Electron micrographs of negatively stained preparations of H. pylori 17874 cells. (A) Flagellated wild type; (B) flagellated virB11 mutant; (C) aflagellate fliI mutant; (D) aflagellate fliQ mutant. Magnification, ×11,200; bar, 1 μm.
No flagella could be detected in the fliI and fliQ mutants. Residual flagellation in less than 1% of the cells has been found in an H. pylori N6 fliI mutant (28). However, we did not find any flagellated cells in our fliI mutant culture. Very rarely we observed unattached membranous structures. The fact that the virB11 mutant was motile and flagellated, while the fliI and fliQ mutants had lost these properties, argues in favor of nonpolarity of the cat insertions.
Effect of mutagenesis on protein expression.
Whole-cell lysates of liquid H. pylori 17874 wild-type and three mutant cultures were prepared and analyzed by Western blotting. Detection with a polyclonal antibody, raised against purified flagellar filaments, demonstrated that expression of both flagellins and the hook protein FlgE was affected by the mutagenesis. In the virB11 mutant, slightly elevated levels of these proteins were detected. In contrast, expression of the flagellins and the hook protein was dramatically reduced in the fliI and fliQ mutants (Fig. 8). Investigation of fractionated samples showed a similar reduction of flagellin and hook protein levels in the supernatants, the envelope protein fraction, and the insoluble protein complexes of the fliI and fliQ mutants, whereas reduction was not as profound in the cytosolic fraction (data not shown). However, the overall level of reduction was not constant in the fliI mutant. Repeated passage of the fliI mutant resulted in flagellin and FlgE levels that were still reduced but higher than in a freshly isolated fliI mutant culture (Fig. 8).
FIG. 8.
Western blot analysis of H. pylori 17874 cultures with polyclonal anti-Fla antibodies. (A) Cells grown for 24 h in liquid medium after one previous passage; (B) cells grown for 24 h in liquid medium after three previous passages. Lanes in both panels: 1, wild type; 2, virB11 mutant; 3, fliI mutant; 4, fliQ mutant. The increased production of flagellins upon additional subculture of the fliI mutant is evident.
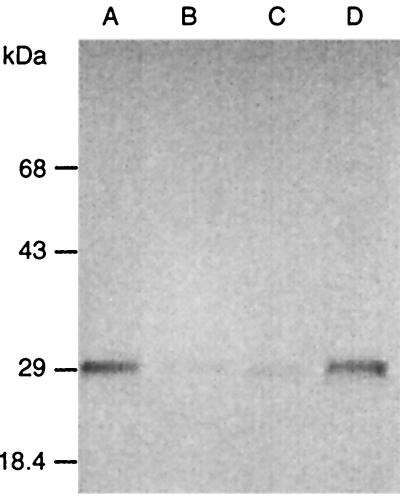
In a preliminary effort to identify proteins whose expression might be changed by mutation of virB11, production of a number of virulence-related proteins was examined by Western immunoblotting. Expression of the cytotoxin-associated protein CagA, the vacuolating cytotoxin VacA, the urease subunit UreB, and the outer membrane protein HopB was not affected by any of the mutations (data not shown). However, the expression of OMP4 (a member of the outer membrane protein family in H. pylori, encoded by TIGR HP0127) was significantly reduced in the virB11 and fliI mutants compared to the wild type or the fliQ mutant (Fig. 9). We did not detect OMP4 in supernatant fractions from the H. pylori 17874 wild-type and mutant strain cultures (not shown).
FIG. 9.
Expression of OMP4 is affected in both the H. pylori virB11 and fliI mutants. Western blot analysis was performed with a monoclonal anti-OMP4 antibody on whole-cell lysates of H. pylori 17874 cells grown in liquid medium for 24 h. Lanes: A, wild type; B, virB11 mutant; C, fliI mutant; D, fliQ mutant.
