Abstract
The inflammatory response in bacterial meningitis is mediated by cytokines, such as tumor necrosis factor alpha (TNF-α) and interleukin-1 (IL-1), which are produced in the subarachnoid space by different cells, e.g., leukocytes, astrocytes, and microglia. The recruitment of leukocytes into the cerebrospinal fluid (CSF) has been shown to contribute to the neurological damage in this disease, a process which could be enhanced by treatment with antibiotics. In this study, we have used a rabbit meningitis model for two sets of experiments with intracisternal (i.c.) injections of Streptococcus pneumoniae. First, pneumococcal cell wall (PCW) components were injected i.c., inducing an inflammatory response with pleocytosis and increased levels of CSF TNF-α) and IL-1 at 6 and 12 h after PCW injection. Treatment with fucoidin, known to inhibit leukocyte rolling, abolished pleocytosis and inhibited the release of TNF-α and IL-1. In the second experiment, live pneumococcal bacteria were injected i.c. and treatment with one dose of ampicillin (40 mg/kg of body weight intravenously) was given 16 h after induction of meningitis, causing a sevenfold increase in CSF leukocytes over a 4-h period. CSF IL-1 levels at 16 h were high but did not increase further at 20 h. Also, CSF TNF-α levels were high at 16 h and tended to increase at 20 h. Fucoidin treatment prevented the antibiotic-induced increase of CSF leukocytes but had no effect on the TNF-α and IL-1 levels. Taken together, fucoidin reduced CSF TNF-α and IL-1 levels in acute bacterial meningitis induced by PCW fragments but had no effect later in the course of the disease, when live bacteria were used and an inflammatory increase was caused by a dose of antibiotics.
In bacterial meningitis, the meningeal inflammatory reaction most likely contributes to the central nervous system (CNS) injury associated with this disease, and one part of this process is the deleterious effects of the cerebrospinal fluid (CSF) leukocytes and their cytotoxic products (19). Consequently, inhibition of leukocyte recruitment into the subarachnoid space has been shown to reduce mortality in experimental meningitis (16, 20). The process of inflammatory leukocyte recruitment involves several sequential steps: margination and rolling of leukocytes along vascular endothelium, firm adhesion to the endothelial cells, and subsequent diapedesis through the vessel wall (2). Leukocyte rolling is mediated by adhesion molecules of the selectin family (2), while stationary leukocyte adhesion is critically dependent on leukocytic integrins, such as CD11 and CD18 (2). Accordingly, the polysaccharide fucoidin, which blocks L- and P-selectin function, inhibits leukocyte rolling (9, 23), and monoclonal antibodies directed against CD11 and CD18 block firm leukocyte adhesion (1). With regard to bacterial meningitis, we have recently shown that fucoidin treatment is an effective way to attenuate meningeal inflammation induced by pneumcoccal cell wall (PCW) fragments (6) and furthermore, it prevents antibiotic-induced CSF leukocyte accumulation in rabbits inoculated with live pneumococcal bacteria (7).
During the onset of meningitis caused by both gram-negative and gram-positive bacteria, the proinflammatory cytokines tumor necrosis factor alpha (TNF-α) and interleukin-1 (IL-1) are present in the CSF (18, 24). These cytokines can be produced by different cells, including leukocytes and cells within the CNS, such as astrocytes and microglia (22). The cytokines play an important role in the pathophysiology of bacterial meningitis. Thus, TNF-α contributes to the accumulation of leukocytes in the CSF, brain edema (17), blood-brain barrier damage (18), and damage of cells within the CNS (22). IL-1 also contributes to disruption of the blood-brain barrier and CSF leukocyte recruitment and stimulates the production of other cytokines, such as IL-6 and TNF-α (22). Moreover, in patients with bacterial meningitis, high CSF IL-1 levels correlate with neurological complications (22). There is also evidence that treatment of bacterial meningitis with antibiotics has the capability to increase CSF cytokine concentrations, most likely because antibiotic-induced bacterial lysis liberates large amounts of harmful bacterial products in the CSF (13). For example, in experimental pneumococcal meningitis, Tuomanen et al. have demonstrated an increase in meningeal inflammation after ampicillin treatment, characterized by a massive influx of leukocytes and elevated levels of protein and lactate in the CSF (20, 21). In the same type of model with pneumococcal bacteria, others have demonstrated elevated CSF TNF-α levels after antibiotic treatment (14). Similar effects of antibiotics have also been demonstrated in experimental Haemophilus influenzae meningitis (13, 16). However, the cellular source of the cytokines (blood leukocytes or stationary CNS cells) is still unclear.
