Abstract
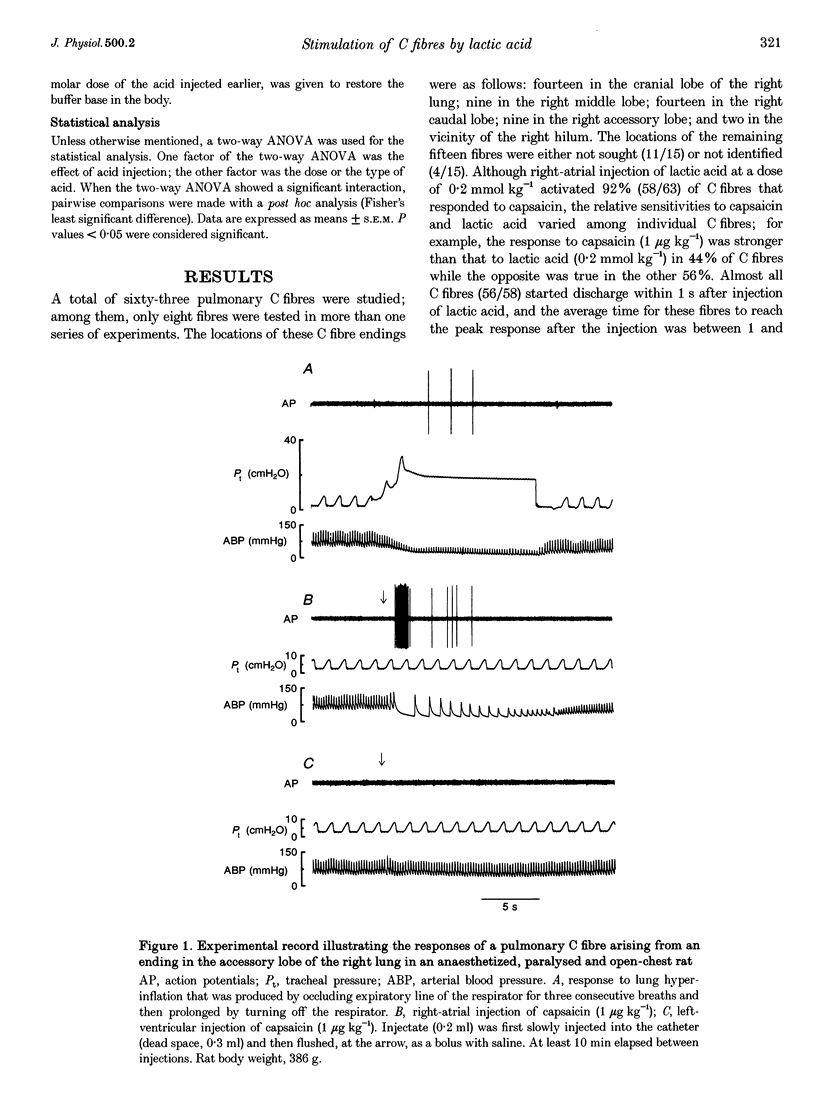
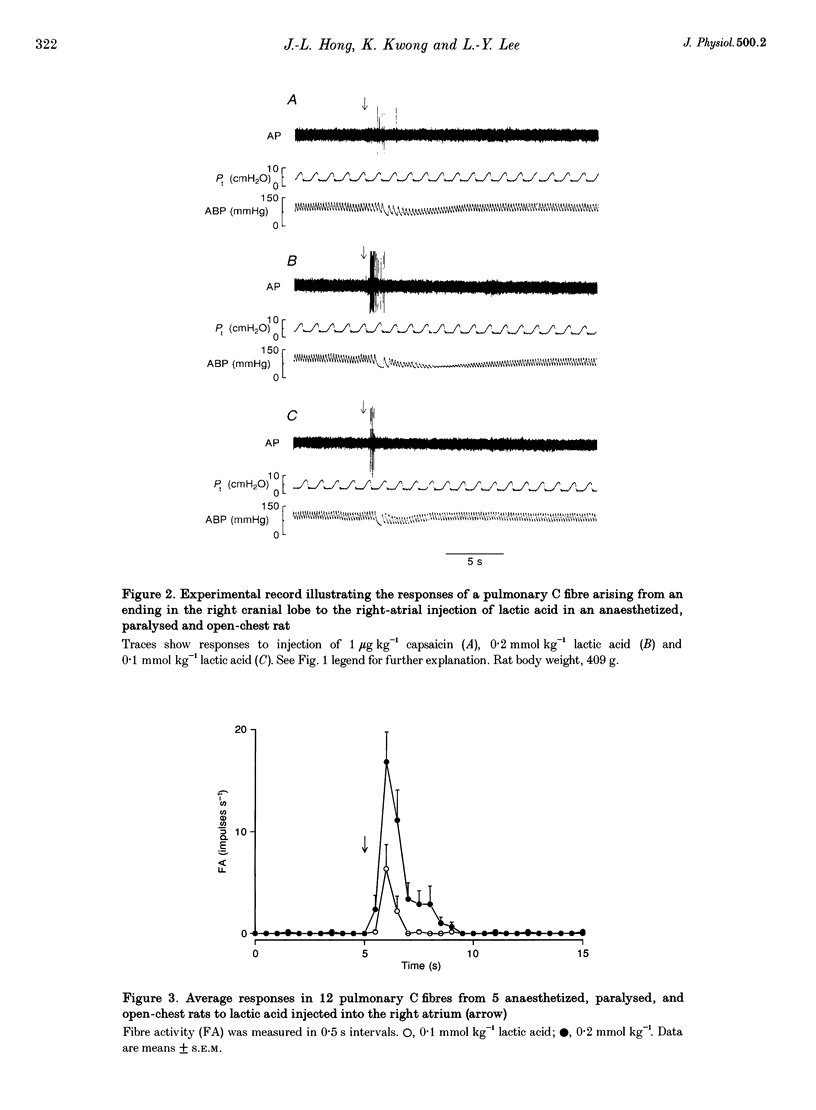
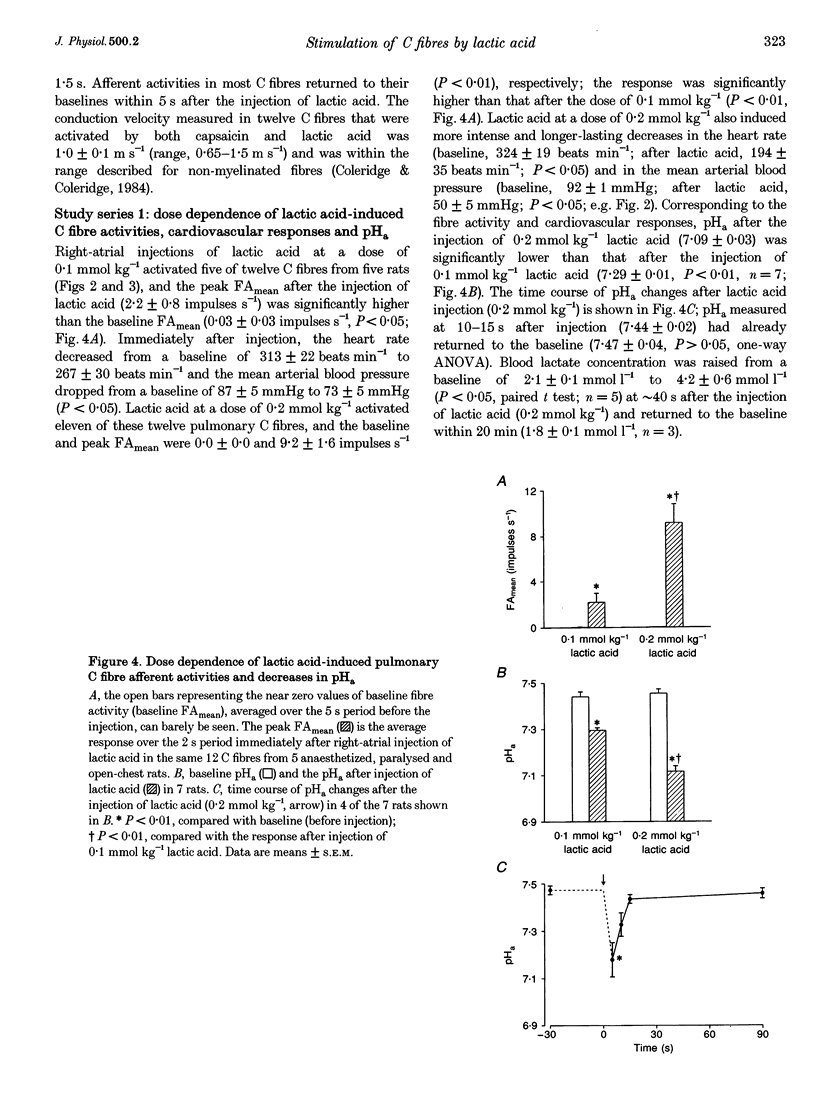
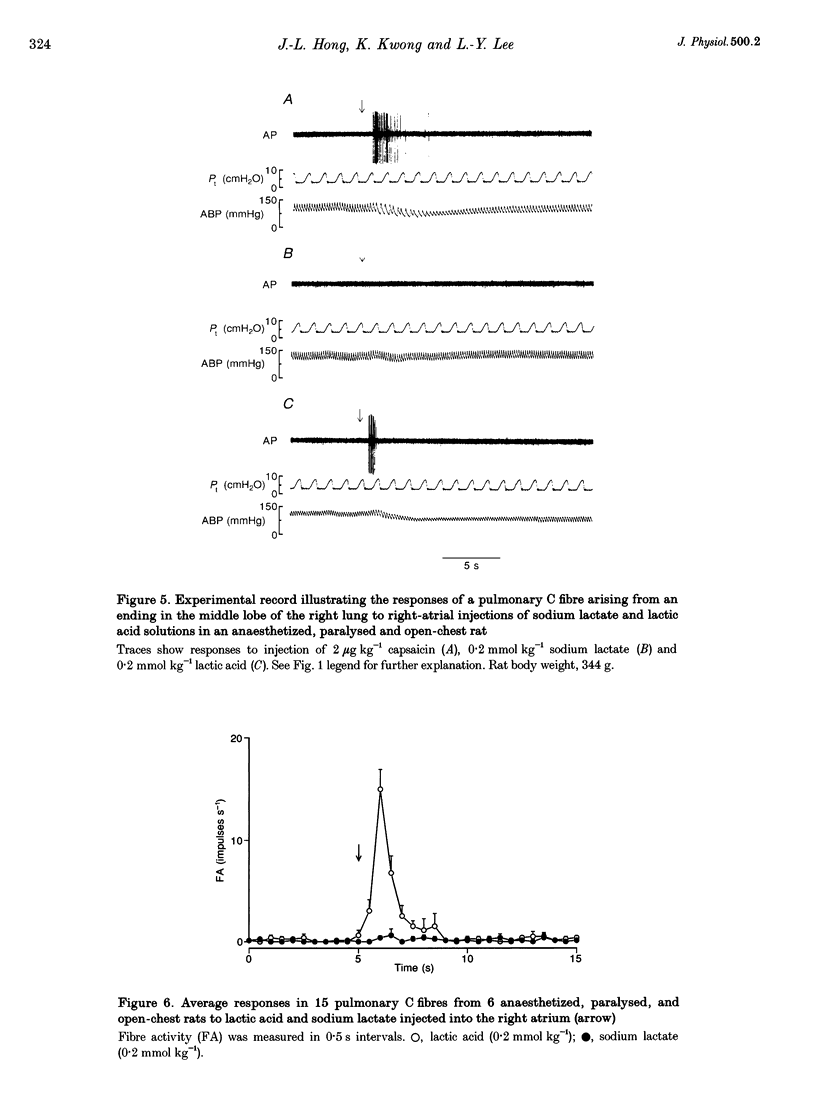
1. The contributions of H+ and lactate ions to the stimulation of single pulmonary C fibres by lactic acid were examined in anaesthetized and artificially ventilated rats. 2. Lactic acid injected into the right atrium caused a transient decrease in arterial blood pH (pHa) and a short but intense burst of afferent activities in pulmonary C fibres, whereas sodium lactate had no effect. The fibre activity usually reached a peak within 1-1.5 s, with an onset latency of < 1 s, and returned to the baseline in 5 s. 3. The injection of hydrochloric acid at the same pH as that of lactic acid did not significantly decrease pHa, nor did it stimulate any C fibres studied. 4. Formic acid has a pKa value (the negative logarithm of the dissociation constant) almost identical to that of lactic acid; thus, its injection decreased pHa to the same degree as did the injection of lactic acid. However, the response of C fibres to lactic acid