DISCUSSION
In this study we investigated an operon of the H. pylori genome which links obviously essential genes with predicted housekeeping functions (ileS and murB) with elements necessary for motility (fliI and fliQ) and possibly virulence factor presentation (virB11). The genetic linkage between an element likely to be involved in virulence factor export (virB11) and genes necessary for transport of flagellar proteins (fliI and fliQ) is exciting. Based on sequence similarities, flagellar export components have been compared with virulence factor export systems, specifically type III protein transport (23; for reviews, see references 33, 38, and 55). However, the physical and transcriptional linkage of a gene sharing extensive identity with nucleoprotein transfer energizers and genes required for flagellar protein export has not previously been described for other bacteria.
The two flagellar genes in this study, fliI and fliQ, are not usually part of the same operon. Typically, a fliI homolog is preceded by the gene for a flagellar export apparatus candidate fliH and followed by a gene fliJ encoding a presumptive flagellar chaperone (50). Interestingly, a fliJ homolog was not annotated in the H. pylori 26695 genome, and the fliH gene was identified in a separate operon, 650 kb away from fliI. The fliQ gene is typically flanked by fliP and fliR, the precise functions of which are not identified (35, 40). Homologs of these genes are found at distant loci in the H. pylori 26695 genome. These peculiarities underline how H. pylori defies established patterns of clustered flagellar gene organization in the family Enterobacteriaeceae.
An annotated virB11 gene in the H. pylori 26695 genome is located in the cag pathogenicity island, surrounded by genes with significant sequence identities to other components of the A. tumefaciens vir gene cluster (2, 53). However, the cag pathogenicity island virB11 gene product does not exhibit higher identities to A. tumefaciens VirB11 than its counterpart encoded within the flagellar export operon. We currently assume overlapping functions of these two similar elements in the context of unidirectional virulence factor transport.
The conservation of the described operon in H. pylori is remarkable for an organism originally noted for its genetic variability (7, 52). This conservation may be more consistent with a recent report of relatively conserved overall coding capacity (20). In all 18 strains of H. pylori examined, we found the flagellar export locus to be conserved. In contrast, the genes immediately up- and downstream of the described operon were less conserved. Clearly, the genetic linkage of ORF03, ileS, the flagellar export genes, and murB has been positively selected in H. pylori, presumably because of successful contribution of this arrangement to pathogenicity or a favorable stoichiometry of gene products.
We demonstrated coexpression of all elements of the described operon in in vitro-grown cells by means of RT-PCR. Unfortunately, Northern blotting attempts failed to detect the message, probably because of a combination of a relatively large size of the transcript (>6 kb) and low copy number. Ignoring possible effects of either premature mRNA termination, processing, or different translation efficiencies, the expression of fliI and fliQ would be expected to be stoichiometrically equal to that of the other gene products encoded by the cistronic mRNA. This contrasts with the current model of transcriptional regulation of class II genes in Enterobacteriaeceae and is consistent with the failure to annotate homologs of the established class II transcription regulator genes, flhC and flhD (36), in the H. pylori 26695 genome.
The primer extension analysis mapped the transcription start site of the operon to the A residue of the initiation codon for ORF03, making it unlikely that this gene is actually expressed. It is still possible that another promoter for ORF03 is up-regulated under alternative conditions, such as during infection or stress. The promoter tentatively inferred for the export operon (Fig. 4) has a −10 hexamer almost identical to the consensus sequence (22) for Eς70, the major RNA polymerase in E. coli, and a reasonable −35 sequence. Despite the availability of the genome sequence of H. pylori, few promoters have been mapped and consensus sequences are not established. On the basis of this study, promoters for essential housekeeping genes may closely resemble those recognized by Eς70. It is not obvious why the indicated promoter is so far away from the first gene definitely expressed (ileS), but it is unequivocally clear from primer extension using the primer internal to ileS that most of the transcription is driven by this promoter.
To our knowledge, this is the first published example of direct transcription analysis of H. pylori genes by RT-PCR on biopsies from gastritis patients and infected mice. These findings prove active transcription of the protein export locus during infection, supporting the involvement of these gene products in pathogenesis. This observation is consistent with the established requirement of bacterial motility for colonization (12, 13). It will be interesting to complement these data with experiments to test the ability of H. pylori SS1 virB11, fliI, and fliQ mutants to colonize mice.