In the present study, we examined whether inhibition of CSF leukocyte recruitment with fucoidin has the capacity to inhibit the production of CSF TNF-α and IL-1 in rabbits with meningitis induced by PCW fragments and in animals inoculated with live pneumococcal bacteria and then treated with ampicillin.
MATERIALS AND METHODS
Bacterial strain.
Streptococcus pneumoniae III, type III, a gift from Elaine Tuomanen, was cultured on blood agar plates and suspended in pyrogen-free saline before injection.
Preparation of pneumococcal antigen.
An unencapsulated strain (CSR-SCS-2 clone 1) of S. pneumoniae (“Cesam strain,” from the Department of Bacteriology, Karolinska Institutet) was cultured overnight on blood agar plates, suspended in pyrogen-free saline, and heat inactivated by being boiled for 10 min. The optical density of the final antigen solution was adjusted to a viable count of 106 CFU/ml.
Experimental meningitis model with PCW.
A previously described meningitis model in female New Zealand White rabbits (3.5 to 4.5 kg) was used (10). Briefly, 0.25 ml of CSF was collected from the cisterna magna, after which an equal volume of the pneumococcal antigen suspension was injected. Subsequent CSF samples (0.25 ml) were taken after 6 and 12 h. In a group of five rabbits, fucoidin (10 mg/kg of body weight; Sigma Chemical Co., St. Louis, Mo.) was administered intravenously (i.v.) at −5 min and at 2 and 4 h relative to the intracisternal (i.c.) introduction of antigen. A control group of six rabbits received an i.c. injection of antigen and i.v. treatment with a vehicle. Fucoidin was dissolved in sterile phosphate-buffered saline (10 mg/ml at pH 7.3) and passed through a 0.2-μm-pore-size sterile filter prior to i.v. administration. Fucoidin has been found to block leukocyte rolling in a dose-dependent manner, without interfering with the process of firm leukocyte adhesion per se (9). Our choice of dose and regimen are based on previous studies (6).
Experimental meningitis model with live pneumococcal bacteria.
In the same model described above, a 0.25-ml saline suspension containing an inoculum of live pneumococci (105 CFU/ml) was injected i.c. in 15 rabbits. Sixteen hours after inoculation of the bacterial, 10 animals received an i.v. injection of ampicillin (40 mg/kg) (Doktacillin; Astra AB, Södertälje, Sweden). Five of these rabbits received ampicillin only, and five rabbits were treated with fucoidin (10 mg/kg i.v.), given 5 min before the ampicillin dose and 2 h thereafter. Five rabbits served as controls, i.e., they were inoculated with bacteria but did not receive any treatment. In all animals, aliquots of CSF were obtained just before inoculation with pneumococci and at 16 and 20 h thereafter. The rationale for choosing these time points was based on previous studies (7).
In both sets of experiments, total and differential leukocyte counts of CSF were done immediately after collection. The remaining CSF was centrifuged (1,200 × g; 10 min), and the supernatants were stored at −70°C until they were assayed for TNF-α and IL-1.
During each cisternal puncture, the animals were anesthetized with 0.25 ml of fluanison-fentanyl (Hypnorm Vet 10; 0.2 mg/ml) (Janssen Pharmaceutica, Beerse, Belgium)/kg and 0.25 ml of diazepam (Stesolid; 5 mg/ml) (Dumex, Kabi Pharmacia, Sweden)/kg intramuscularly. The experiments were approved by an animal ethics committee at Södra Roslags Court House. All animals were euthanized within 24 h after inoculation with bacterial.
Assays for TNF-α and IL-1.