was 134% stronger than that to formic acid. 5. We conclude that H+ is primarily responsible for the activation of pulmonary C fibres by lactic acid, probably through a direct effect of H+ on these afferent endings. The lactate ion, by itself, does not activate C fibres, but it seems to potentiate the stimulatory effect of H+ on these afferents.
Full text
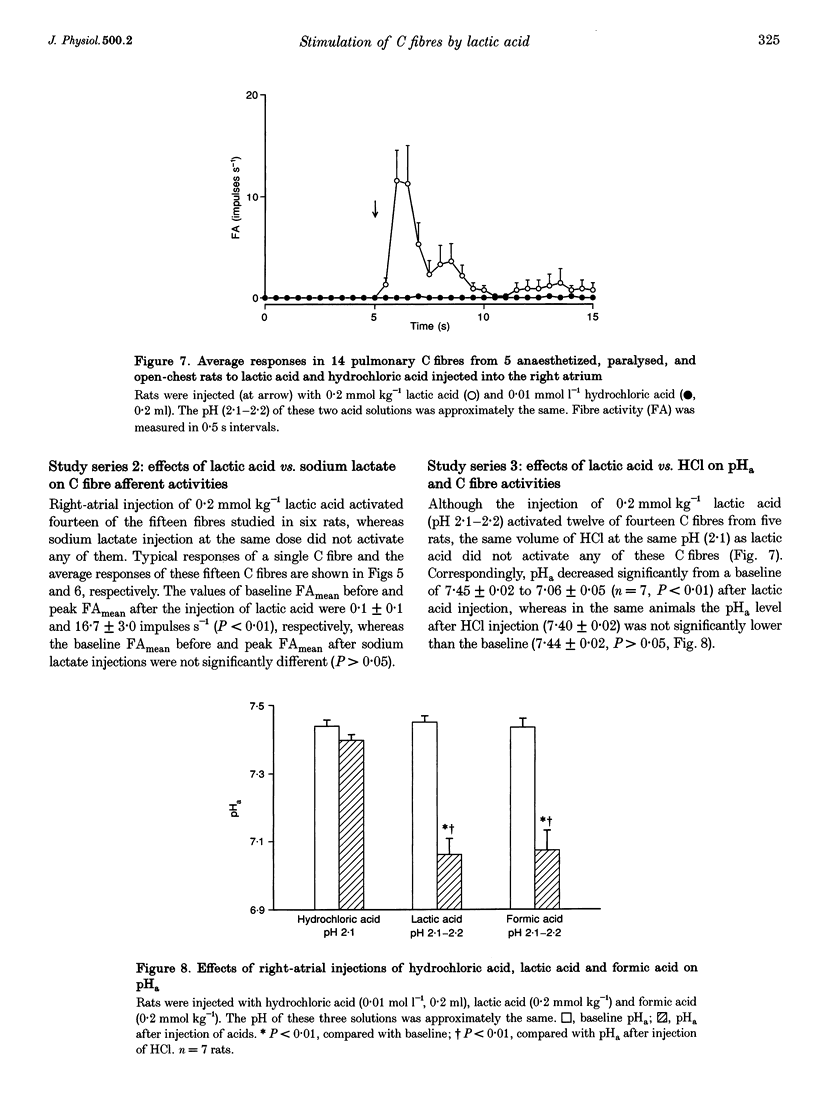
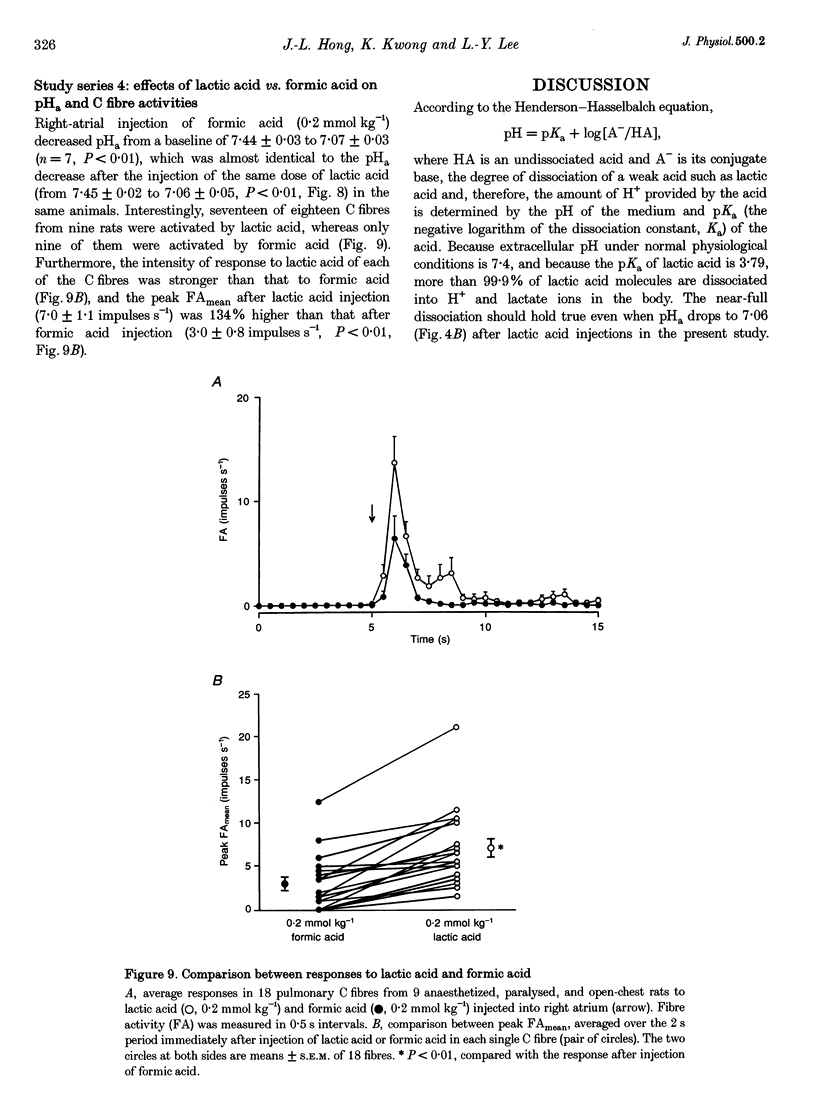
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allott C. P., Evans D. P., Marshall P. W. A model of irritant-induced bronchoconstriction in the spontaneously breathing guinea-pig. Br J Pharmacol. 1980;71(1):165–168. doi: 10.1111/j.1476-5381.1980.tb10921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleridge H. M., Coleridge J. C. Pulmonary reflexes: neural mechanisms of pulmonary defense. Annu Rev Physiol. 1994;56:69–91. doi: 10.1146/annurev.ph.56.030194.000441. [DOI] [PubMed] [Google Scholar]
- DAWES G. S., COMROE J. H., Jr Chemoreflexes from the heart and lungs. Physiol Rev. 1954 Apr;34(2):167–201. doi: 10.1152/physrev.1954.34.2.167. [DOI] [PubMed] [Google Scholar]