Analysis of these H. pylori 17874 mutants resulted in some unexpected insights into respective phenotypes. The virB11 knockout did not inhibit flagellar biosynthesis. The slightly greater motility consistently observed, and the elevated production levels of flagellin and the hook protein, may be explained by the introduction of an additional promoter element upstream of fliI, namely, that of the cat cassette. The resulting increase in FliI production may have triggered a positive feedback to production of its substrates, the class III flagellar proteins. However, we did not detect more, or longer, flagella in the virB11 mutant than in the wild type. VirB11 may also compete with FliI for ATP and thus lower the efficiency of the transport process mediated by FliI.
The nonmotile fliI mutant strain produced less flagellin and FlgE than the wild type, which was also observed by Jenks et al. (28). However, we were not able to confirm the observation of Jenks and coworkers that some fliI mutant cells retained their ability to produce flagella. We suggest that an early block of the flagellar assembly pathway occurs in an H. pylori fliI mutant. A second, less efficient energy transducer may, however, adapt to functioning in flagellar export, enabling fliI mutants to gradually regain flagellation. Construction of the export channel (and subsequently the hook and filament) would still proceed, but more slowly, in a fliI mutant, resulting in gradual leakiness of this mutation with respect to the negative feedback on flagellin production. This may explain the results of Jenks and coworkers. In support of this hypothesis, an S. typhimurium fliI mutant was able to rebuild sheared flagella, although more slowly than the wild type (57).
In the fliQ mutant, nonmotility and loss of flagellation were observed, clearly establishing the FliQ protein as an essential component in flagellar biosynthesis. The reduction of flagellin and FlgE production was more stable than in the fliI mutant. FliQ may be necessary for construction of the channel through which both membrane-associated and exterior flagellar proteins would be transported in order to form the flagellum. If this component was missing, the channel could not be constructed; this would be sensed, and a regulator would then shut off flagellin and hook protein production.
Similarities between components of the flagellar export apparatus and systems for virulence factor presentation in a number of organisms are well established. However, a direct cross talk between these systems has not yet been demonstrated. The production of a protein of the Hop/BAB family, OMP4, is clearly affected in both the H. pylori virB11 and fliI mutants. Although OMP4 has not been proven to be a virulence factor, its sequence similarity to established adhesins makes it a likely candidate. The effect of a fliI mutation on OMP4 expression might illustrate a possible role of FliI in the transport of other, nonflagellar components. We believe this to be the first experimental evidence for an overlapping function of virulence factor transport proteins and flagellar export components. However, the lack of influence of these mutations on HopB production suggests that not all outer membrane protein export of H. pylori is affected by FliI function, so the mechanism whereby OMP4 production is reduced warrants further investigation. Further experiments will also be conducted to investigate effects of a virB11 and fliI mutation on production and localization of other members of the outer membrane protein family in H. pylori.
ACKNOWLEDGMENTS
This work was supported by grants to P.W.O. from the Marsden Fund, administered by the Royal Society of New Zealand, and the Massey University Research Fund. S.P. was the recipient of a Massey University doctoral scholarship.
We thank D. Hopcroft from The Horticultural and Food Research Institute of New Zealand for electron microscopy experiments.
REFERENCES
- 1.Aizawa S I. Flagellar assembly in Salmonella typhimurium. Mol Microbiol. 1996;19:1–5. doi: 10.1046/j.1365-2958.1996.344874.x. [DOI] [PubMed] [Google Scholar]
- 2.Akopyants N S, Clifton S W, Kersulyte D, Crabtree J E, Youree B E, Reece C A, Bukanov N O, Drazek E S, Roe B A, Berg D E. Analyses of the cag pathogenicity island of Helicobacter pylori. Mol Microbiol. 1998;28:37–53. doi: 10.1046/j.1365-2958.1998.00770.x. [DOI] [PubMed] [Google Scholar]
- 3.Alm, R. A. 1998. Personal communication.
