Abstract
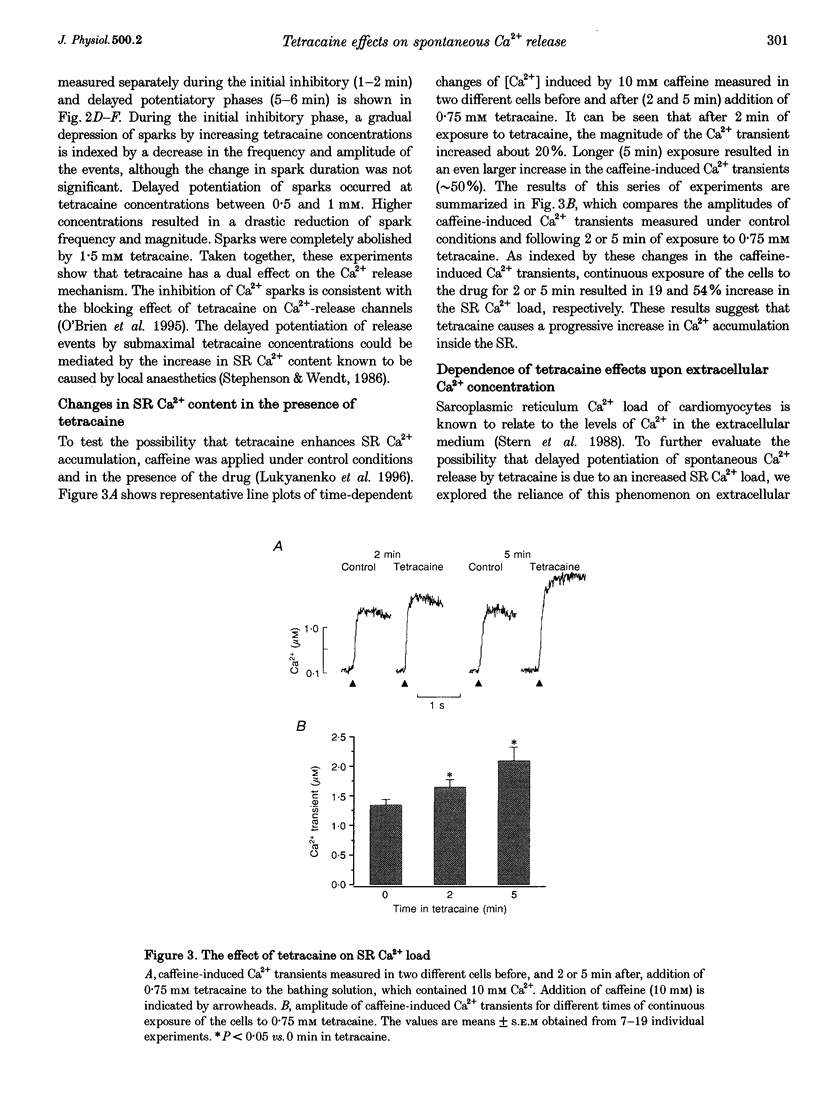
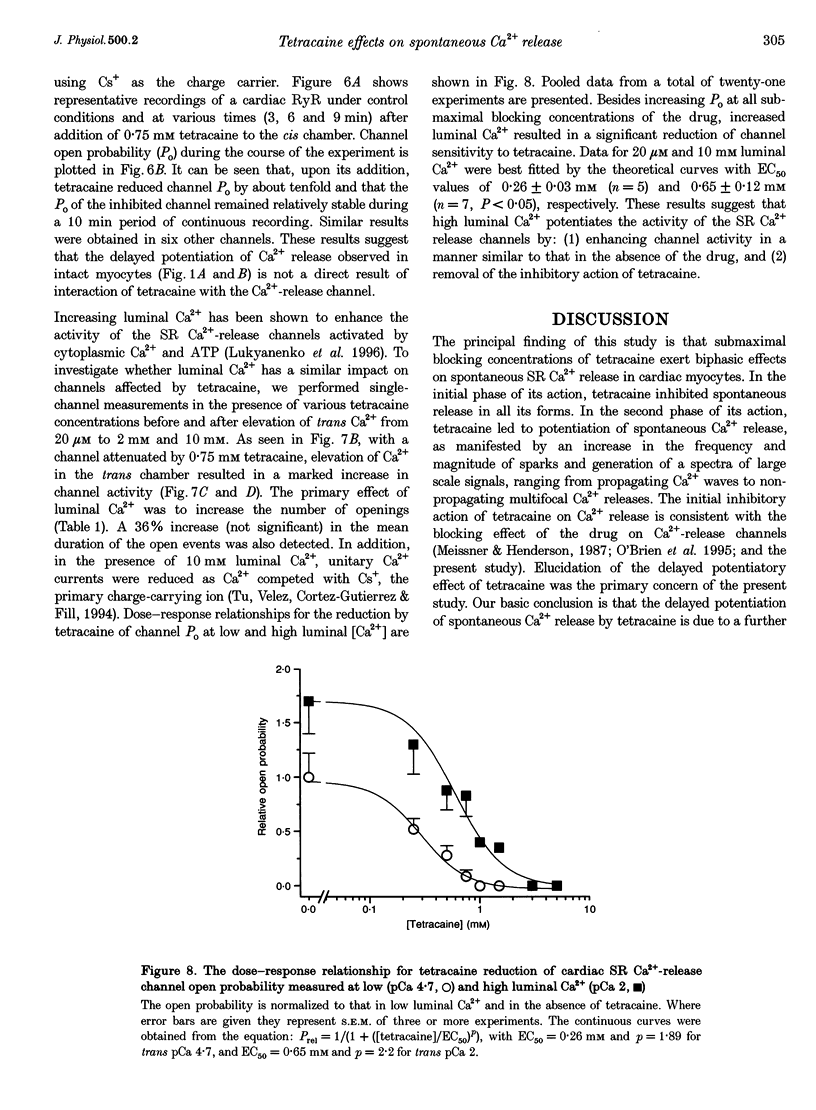
1. Confocal microfluorometry was used to study the effects of tetracaine on spontaneous Ca2+ release from the sarcoplasmic reticulum (SR) in isolated rat ventricular myocytes. 2. At low concentrations (0.25-1.25 mM), tetracaine caused an initial inhibition of spontaneous release events (Ca2+ sparks) and Ca2+ waves, which was followed by a gradual increase in Ca2+ release activity. The frequency and magnitude of sparks were first decreased and then increased with respect to control levels. At high concentrations (> 1.25 mM), tetracaine abolished all forms of spontaneous release. 3. Exposure of the myocytes to tetracaine resulted in a gradual increase in the SR Ca2+ load as indexed by changes in the magnitude of caffeine-induced Ca2+ transients. 4. In cardiac SR Ca(2+)-release channels incorporated into lipid bilayers, tetracaine (> 0.25 mM) induced a steady inhibition of channel activity. Addition of millimolar Ca2+ to the luminal side of the channel caused an increase in channel open probability under control conditions as well as in the presence of various concentrations of tetracaine. 5. We conclude that the primary effect of tetracaine on SR Ca(2+)-release channels is inhibition of channel activity both in vitro and in situ. The ability of tetracaine to reduce spark magnitude suggests that these events are not due to activation of single channels or non-reducible clusters of channels and, therefore, supports the multichannel origin of sparks. We propose that the paradoxical late potentiation of release by submaximal concentrations of tetracaine is caused by a gradual increase in SR Ca2+ load and subsequent activation of the Ca(2+)-release channels by Ca2+ inside the SR.