- Forsberg K., Karlsson J. A., Theodorsson E., Lundberg J. M., Persson C. G. Cough and bronchoconstriction mediated by capsaicin-sensitive sensory neurons in the guinea-pig. Pulm Pharmacol. 1988;1(1):33–39. doi: 10.1016/0952-0600(88)90008-7. [DOI] [PubMed] [Google Scholar]
- Fox A. J., Urban L., Barnes P. J., Dray A. Effects of capsazepine against capsaicin- and proton-evoked excitation of single airway C-fibres and vagus nerve from the guinea-pig. Neuroscience. 1995 Aug;67(3):741–752. doi: 10.1016/0306-4522(95)00115-y. [DOI] [PubMed] [Google Scholar]
- Franco-Cereceda A., Källner G., Lundberg J. M. Cyclo-oxygenase products released by low pH have capsaicin-like actions on sensory nerves in the isolated guinea pig heart. Cardiovasc Res. 1994 Mar;28(3):365–369. doi: 10.1093/cvr/28.3.365. [DOI] [PubMed] [Google Scholar]
- Franco-Cereceda A., Lundberg J. M. Capsazepine inhibits low pH- and lactic acid-evoked release of calcitonin gene-related peptide from sensory nerves in guinea-pig heart. Eur J Pharmacol. 1992 Oct 6;221(1):183–184. doi: 10.1016/0014-2999(92)90792-3. [DOI] [PubMed] [Google Scholar]
