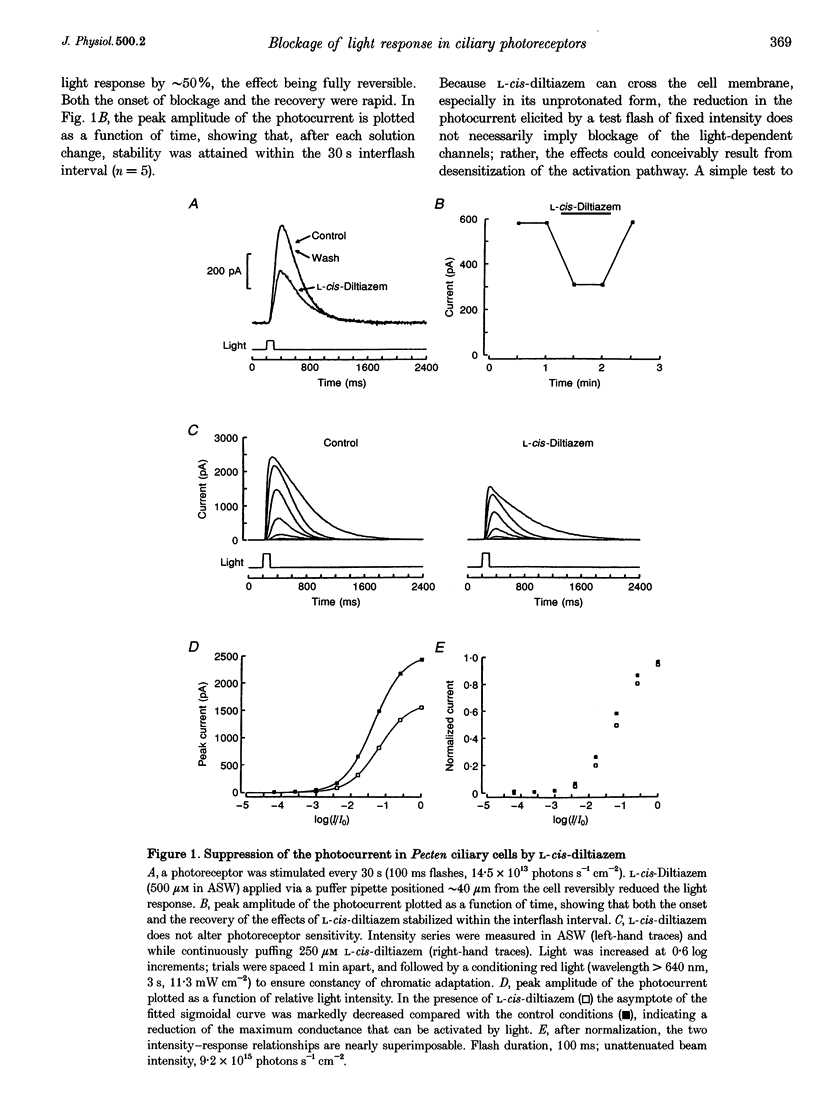