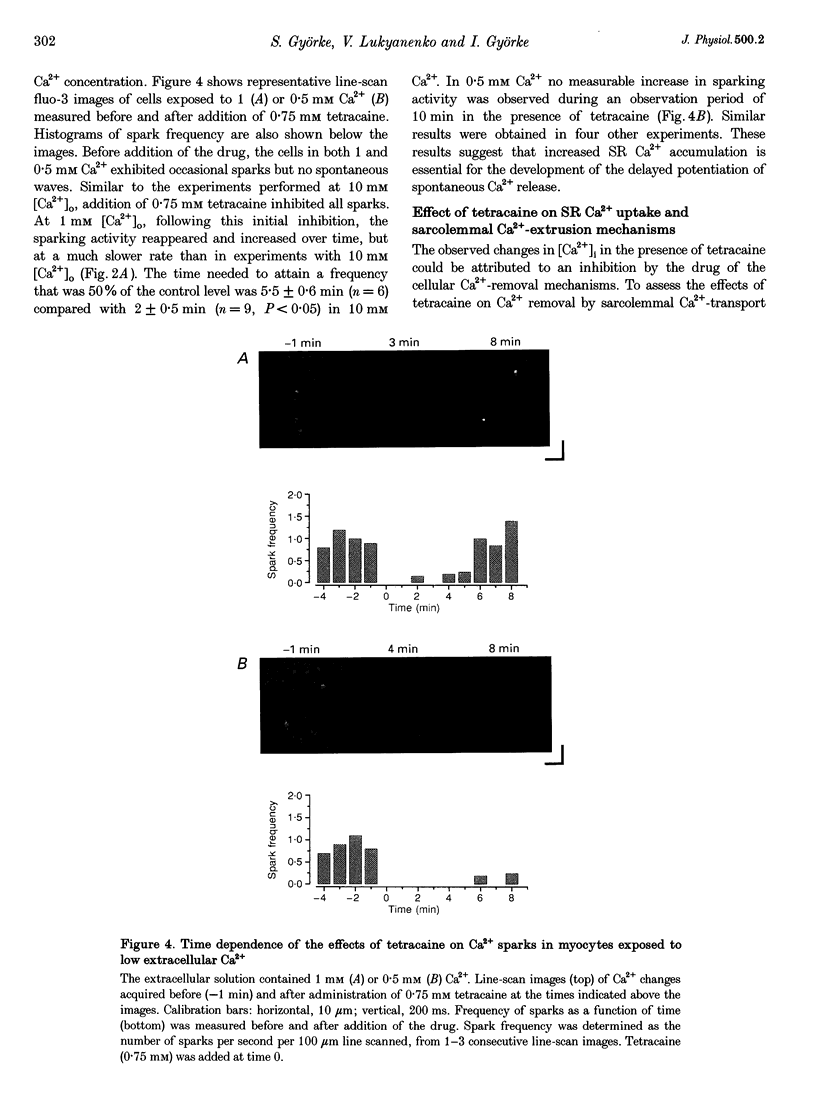
Full text
PDF

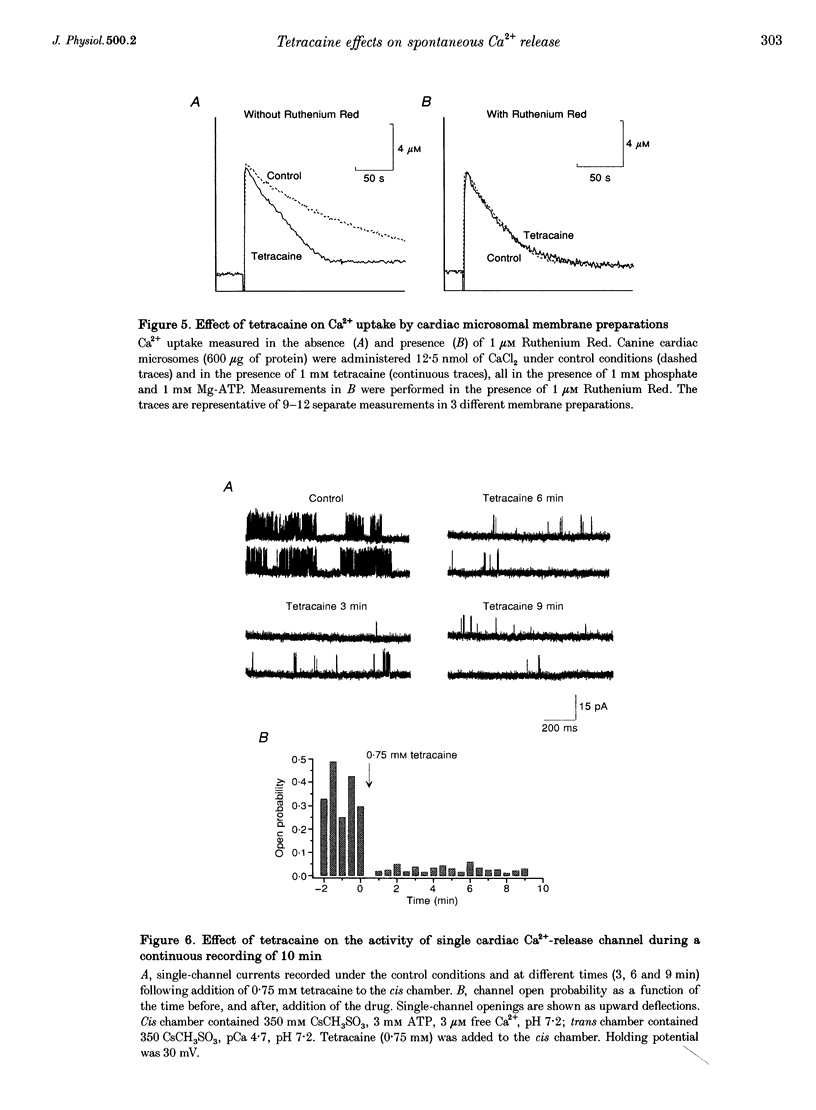
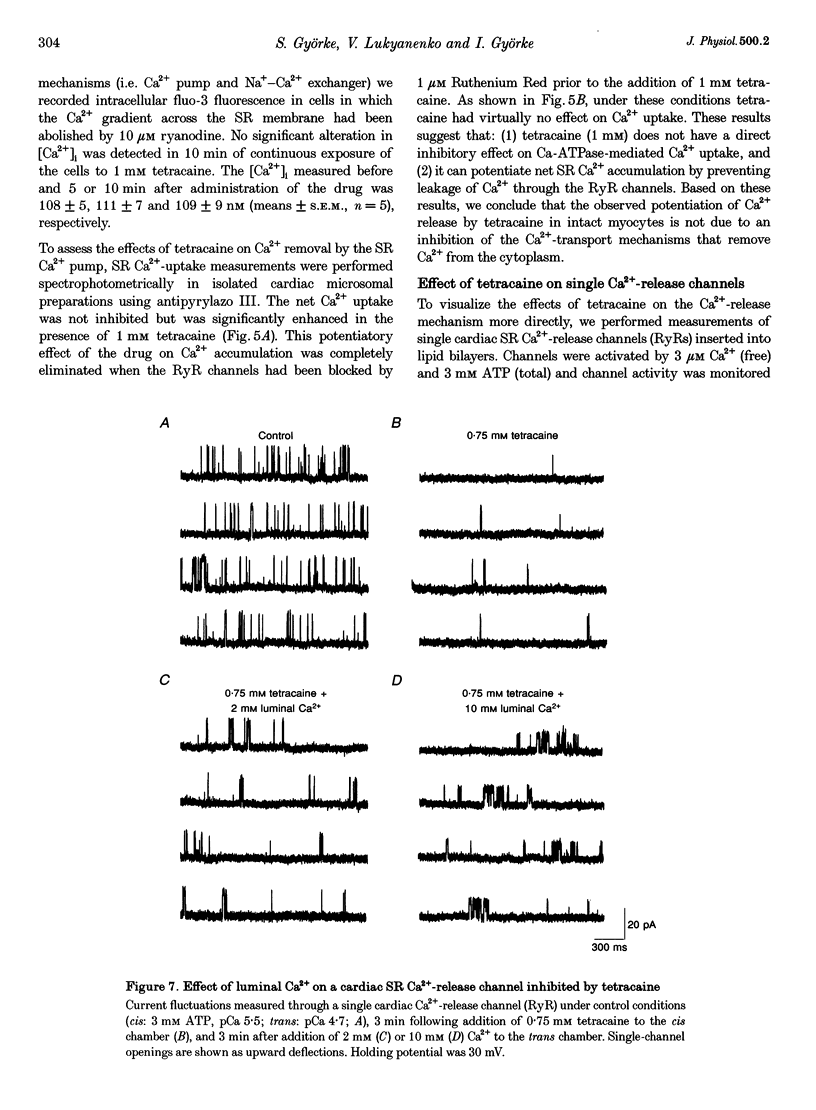




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen D. G., Morris P. G., Orchard C. H., Pirolo J. S. A nuclear magnetic resonance study of metabolism in the ferret heart during hypoxia and inhibition of glycolysis. J Physiol. 1985 Apr;361:185–204. doi: 10.1113/jphysiol.1985.sp015640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassani J. W., Yuan W., Bers D. M. Fractional SR Ca release is regulated by trigger Ca and SR Ca content in cardiac myocytes. Am J Physiol. 1995 May;268(5 Pt 1):C1313–C1319. doi: 10.1152/ajpcell.1995.268.5.C1313. [DOI] [PubMed] [Google Scholar]
- Chamberlain B. K., Volpe P., Fleischer S. Inhibition of calcium-induced calcium release from purified cardiac sarcoplasmic reticulum vesicles. J Biol Chem. 1984 Jun 25;259(12):7547–7553. [PubMed] [Google Scholar]
- Chapman R. A., Miller D. J. The effects of caffeine on the contraction of the frog heart. J Physiol. 1974 Nov;242(3):589–613. doi: 10.1113/jphysiol.1974.sp010725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H., Lederer M. R., Lederer W. J., Cannell M. B. Calcium sparks and [Ca2+]i waves in cardiac myocytes. Am J Physiol. 1996 Jan;270(1 Pt 1):C148–C159. doi: 10.1152/ajpcell.1996.270.1.C148. [DOI] [PubMed] [Google Scholar]
- Cheng H., Lederer W. J., Cannell M. B. Calcium sparks: elementary events underlying excitation-contraction coupling in heart muscle. Science. 1993 Oct 29;262(5134):740–744. doi: 10.1126/science.8235594. [DOI] [PubMed] [Google Scholar]
- Dettbarn C., Györke S., Palade P. Many agonists induce "quantal" Ca2+ release or adaptive behavior in muscle ryanodine receptors. Mol Pharmacol. 1994 Sep;46(3):502–507. [PubMed] [Google Scholar]
- Engel J., Sowerby A. J., Finch S. A., Fechner M., Stier A. Temperature dependence of Ca2+ wave properties in cardiomyocytes: implications for the mechanism of autocatalytic Ca2+ release in wave propagation. Biophys J. 1995 Jan;68(1):40–45. doi: 10.1016/S0006-3495(95)80196-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A. Two kinds of calcium-induced release of calcium from the sarcoplasmic reticulum of skinned cardiac cells. Adv Exp Med Biol. 1992;311:245–262. doi: 10.1007/978-1-4615-3362-7_18. [DOI] [PubMed] [Google Scholar]