TNF-α was measured by its cytotoxic effect on the mouse fibrosarcoma cell line WEHI 164 clone 13 (3). Cell viability was measured after 20 h by a colorimetric MTT (tetrazolium) assay (11). Human recombinant TNF-α (Genentech Inc., South San Francisco, Calif.) was used as a standard. CSF was assayed in duplicate in serial dilutions from 1:8 to 1:528. The detection limit was 15 pg of TNF-α per ml of CSF.
IL-1 was measured in a two-stage assay with the IL-1-responsive mouse T-cell line EL-4 6.1 clone NOB-I (5) and the IL-2-dependent mouse T-cell line HT-2 (12). The EL-4 cells produce IL-2 in response to IL-1 stimulation, and IL-2 production is measured by the proliferation of the IL-2-dependent HT-2 cells. The medium used was RPMI 1640 supplemented with l-glutamine (0.1 mg/ml), gentamicin (40 μg/ml), 10% fetal calf serum, and 2-mercaptoethanol (25 μmol/liter). Serial dilutions of CSF were added in duplicate to flat-bottom 96-well microtiter plates, and the volume was adjusted with medium to 100 μl per well. The EL-4 cells were washed once in Hanks’ balanced salt solution, resuspended in medium, and incubated at 2 × 105 per well, producing a final volume of 200 μl per well. After 24 h of incubation, 100 μl of supernatant was transferred from each well into a replicate microtiter plate. The HT-2 cells were washed three times in Hanks’ balanced salt solution, resuspended in medium, and distributed in the microtiter plates at a concentration of 0.15 × 105 per well, giving a final volume of 200 μl per well. After 20 h of incubation, the cell growth was measured by the colorimetric MTT assay. Human recombinant IL-1α (Glaxo, Geneva, Switzerland) was included as a standard. CSF was assayed in dilutions from 1:4 to 1:256. The detection limit was 8 pg of IL-1 per ml of CSF. Some samples containing IL-1 activity were retested in the HT-2 assay to ascertain if the activity could be caused by the presence of IL-2 in the sample.
Statistical analysis.
Results were analyzed by the Wilcoxon signed-rank test or the Mann-Whitney rank sum test. Differences were considered significant when P was less than 0.05. Data are expressed as mean ± standard error.
RESULTS
Experimental meningitis with PCW.
In the experiment with i.c. injection of PCW fragments, the mean CSF leukocyte counts in the control animals increased substantially at both 6 and 12 h (Table 1). In the group of rabbits treated with fucoidin (10 mg/kg, given 5 min before and 2 and 4 h after antigen challenge), the corresponding mean CSF leukocyte counts were very low at 6 h and slightly raised at 12 h (i.e., 8 h after the end of fucoidin treatment) (Table 1).
TABLE 1.
Mean CSF leukocyte counts and CSF IL-1 and TNF-α concentrations in rabbits injected i.c. with PCW fragments, with and without fucoidin treatmenta
| CSF index | PCW
|
PCW + fucoidin
|
||||
|---|---|---|---|---|---|---|
| 0 h | 6 h | 12 h | 0 h | 6 h | 12 h | |
| Leukocytes (106/liter) | 1 ± 1.5 | 1,703 ± 295 | 2,048 ± 443 | 2 ± 2.6 | 3 ± 3.6b | 148 ± 136c |
| Polynuclear | 0 | 887 ± 223 | 635 ± 423 | 0 | 0b | 68 ± 86c |
| Mononuclear | 1 ± 1.5 | 808 ± 134 | 1,337 ± 122 | 2 ± 2.6 | 3 ± 3.6b | 55 ± 50c |
| IL-1 (pg/liter) | <0.008 | 141 ± 32 | 97 ± 28 | 7.4 ± 5 | 20 ± 7b | 31 ± 14 |
| TNF-α (pg/liter) | <0.015 | 333 ± 182 | 260 ± 211 | <0.015 | 184 ± 116 | 0c |
PCW fragments were injected i.c. at 0 h. Fucoidin (10 mg/kg i.v.) was given 5 min prior to injection of PCW fragments and 2 and 4 h thereafter. The data are expressed as means ± standard errors of the mean.