- 4.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 5.Blaser M J. Helicobacter pylori: microbiology of a ‘slow’ bacterial infection. Trends Microbiol. 1993;1:255–260. doi: 10.1016/0966-842x(93)90047-u. [DOI] [PubMed] [Google Scholar]
- 6.Blattner F R, Plunkett G R, Bloch C A, Perna N T, Burland V, Riley M, Collado-Vides J, Glasner J D, Rode C K, Mayhew G F, Gregor J, Davis N W, Kirkpatrick H A, Goeden M A, Rose D J, Mau B, Shao Y. The complete genome sequence of Escherichia coli K-12. Science. 1997;277:1453–1474. doi: 10.1126/science.277.5331.1453. [DOI] [PubMed] [Google Scholar]
- 7.Bukanov N O, Berg D E. Ordered cosmid library and high resolution physical-genetic map of Helicobacter pylori strain NCTC 11638. Mol Microbiol. 1994;11:509–523. doi: 10.1111/j.1365-2958.1994.tb00332.x. [DOI] [PubMed] [Google Scholar]
- 8.Campbell S, Fraser A, Holliss B, Schmid J, O’Toole P W. Evidence for ethnic tropism of Helicobacter pylori. Infect Immun. 1997;65:3708–3712. doi: 10.1128/iai.65.9.3708-3712.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doig P, Trust T J. Identification of surface-exposed outer membrane antigens of Helicobacter pylori. Infect Immun. 1994;62:4526–4533. doi: 10.1128/iai.62.10.4526-4533.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dreyfus G, Williams A W, Kawagishi I, Macnab R M. Genetic and biochemical analysis of Salmonella typhimurium FliI, a flagellar protein related to the catalytic subunit of the F0F1 ATPase and to virulence proteins of mammalian and plant pathogens. J Bacteriol. 1993;175:3131–3138. doi: 10.1128/jb.175.10.3131-3138.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dunn B E, Cohen H, Blaser M J. Helicobacter pylori. Clin Microbiol Rev. 1997;10:720–741. doi: 10.1128/cmr.10.4.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eaton K, Morgan D R, Krakowka S. Motility as a factor in the colonisation of gnotobiotic piglets by Helicobacter pylori. J Med Microbiol. 1992;37:123–127. doi: 10.1099/00222615-37-2-123. [DOI] [PubMed] [Google Scholar]
- 13.Eaton K A, Suerbaum S, Josenhans C, Krakowka S. Colonization of gnotobiotic piglets by Helicobacter pylori deficient in two flagellin genes. Infect Immun. 1996;64:2445–2448. doi: 10.1128/iai.64.7.2445-2448.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fan F, Macnab R M. Enzymatic characterization of FliI—an ATPase involved in flagellar assembly in Salmonella typhimurium. J Biol Chem. 1996;271:31981–31988. doi: 10.1074/jbc.271.50.31981. [DOI] [PubMed] [Google Scholar]
- 15.Fenselau S, Balbo I, Bonas U. Determinants of pathogenicity in Xanthomonas campestris pv. vesicatoria are related to proteins involved in secretion in bacterial pathogens of animals. Mol Plant Microbe Interact. 1992;5:390–396. doi: 10.1094/mpmi-5-390. [DOI] [PubMed] [Google Scholar]
- 16.Ge Y G, Old I G, Girons I S, Charon N W. Molecular characterization of a large Borrelia burgdorferi motility operon which is initiated by a consensus sigma 70 promoter. J Bacteriol. 1997;179:2289–2299. doi: 10.1128/jb.179.7.2289-2299.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ge Z, Taylor D E. H. pylori DNA transformation by natural competence and electroporation. In: Clayton C L, Mobley H L T, editors. Helicobacter pylori protocols. Totowa, N.J: Humana Press; 1997. pp. 145–152. [DOI] [PubMed] [Google Scholar]