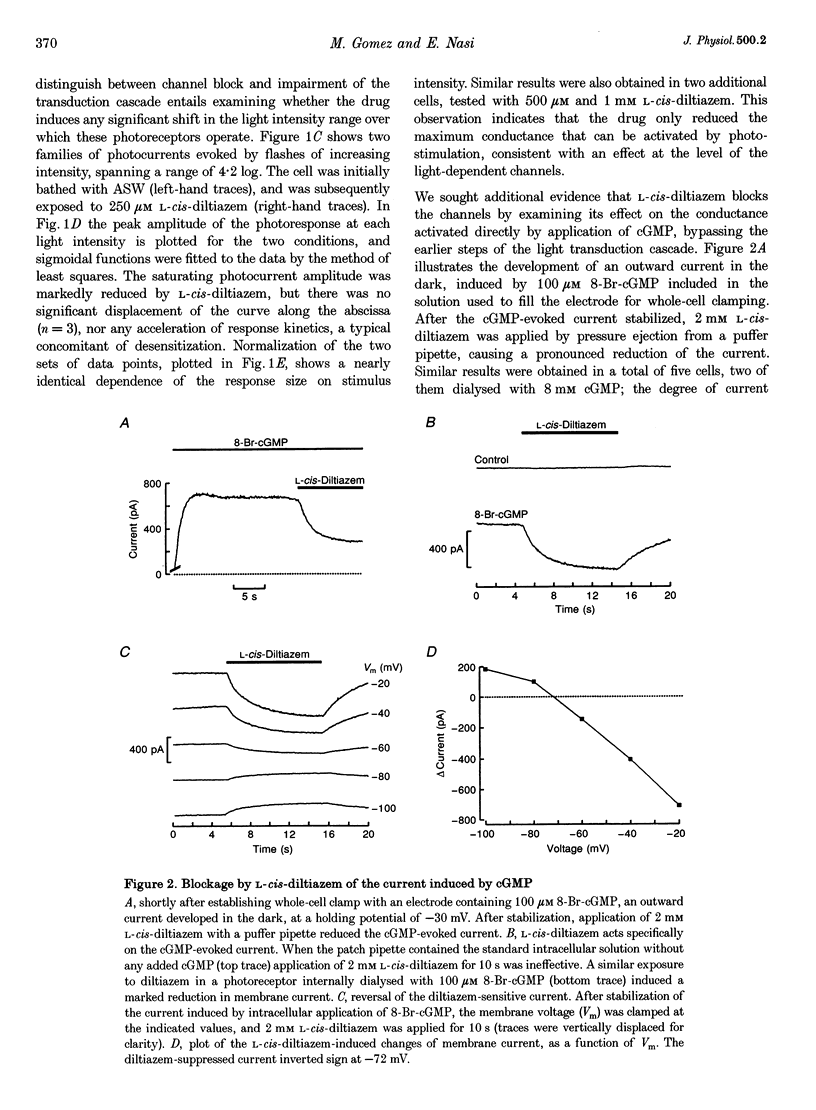
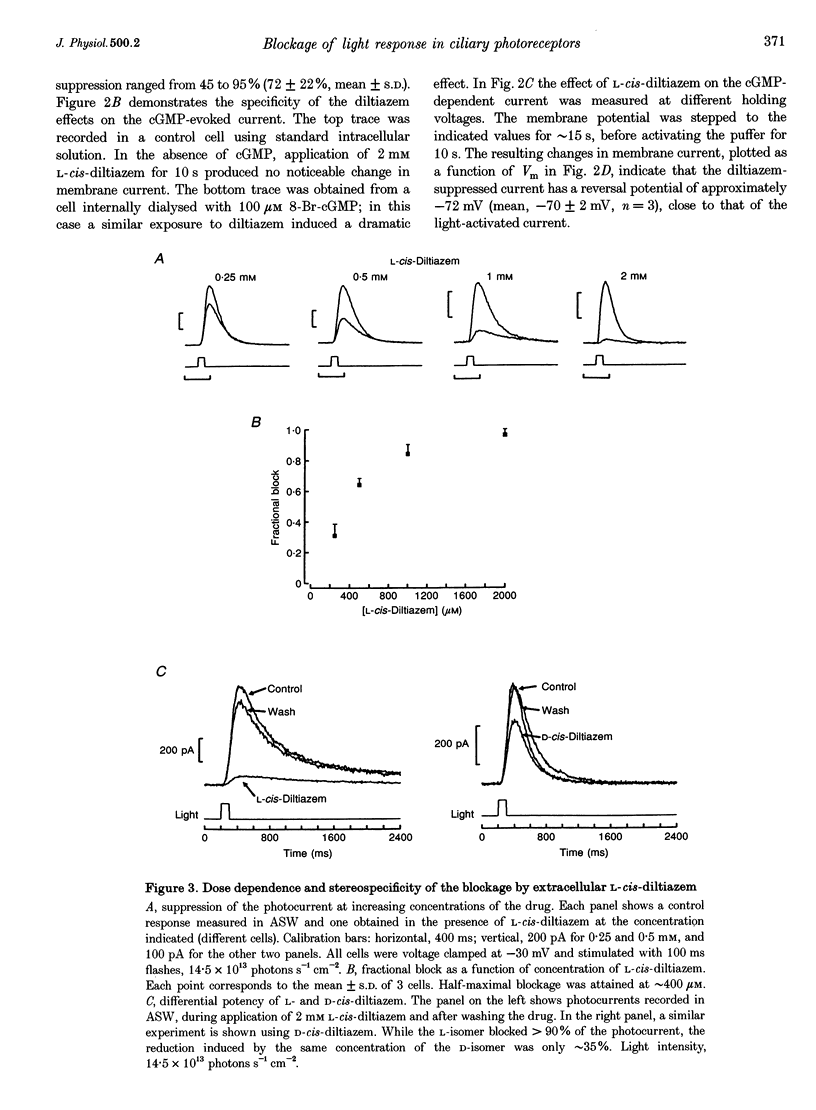