- Iturriaga R., Rumsey W. L., Lahiri S., Spergel D., Wilson D. F. Intracellular pH and oxygen chemoreception in the cat carotid body in vitro. J Appl Physiol (1985) 1992 Jun;72(6):2259–2266. doi: 10.1152/jappl.1992.72.6.2259. [DOI] [PubMed] [Google Scholar]
- Lee L. Y., Morton R. F., Lundberg J. M. Pulmonary chemoreflexes elicited by intravenous injection of lactic acid in anesthetized rats. J Appl Physiol (1985) 1996 Dec;81(6):2349–2357. doi: 10.1152/jappl.1996.81.6.2349. [DOI] [PubMed] [Google Scholar]
- Lee L. Y., Morton R. F. Pulmonary chemoreflex sensitivity is enhanced by prostaglandin E2 in anesthetized rats. J Appl Physiol (1985) 1995 Nov;79(5):1679–1686. doi: 10.1152/jappl.1995.79.5.1679. [DOI] [PubMed] [Google Scholar]
- Longhurst J. C., Dittman L. E. Hypoxia, bradykinin, and prostaglandins stimulate ischemically sensitive visceral afferents. Am J Physiol. 1987 Sep;253(3 Pt 2):H556–H567. doi: 10.1152/ajpheart.1987.253.3.H556. [DOI] [PubMed] [Google Scholar]