P < 0.05 versus PCW at 6 h.
P < 0.05 versus PCW at 12 h.
In the control group, PCW injection increased the mean CSF IL-1 concentration at 6 and 12 h (Table 1). In the animals treated with fucoidin, the mean CSF IL-1 values were significantly reduced at 6 h and tended to be reduced at 12 h (Table 1). IL-1 was detected in the CSF of all rabbits after PCW injection.
PCW challenge also increased the mean CSF TNF-α levels in control rabbits at 6 and 12 h. Fucoidin treatment tended to reduce the mean CSF TNF-α levels at 6 h (not significant), while the reduction was significant at 12 h (Table 1). However, TNF-α was detected in only two animals in the treatment group, both at 6 h. In contrast, the control group had detectable TNF-α in all samples at both time points after PCW injection.
Experimental meningitis with live pneumococcal bacteria.
In the second set of experiments, where live pneumococci were injected i.c., an i.v. dose of ampicillin (40 mg/kg 16 h after inoculation with bacteria elicited a sevenfold increase of the mean CSF leukocyte count 4 h later. If a dose of fucoidin was given i.v. 5 min before the ampicillin and 2 h thereafter, the CSF leukocyte influx was prevented and the 20-h value was even lower than the 16-h value (Table 2). In the five animals serving as controls (inoculated with bacteria without treatment with ampicillin or fucoidin), the CSF leukocyte counts were 0, 960 × 106 ± 326 × 106, and 662 × 106 ± 160 × 106/ml at 0, 16, and 20 h, respectively (data not shown).
TABLE 2.
Mean CSF leukocyte counts and CSF IL-1 and TNF-α concentrations in rabbits injected i.c. with live pneumococci and treated with ampicillin or ampicillin and fucoidina
| CSF index | Pneumococci + ampicillin
|
Pneumococci + ampicillin + fucoidin
|
||||
|---|---|---|---|---|---|---|
| 0 h | 16 h | 20 h | 0 h | 16 h | 20 h | |
| Leukocytes (106/liter) | 0 | 1,798 ± 856 | 13,322 ± 5,219 | 3 ± 3.8 | 1,170 ± 738 | 664 ± 343b |
| Polynuclear | 0 | 1,218 ± 532 | 10,406 ± 5,931 | 0 | 938 ± 638 | 520 ± 259b |
| Mononuclear | 0 | 852 ± 439 | 2,916 ± 1,563 | 3 ± 3.8 | 232 ± 108 | 144 ± 89b |
| IL-1 (pg/liter) | 3 ± 2 | 638 ± 221 | 661 ± 264 | 2 ± 2 | 839 ± 232 | 1,010 ± 264 |
| TNF-α (pg/liter) | <0.015 | 906 ± 874 | 3,540 ± 2,418 | <0.015 | 1,579 ± 453 | 6,910 ± 5,963 |
Pneumococci were injected i.c. at 0 h. Ampicillin (40 mg/kg i.v.) was injected at 16 h. Fucoidin (10 mg/kg i.v.) was given 5 min before ampicillin and 2 h thereafter. Data are expressed as means ± standard errors of the mean.
P < 0.05 versus ampicillin only at 20 h.
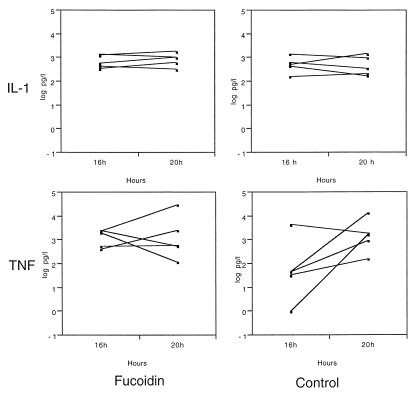
The CSF IL-1 concentration at 16 h after inoculation with bacteria was increased in all 10 animals. Treatment with ampicillin at the 16-h time point did not elevate the IL-1 levels as measured 4 h later. Moreover, treatment with fucoidin had no effect on the CSF IL-1 levels (Table 2). The individual values for each animal at 16 and 20 h are presented in Fig. 1, showing the narrow range of values in both treatment groups at both time points.