- 18.Geis G, Leying H, Suerbaum S, Mai U, Opferkuch W. Ultrastructure and chemical analysis of Campylobacter pylori flagella. J Clin Microbiol. 1989;27:436–441. doi: 10.1128/jcm.27.3.436-441.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gilbert J V, Ramakrishna J, Sunderman F W, Jr, Wright A, Plaut A G. Protein Hpn: cloning and characterization of a histidine-rich metal-binding polypeptide in Helicobacter pylori and Helicobacter mustelae. Infect Immun. 1995;63:2682–2688. doi: 10.1128/iai.63.7.2682-2688.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hancock R E, Alm R, Bina J, Trust T. Helicobacter pylori: a surprisingly conserved bacterium. Nat Biotechnol. 1998;16:216–217. doi: 10.1038/nbt0398-216. [DOI] [PubMed] [Google Scholar]
- 21.Hardham J M, Frye J G, Stamm L V. Identification and sequences of the Treponema pallidum fliM′, fliY, fliP, fliQ, fliR and flhB′ genes. Gene. 1995;166:57–64. doi: 10.1016/0378-1119(95)00583-x. [DOI] [PubMed] [Google Scholar]
- 22.Hawley D K, McClure W R. Compilation and analysis of Escherichia coli promoter DNA sequences. Nucleic Acids Res. 1983;11:2237–2255. doi: 10.1093/nar/11.8.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He S Y. Hrp-controlled interkingdom protein transport: learning from flagellar assembly? Trends Microbiol. 1997;5:489–495. doi: 10.1016/S0966-842X(97)01163-3. [DOI] [PubMed] [Google Scholar]
- 24.Higgins D G, Sharp P M. Fast and sensitive multiple sequence alignments on a microcomputer. CABIOS Commun. 1989;5:151–153. doi: 10.1093/bioinformatics/5.2.151. [DOI] [PubMed] [Google Scholar]
- 25.Huang J, O’Toole P W, Doig P, Trust T J. Stimulation of interleukin-8 production in epithelial cell lines by Helicobacter pylori. Infect Immun. 1995;63:1732–1738. doi: 10.1128/iai.63.5.1732-1738.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hughes K T, Gillen K L, Semon M J, Karlinsey J E. Sensing structural intermediates in bacterial flagellar assembly by export of a negative regulator. Science. 1993;262:1277–1280. doi: 10.1126/science.8235660. [DOI] [PubMed] [Google Scholar]
- 27.Hussel T, Isacsson P G, Crabtree J E, Spencer J. The response of cells from low grade B-cell gastric lymphomas of mucosa-associated lymphoid tissue to Helicobacter pylori. Lancet. 1993;342:571–574. doi: 10.1016/0140-6736(93)91408-e. [DOI] [PubMed] [Google Scholar]
- 28.Jenks P J, Foynes S, Ward S J, Constantinidou C, Penn C W, Wren B W. A flagellar-specific ATPase (FliI) is necessary for flagellar export in Helicobacter pylori. FEMS Microbiol Lett. 1997;152:205–211. doi: 10.1111/j.1574-6968.1997.tb10429.x. [DOI] [PubMed] [Google Scholar]
- 29.Josenhans C, Labigne A, Suerbaum S. Comparative ultrastructural and functional studies of Helicobacter pylori and Helicobacter mustelae flagellin mutants: both flagellin subunits, FlaA and FlaB, are necessary for full motility in Helicobacter species. J Bacteriol. 1995;177:3010–3020. doi: 10.1128/jb.177.11.3010-3020.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kostrzynska M, Betts J D, Austin J W, Trust T J. Identification, characterization, and spatial localization of two flagellin species in Helicobacter pylori flagella. J Bacteriol. 1991;173:937–946. doi: 10.1128/jb.173.3.937-946.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kunst F, Ogasawara N, Moszer I, Albertini A M, Alloni G, Azevedo V, Bertero M G, Bessieres P, Bolotin A, Borchert S, Borriss R, Boursier L, Brans A, Braun M, Brignell S C, Bron S, Brouillet S, Bruschi C V, Caldwell B, Capuano V, Carter N M, Choi S K, Codani J J, Connerton I F, Danchin A, et al. The complete genome sequence of the gram-positive bacterium Bacillus subtilis. Nature. 1997;390:249–256. doi: 10.1038/36786. [DOI] [PubMed] [Google Scholar]
- 32.Lee A, Orourke J, Deungria M C, Robertson B, Daskalopoulos G, Dixon M F. A standardized mouse model of Helicobacter pylori infection—introducing the Sydney strain. Gastroenterology. 1997;112:1386–1397. doi: 10.1016/s0016-5085(97)70155-0. [DOI] [PubMed] [Google Scholar]