- Fink R. H., Stephenson D. G. Ca2+-movements in muscle modulated by the state of K+-channels in the sarcoplasmic reticulum membranes. Pflugers Arch. 1987 Aug;409(4-5):374–380. doi: 10.1007/BF00583791. [DOI] [PubMed] [Google Scholar]
- Fink R. H., Veigel C. Calcium uptake and release modulated by counter-ion conductances in the sarcoplasmic reticulum of skeletal muscle. Acta Physiol Scand. 1996 Mar;156(3):387–396. doi: 10.1046/j.1365-201X.1996.212000.x. [DOI] [PubMed] [Google Scholar]
- Gill D. L., Grollman E. F., Kohn L. D. Calcium transport mechanisms in membrane vesicles from guinea pig brain synaptosomes. J Biol Chem. 1981 Jan 10;256(1):184–192. [PubMed] [Google Scholar]
- Györke S., Vélez P., Suárez-Isla B., Fill M. Activation of single cardiac and skeletal ryanodine receptor channels by flash photolysis of caged Ca2+. Biophys J. 1994 Jun;66(6):1879–1886. doi: 10.1016/S0006-3495(94)80981-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann-Frank A., Lehmann-Horn F. Regulation of the purified Ca2+ release channel/ryanodine receptor complex of skeletal muscle sarcoplasmic reticulum by luminal calcium. Pflugers Arch. 1996 May;432(1):155–157. doi: 10.1007/s004240050117. [DOI] [PubMed] [Google Scholar]
- Hunter D. R., Haworth R. A., Berkoff H. A. Cellular calcium turnover in the perfused rat heart: modulation by caffeine and procaine. Circ Res. 1982 Sep;51(3):363–370. doi: 10.1161/01.res.51.3.363. [DOI] [PubMed] [Google Scholar]
- Lipp P., Niggli E. Modulation of Ca2+ release in cultured neonatal rat cardiac myocytes. Insight from subcellular release patterns revealed by confocal microscopy. Circ Res. 1994 May;74(5):979–990. doi: 10.1161/01.res.74.5.979. [DOI] [PubMed] [Google Scholar]
- Lipp P., Niggli E. Submicroscopic calcium signals as fundamental events of excitation--contraction coupling in guinea-pig cardiac myocytes. J Physiol. 1996 Apr 1;492(Pt 1):31–38. doi: 10.1113/jphysiol.1996.sp021286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukyanenko V., Györke I., Györke S. Regulation of calcium release by calcium inside the sarcoplasmic reticulum in ventricular myocytes. Pflugers Arch. 1996 Oct;432(6):1047–1054. doi: 10.1007/s004240050233. [DOI] [PubMed] [Google Scholar]