FIG. 1.
CSF IL-1 and TNF-α concentrations in rabbits injected i.c. with live pneumococci and treated with a dose of ampicillin (40 mg/kg i.v.) after 16 h. One group (Control) received ampicillin only, and the other group received treatment with fucoidin (10 mg/kg i.v.). The values for each animal are shown; n = 5 in each group.
The mean CSF TNF-α concentration 4 h after ampicillin treatment only tended to be increased in both treatment groups, and fucoidin treatment did not affect the TNF-α levels (Table 2). However, one fucoidin-treated animal had a pronounced CSF TNF-α activity (30,700 pg/liter) in the 20-h sample, causing the substantially increased mean value. The individual values for each animal at 16 and 20 h are presented in Fig. 1, showing the wide range of TNF-α values, particularly in the control group. All rabbits except one (16-h sample) had detectable TNF-α levels in all samples after inoculation with bacteria.
DISCUSSION
In this study, as in a previous one (6), we found that pretreatment with fucoidin prevented the accumulation of leukocytes in the subarachnoid space during the first 6 h of experimental meningitis induced with PCW components. In parallel, fucoidin treatment significantly attenuated the increased CSF levels of the proinflammatory cytokine IL-1 while only tending to reduce TNF-α levels. At 12 h, however, fucoidin treatment abolished TNF-α release in all animals. In the second part of this study, where meningitis was established with live pneumococci, a sevenfold increase in CSF leukocytosis occurred after a dose of ampicillin. This increased leukocyte influx was also prevented with fucoidin treatment, consistent with our previous results (7). In these experiments the mean TNF-α level increased fourfold, although not significantly, while the mean IL-1 activity remained unchanged 4 h after the dose of ampicillin.
In the first set of experiments with PCW fragments, the reduced levels of CSF TNF-α and IL-1 appeared to correlate with the inhibition of CSF leukocyte accumulation, indicating that leukocytes (directly or indirectly) were involved in the production of these inflammatory cytokines. In contrast, no such correlation could be demonstrated in the second set of experiments with live pneumococci. Thus, despite a significant inhibition of CSF leukocyte influx after a dose of ampicillin in fucoidin-treated animals, no significant inhibition of CSF cytokines was observed, suggesting that leukocytes might be less important for cytokine production in this later phase of meningitis. This is in line with a meningitis study by Saéz-Llorens et al. (16) showing that treatment with an anti-CD18 monoclonal antibody of rabbits inoculated with H. influenzae and treated with antibiotics did not inhibit CSF TNF-α formation, despite significant attenuation of the CSF inflammatory burst, including pleocytosis (16).
It is not clear why leukocyte blocking with fucoidin partially inhibited TNF-α and IL-1 production in our experiments with PCW but not in the experiments with live bacteria. However, it is clear that several types of cells possibly involved in the pathophysiological process in bacterial meningitis can produce TNF-α and/or IL-1 upon stimulation with, e.g., pneumococci. For example, it has been demonstrated that both monocytes and CNS cells (astrocytes and microglia) produce IL-1β and TNF-α in response to PCW components and other stimuli (4, 8, 15, 22). Moreover, in studies of experimental pneumococcal meningitis in rabbits, it has been shown that systemic monocyte depletion reduced CSF IL-1β levels whereas CSF TNF-α concentrations were not different from those of controls (25). Thus, one explanation for the different effects of fucoidin in our acute experiment with PCW and the prolonged experiments with live bacteria could be that the major source of these cytokines in the acute phase of meningitis is leukocytes and that other cells, like astrocytes and microglia, reach full cytokine production later in the course of pneumococcal meningitis. However, further studies are needed to elucidate the exact cellular sources and release kinetics of cytokines in both experimental and clinical meningitis.