- 33.Lee C A. Type III secretion systems: machines to deliver bacterial proteins into eukaryotic cells? Trends Microbiol. 1997;5:148–156. doi: 10.1016/S0966-842X(97)01029-9. [DOI] [PubMed] [Google Scholar]
- 34.Leying H, Suerbaum S, Geis G, Haas R. Cloning and genetic characterization of a Helicobacter pylori flagellin gene. Mol Microbiol. 1992;6:2863–2874. doi: 10.1111/j.1365-2958.1992.tb01466.x. [DOI] [PubMed] [Google Scholar]
- 35.Macnab R M. Genetics and biogenesis of bacterial flagella. Ann Rev Genet. 1992;26:131–158. doi: 10.1146/annurev.ge.26.120192.001023. [DOI] [PubMed] [Google Scholar]
- 36.Macnab R M. Flagella and motility. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C: ASM Press; 1996. pp. 123–145. [Google Scholar]
- 37.Malakooti J, Ely B, Matsumura P. Molecular characterization, nucleotide sequence, and expression of the fliO, fliP, fliQ, and fliR genes of Escherichia coli. J Bacteriol. 1994;176:189–197. doi: 10.1128/jb.176.1.189-197.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mecsas J, Strauss E J. Molecular mechanisms of bacterial virulence—type III secretion and pathogenicity islands. Emerging Infect Dis. 1996;2:271–288. doi: 10.3201/eid0204.960403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Minamino T, Iino T, Kutuskake K. Molecular characterization of the Salmonella typhimurium flhB operon and its protein products. J Bacteriol. 1994;176:7630–7637. doi: 10.1128/jb.176.24.7630-7637.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ohnishi K, Fan F, Schoenhals G J, Kihara M, Macnab R M. The FliO, FliP, FliQ, and FliR proteins of Salmonella typhimurium: putative components for flagellar assembly. J Bacteriol. 1997;179:6092–6099. doi: 10.1128/jb.179.19.6092-6099.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O’Toole P W, Austin J W, Trust T J. Identification and molecular characterization of a major ring-forming surface protein from the gastric pathogen Helicobacter mustelae. Mol Microbiol. 1994;11:349–361. doi: 10.1111/j.1365-2958.1994.tb00315.x. [DOI] [PubMed] [Google Scholar]
- 42.O’Toole P W, Janzon L, Doig P, Huang J, Kostrzynska M, Trust T J. The putative neuraminyllactose-binding hemagglutinin HpaA of Helicobacter pylori CCUG 17874 is a lipoprotein. J Bacteriol. 1995;177:6049–6057. doi: 10.1128/jb.177.21.6049-6057.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O’Toole P W, Kostrzynska M, Trust T J. Non-motile mutants of Helicobacter pylori and Helicobacter mustelae defective in flagellar hook production. Mol Microbiol. 1994;14:691–703. doi: 10.1111/j.1365-2958.1994.tb01307.x. [DOI] [PubMed] [Google Scholar]
- 44.O’Toole P W, Logan S M, Kostrzynska M, Wadström T, Trust T J. Isolation and biochemical and molecular analyses of a species-specific protein antigen from the gastric pathogen Helicobacter pylori. J Bacteriol. 1991;173:505–513. doi: 10.1128/jb.173.2.505-513.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rowland S L, Errington J, Wake R G. The Bacillus subtilis cell-division 135–137 degrees region contains an essential ORF with significant similarity to murB and a dispensable sbp gene. Gene. 1995;164:113–116. doi: 10.1016/0378-1119(95)00467-k. [DOI] [PubMed] [Google Scholar]
- 46.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 47.Schmitz A, Josenhans C, Suerbaum S. Cloning and characterization of the Helicobacter pylori flbA gene, which codes for a membrane protein involved in coordinated expression of flagellar genes. J Bacteriol. 1997;179:987–997. doi: 10.1128/jb.179.4.987-997.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Segal E D, Tompkins L S. Transformation of Helicobacter pylori by electroporation. BioTechniques. 1993;14:225–226. [PubMed] [Google Scholar]