- Longhurst J. C., Rotto D. M., Kaufman M. P., Stahl G. L. Ischemically sensitive abdominal visceral afferents: response to cyclooxygenase blockade. Am J Physiol. 1991 Dec;261(6 Pt 2):H2075–H2081. doi: 10.1152/ajpheart.1991.261.6.H2075. [DOI] [PubMed] [Google Scholar]
- Lou Y. P., Lundberg J. M. Inhibition of low pH evoked activation of airway sensory nerves by capsazepine, a novel capsaicin-receptor antagonist. Biochem Biophys Res Commun. 1992 Nov 30;189(1):537–544. doi: 10.1016/0006-291x(92)91591-d. [DOI] [PubMed] [Google Scholar]
- Lundberg J. M., Saria A. Polypeptide-containing neurons in airway smooth muscle. Annu Rev Physiol. 1987;49:557–572. doi: 10.1146/annurev.ph.49.030187.003013. [DOI] [PubMed] [Google Scholar]
- Mizock B. A., Falk J. L. Lactic acidosis in critical illness. Crit Care Med. 1992 Jan;20(1):80–93. doi: 10.1097/00003246-199201000-00020. [DOI] [PubMed] [Google Scholar]
- Poole R. C., Halestrap A. P. Transport of lactate and other monocarboxylates across mammalian plasma membranes. Am J Physiol. 1993 Apr;264(4 Pt 1):C761–C782. doi: 10.1152/ajpcell.1993.264.4.C761. [DOI] [PubMed] [Google Scholar]