In summary, we have documented that blocking of CSF leukocyte accumulation with the polysaccharide fucoidin has the capacity to inhibit CSF levels of TNF-α and IL-1 in experimental meningitis induced with PCW fragments. However, fucoidin treatment had no significant effect on TNF-α or IL-1 levels after a dose of antibiotics 16 h after the induction of meningitis with live pneumococci, although the burst of pleocytosis was prevented.
ACKNOWLEDGMENTS
We thank Gudmundur Axelsson for valuable help with statistics.
This study was supported by the Swedish Society for Medical Research, the Swedish Medical Research Council (14X-4342), the Swedish Foundation for Health Care Sciences and Allergy Research (A95093), and Clas Groschinski’s Memorial Foundation.
REFERENCES
- 1.Arfors K E, Lundberg C, Lindbom L, Lundberg K, Beatty P G, Harlan J M. A monoclonal antibody to the membrane glycoprotein complex CD18 inhibits polymorphonuclear leukocyte accumulation and plasma leakage in vivo. Blood. 1987;69:338–340. [PubMed] [Google Scholar]
- 2.Carlos, T. M., and J. M. Harlan. 1994. Leukocyte-endothelial adhesion molecules. Blood 2068–2101. [PubMed]
- 3.Espevik T, Nissen-Meyer J. A highly sensitive cell line, WEHI 164 clone 13, for measuring cytotoxic factor/tumor necrosis factor from human monocytes. J Immunol Methods. 1986;95:99–103. doi: 10.1016/0022-1759(86)90322-4. [DOI] [PubMed] [Google Scholar]
- 4.Freyer D, Weih M, Weber J R, Bürger W, Scholz P, Manz R, Ziegenhorn A, Angstwurm K, Dirnagl U. Pneumococcal cell wall components induce nitric oxide synthase and TNF-α in astroglial-enriched cultures. Glia. 1996;16:1–6. doi: 10.1002/(SICI)1098-1136(199601)16:1<1::AID-GLIA1>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 5.Gearing A J H, Bird C R, Bristow A, Poole S, Thorpe R. A simple sensitive bioassay for interleukin-1 which is unresponsive to 103 U/ml of interleukin-2. J Immunol Methods. 1987;99:7–11. doi: 10.1016/0022-1759(87)90025-1. [DOI] [PubMed] [Google Scholar]
- 6.Granert C, Raud J, Xie X, Lindquist L, Lindbom L. Inhibition of leukocyte rolling with polysaccharide fucoidin prevents pleocytosis in experimental meningitis in the rabbit. J Clin Investig. 1994;93:929–936. doi: 10.1172/JCI117098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Granert C, Raud J, Lindquist L. The polysaccharide fucoidin inhibits the antibiotic-induced inflammatory cascade in experimental pneumococcal meningitis. Clin Diagn Lab Immunol. 1998;5:322–324. doi: 10.1128/cdli.5.3.322-324.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heumann D, Barras C, Severin A, Glauser M P, Tomasz A. Gram-positive cell walls stimulate synthesis of tumor necrosis factor alpha and interleukin-6 by human monocytes. Infect Immun. 1994;62:2715–2721. doi: 10.1128/iai.62.7.2715-2721.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lindbom L, Xie X, Raud J, Hedqvist P. Chemoattractant-induced firm adhesion of leukocytes to vascular endothelium in vivo is critically dependent on initial leukocyte rolling. Acta Physiol Scand. 1992;146:415–421. doi: 10.1111/j.1748-1716.1992.tb09442.x. [DOI] [PubMed] [Google Scholar]
- 10.Lindquist L, Lundbergh P, Hedström K G, Hansson L O, Hultman E. Experimental bacterial meningitis in the rabbit: cerebrospinal fluid changes and its relation to leukocyte response. Scand J Infect Dis. 1987;19:263–270. doi: 10.3109/00365548709032409. [DOI] [PubMed] [Google Scholar]
- 11.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 12.Mosmann T R, Cherwinski H, Bond M W, Giedlin M A, Coffman R L. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–2357. [PubMed] [Google Scholar]