- 49.Silhavy T J. Cell biology—death by lethal injection. Science. 1997;278:1085–1086. doi: 10.1126/science.278.5340.1085. [DOI] [PubMed] [Google Scholar]
- 50.Stephens C, Mohr C, Boyd C, Maddock J, Gober J, Shapiro L. Identification of the fliI and fliJ components of the Caulobacter flagellar type III protein secretion system. J Bacteriol. 1997;179:5355–5365. doi: 10.1128/jb.179.17.5355-5365.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Suerbaum S, Josenhans C, Labigne A. Cloning and genetic characterization of the Helicobacter pylori and Helicobacter mustelae flaB flagellin genes and construction of H. pylori flaA- and flaB-negative mutants by electroporation-mediated allelic exchange. J Bacteriol. 1993;175:3278–3288. doi: 10.1128/jb.175.11.3278-3288.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Taylor D E, Eaton M, Chang N, Salama S M. Construction of a Helicobacter pylori genome map and demonstration of diversity at the genome level. J Bacteriol. 1992;174:6800–6806. doi: 10.1128/jb.174.21.6800-6806.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tomb J F, White O, Kerlavage A R, Clayton R A, Sutton G G, Fleischmann R D, Ketchum K A, Klenk H P, Gill S, Dougherty B A, Nelson K, Quackenbush J, Zhou L X, Kirkness E F, Peterson S, Loftus B, Richardson D, Dodson R, Khalak H G, Glodek A, McKenney K, Fitzegerald L M, Lee N, Adams M D, Hickey E K, et al. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature. 1997;388:539–547. doi: 10.1038/41483. [DOI] [PubMed] [Google Scholar]
- 54.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;74:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Van Gijsegem F, Gough C, Zischek C, Niqueux E, Arlat M, Genin S, Barberis P, German S, Castello P, Boucher C. The hrp gene locus of Pseudomonas solanacearum, which controls the production of a type III secretion system, encodes eight proteins related to components of the bacterial flagellar biogenesis complex. Mol Microbiol. 1995;15:1095–1114. doi: 10.1111/j.1365-2958.1995.tb02284.x. [DOI] [PubMed] [Google Scholar]
- 56.Venkatesan M M, Buysse J M, Oaks E V. Surface presentation of Shigella flexneri invasion plasmid antigens requires the products of the spa locus. J Bacteriol. 1992;174:1990–2001. doi: 10.1128/jb.174.6.1990-2001.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vogler A P, Homma M, Irikura V M, Macnab R M. Salmonella typhimurium mutants defective in flagellar filament regrowth and sequence similarity of FliI to F0F1, vacuolar, and archaebacterial ATPase subunits. J Bacteriol. 1991;173:3564–3572. doi: 10.1128/jb.173.11.3564-3572.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Walker J E, Saraste M, Runswick M J, Gay N J. Distantly related sequences in the alpha- and beta-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1982;1:945–951. doi: 10.1002/j.1460-2075.1982.tb01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.World Health Organization. Schistosomes, liver flukes and Helicobacter pylori. IARC Monogr Eval Carcinog Risks Hum. 1994;61:177–240. [PMC free article] [PubMed] [Google Scholar]
- 60.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- 61.Yao R, Alm R A, Trust T J, Guerry P. Construction of new Campylobacter cloning vectors and a new mutational cat cassette. Gene. 1993;130:127–130. doi: 10.1016/0378-1119(93)90355-7. [DOI] [PubMed] [Google Scholar]
- 62.Zhuang W Y, Shapiro L. Caulobacter FliQ and FliR membrane proteins, required for flagellar biogenesis and cell division, belong to a family of virulence factor export proteins. J Bacteriol. 1995;177:343–356. doi: 10.1128/jb.177.2.343-356.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]