- Meissner G., Henderson J. S. Rapid calcium release from cardiac sarcoplasmic reticulum vesicles is dependent on Ca2+ and is modulated by Mg2+, adenine nucleotide, and calmodulin. J Biol Chem. 1987 Mar 5;262(7):3065–3073. [PubMed] [Google Scholar]
- Niggli E., Lipp P. Subcellular features of calcium signalling in heart muscle: what do we learn? Cardiovasc Res. 1995 Apr;29(4):441–448. [PubMed] [Google Scholar]
- O'Brien J., Valdivia H. H., Block B. A. Physiological differences between the alpha and beta ryanodine receptors of fish skeletal muscle. Biophys J. 1995 Feb;68(2):471–482. doi: 10.1016/S0006-3495(95)80208-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santana L. F., Cheng H., Gómez A. M., Cannell M. B., Lederer W. J. Relation between the sarcolemmal Ca2+ current and Ca2+ sparks and local control theories for cardiac excitation-contraction coupling. Circ Res. 1996 Jan;78(1):166–171. doi: 10.1161/01.res.78.1.166. [DOI] [PubMed] [Google Scholar]
- Sitsapesan R., Williams A. J. Regulation of the gating of the sheep cardiac sarcoplasmic reticulum Ca(2+)-release channel by luminal Ca2+. J Membr Biol. 1994 Feb;137(3):215–226. doi: 10.1007/BF00232590. [DOI] [PubMed] [Google Scholar]
- Stephenson D. G., Wendt I. R. Effects of procaine on calcium accumulation by the sarcoplasmic reticulum of mechanically disrupted rat cardiac muscle. J Physiol. 1986 Apr;373:195–207. doi: 10.1113/jphysiol.1986.sp016042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern M. D., Capogrossi M. C., Lakatta E. G. Spontaneous calcium release from the sarcoplasmic reticulum in myocardial cells: mechanisms and consequences. Cell Calcium. 1988 Dec;9(5-6):247–256. doi: 10.1016/0143-4160(88)90005-x. [DOI] [PubMed] [Google Scholar]
- Stern M. D., Lakatta E. G. Excitation-contraction coupling in the heart: the state of the question. FASEB J. 1992 Sep;6(12):3092–3100. doi: 10.1096/fasebj.6.12.1325933. [DOI] [PubMed] [Google Scholar]