- Satoh H., Lou Y. P., Lundberg J. M. Inhibitory effects of capsazepine and SR 48968 on citric acid-induced bronchoconstriction in guinea-pigs. Eur J Pharmacol. 1993 Jun 4;236(3):367–372. doi: 10.1016/0014-2999(93)90473-u. [DOI] [PubMed] [Google Scholar]
- Schneider U., Poole R. C., Halestrap A. P., Grafe P. Lactate-proton co-transport and its contribution to interstitial acidification during hypoxia in isolated rat spinal roots. Neuroscience. 1993 Apr;53(4):1153–1162. doi: 10.1016/0306-4522(93)90497-4. [DOI] [PubMed] [Google Scholar]
- Shams H., Peskar B. A., Scheid P. Acid infusion elicits thromboxane A2-mediated effects on respiration and pulmonary hemodynamics in the cat. Respir Physiol. 1988 Feb;71(2):169–183. doi: 10.1016/0034-5687(88)90014-x. [DOI] [PubMed] [Google Scholar]
- Stahl G. L., Longhurst J. C. Ischemically sensitive visceral afferents: importance of H+ derived from lactic acid and hypercapnia. Am J Physiol. 1992 Mar;262(3 Pt 2):H748–H753. doi: 10.1152/ajpheart.1992.262.3.H748. [DOI] [PubMed] [Google Scholar]
- Stringer W., Casaburi R., Wasserman K. Acid-base regulation during exercise and recovery in humans. J Appl Physiol (1985) 1992 Mar;72(3):954–961. doi: 10.1152/jappl.1992.72.3.954. [DOI] [PubMed] [Google Scholar]