- 13.Mustafa M M, Ramilo O, Mertsola J, Risser R C, Beutler B, Hansen E J, MacCracken G H., Jr Modulation of inflammation and cachectin activity in relation to treatment of experimental Haemophilus influenzae type b meningitis. J Infect Dis. 1989;160:818–825. doi: 10.1093/infdis/160.5.818. [DOI] [PubMed] [Google Scholar]
- 14.Pfister H W, Fontana A, Täuber M G, Tomasz A, Scheld W M. Mechanisms of brain injury in bacterial meningitis: workshop summary. Clin Infect Dis. 1994;19:463–479. doi: 10.1093/clinids/19.3.463. [DOI] [PubMed] [Google Scholar]
- 15.Riesenfeld-Örn I, Wolpe S, Garcia-Bustos J F, Hoffman M K, Tuomanen E. Production of interleukin-1 but not tumor necrosis factor by human monocytes stimulated with pneumococcal cell surface components. Infect Immun. 1989;57:1890–1893. doi: 10.1128/iai.57.7.1890-1893.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sáez-Llorens X, Jafari H S, Severien C, Parras F, Olsen K D, Hansen E J, Singer I I, McCracken G H., Jr Enhanced attenuation of meningeal inflammation and brain edema by concomitant administration of anti-CD18 monoclonal antibodies and dexamethasone in experimental Haemophilus meningitis. J Clin Investig. 1991;88:2003–2011. doi: 10.1172/JCI115527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saukkonen K, Sande S, Cioffe C, Wolpe S, Sherry B, Cerami A, Tuomanen E. The role of cytokines in the generation of inflammation and tissue damage in experimental Gram-positive meningitis. J Exp Med. 1990;171:439–448. doi: 10.1084/jem.171.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharief M K, Ciardi M, Thompson E J. Blood-brain barrier damage in patients with bacterial meningitis: association with tumor necrosis factor-α but not interleukin-1β. J Infect Dis. 1992;166:350–358. doi: 10.1093/infdis/166.2.350. [DOI] [PubMed] [Google Scholar]
- 19.Täuber M G, Borschberg U, Sande M A. Influence of granulocytes on brain edema, intracranial pressure, and cerebrospinal fluid concentrations of lactate and protein in experimental meningitis. J Infect Dis. 1988;157:456–464. doi: 10.1093/infdis/157.3.456. [DOI] [PubMed] [Google Scholar]
- 20.Tuomanen E, Saukkonen K, Sande S, Cioffe C, Wright S D. Reduction of inflammation, tissue damage, and mortality in bacterial meningitis in rabbits treated with monoclonal antibodies against adhesion-promoting receptors of leukocytes. J Exp Med. 1989;170:959–969. doi: 10.1084/jem.170.3.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tuomanen E, Hengstler B, Rich R, Bray M A, Zak O, Tomasz A. Nonsteroidal anti-inflammatory agents in the therapy for experimental pneumococcal meningitis. J Infect Dis. 1987;155:985–990. doi: 10.1093/infdis/155.5.985. [DOI] [PubMed] [Google Scholar]
- 22.van Furth A M, Roord J J, van Furth R. Roles of proinflammatory and anti-inflammatory cytokines in pathophysiology of bacterial meningitis and effect of adjunctive therapy. Infect Immun. 1996;64:4883–4890. doi: 10.1128/iai.64.12.4883-4890.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Varki A. Selectin ligands. Proc Natl Acad Sci USA. 1994;91:7390–7397. doi: 10.1073/pnas.91.16.7390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Waage A, Halstensen A, Shalaby R, Brandtzaeg P, Kierulf P, Espevik T. Local production of tumor necrosis factor α, interleukin 1, and interleukin 6 in meningococcal meningitis. J Exp Med. 1989;170:1859–1867. doi: 10.1084/jem.170.6.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zysk G, Brück W, Huitinga I, Fischer F R, Flachsbarth F, van Rooijen N, Nau R. Elimination of blood-derived macrophages inhibits the release of interleukin-1 and the entry of leukocytes into the cerebrospinal fluid in experimental pneumococcal meningitis. J Neuroimmunol. 1997;73:77–80. doi: 10.1016/s0165-5728(96)00173-7. [DOI] [PubMed] [Google Scholar]