- Stern M. D. Theory of excitation-contraction coupling in cardiac muscle. Biophys J. 1992 Aug;63(2):497–517. doi: 10.1016/S0006-3495(92)81615-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takuma T., Kuyatt B. L., Baum B. J. Calcium transport mechanisms in basolateral plasma membrane-enriched vesicles from rat parotid gland. Biochem J. 1985 Apr 1;227(1):239–245. doi: 10.1042/bj2270239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trafford A. W., Lipp P., O'Neill S. C., Niggli E., Eisner D. A. Propagating calcium waves initiated by local caffeine application in rat ventricular myocytes. J Physiol. 1995 Dec 1;489(Pt 2):319–326. doi: 10.1113/jphysiol.1995.sp021053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathy A., Meissner G. Sarcoplasmic reticulum lumenal Ca2+ has access to cytosolic activation and inactivation sites of skeletal muscle Ca2+ release channel. Biophys J. 1996 Jun;70(6):2600–2615. doi: 10.1016/S0006-3495(96)79831-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsugorka A., Ríos E., Blatter L. A. Imaging elementary events of calcium release in skeletal muscle cells. Science. 1995 Sep 22;269(5231):1723–1726. doi: 10.1126/science.7569901. [DOI] [PubMed] [Google Scholar]
- Tu Q., Velez P., Cortes-Gutierrez M., Fill M. Surface charge potentiates conduction through the cardiac ryanodine receptor channel. J Gen Physiol. 1994 May;103(5):853–867. doi: 10.1085/jgp.103.5.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wier W. G., Cannell M. B., Berlin J. R., Marban E., Lederer W. J. Cellular and subcellular heterogeneity of [Ca2+]i in single heart cells revealed by fura-2. Science. 1987 Jan 16;235(4786):325–328. doi: 10.1126/science.3798114. [DOI] [PubMed] [Google Scholar]
- Yasui K., Palade P., Györke S. Negative control mechanism with features of adaptation controls Ca2+ release in cardiac myocytes. Biophys J. 1994 Jul;67(1):457–460. doi: 10.1016/S0006-3495(94)80501-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahradníková A., Palade P. Procaine effects on single sarcoplasmic reticulum Ca2+ release channels. Biophys J. 1993 Apr;64(4):991–1003. doi: 10.1016/S0006-3495(93)81465-6. [DOI] [PMC free article] [PubMed] [Google Scholar]