- Thimm F., Baum K. Response of chemosensitive nerve fibers of group III and IV to metabolic changes in rat muscles. Pflugers Arch. 1987 Sep;410(1-2):143–152. doi: 10.1007/BF00581907. [DOI] [PubMed] [Google Scholar]
- Thimm F., Carvalho M., Babka M., Meier zu Verl E. Reflex increases in heart-rate induced by perfusing the hind leg of the rat with solutions containing lactic acid. Pflugers Arch. 1984 Mar;400(3):286–293. doi: 10.1007/BF00581561. [DOI] [PubMed] [Google Scholar]
- Toffaletti J. G. Blood lactate: biochemistry, laboratory methods, and clinical interpretation. Crit Rev Clin Lab Sci. 1991;28(4):253–268. doi: 10.3109/10408369109106865. [DOI] [PubMed] [Google Scholar]
- Trenchard D. CO2/H+ receptors in the lungs of anaesthetized rabbits. Respir Physiol. 1986 Feb;63(2):227–240. doi: 10.1016/0034-5687(86)90116-7. [DOI] [PubMed] [Google Scholar]
- Walz W., Mukerji S. Lactate release from cultured astrocytes and neurons: a comparison. Glia. 1988;1(6):366–370. doi: 10.1002/glia.440010603. [DOI] [PubMed] [Google Scholar]