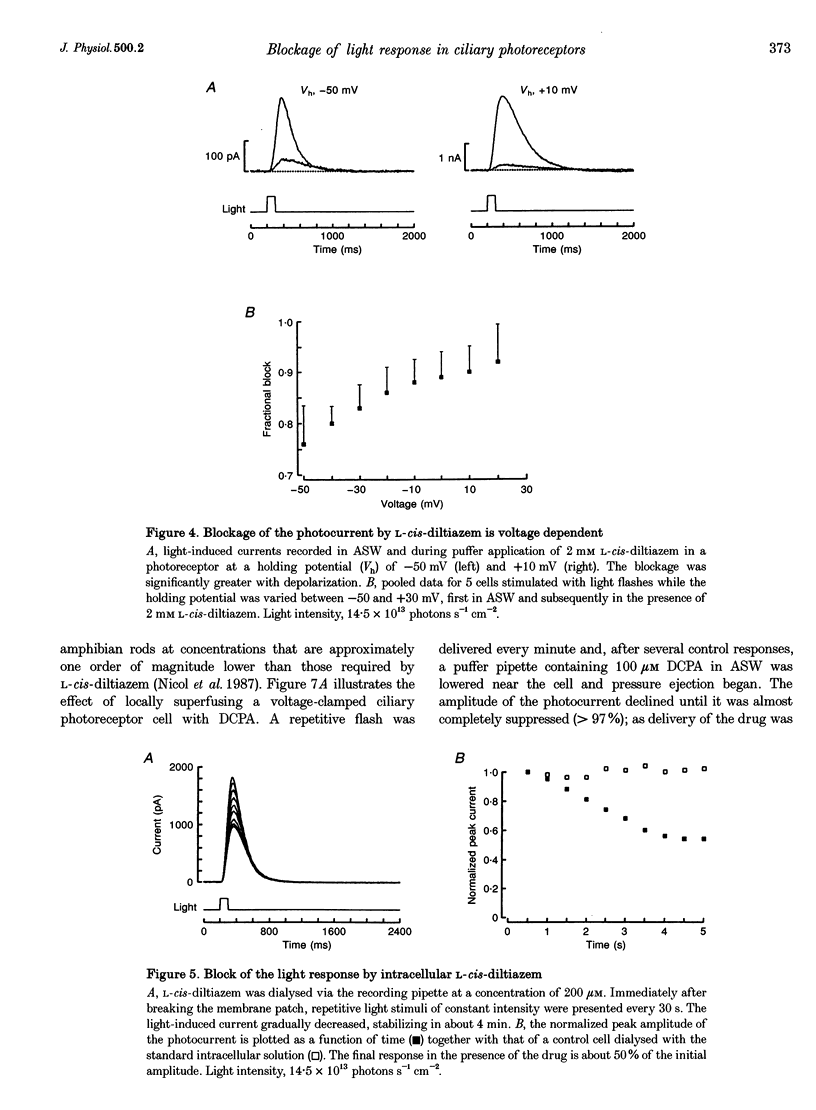
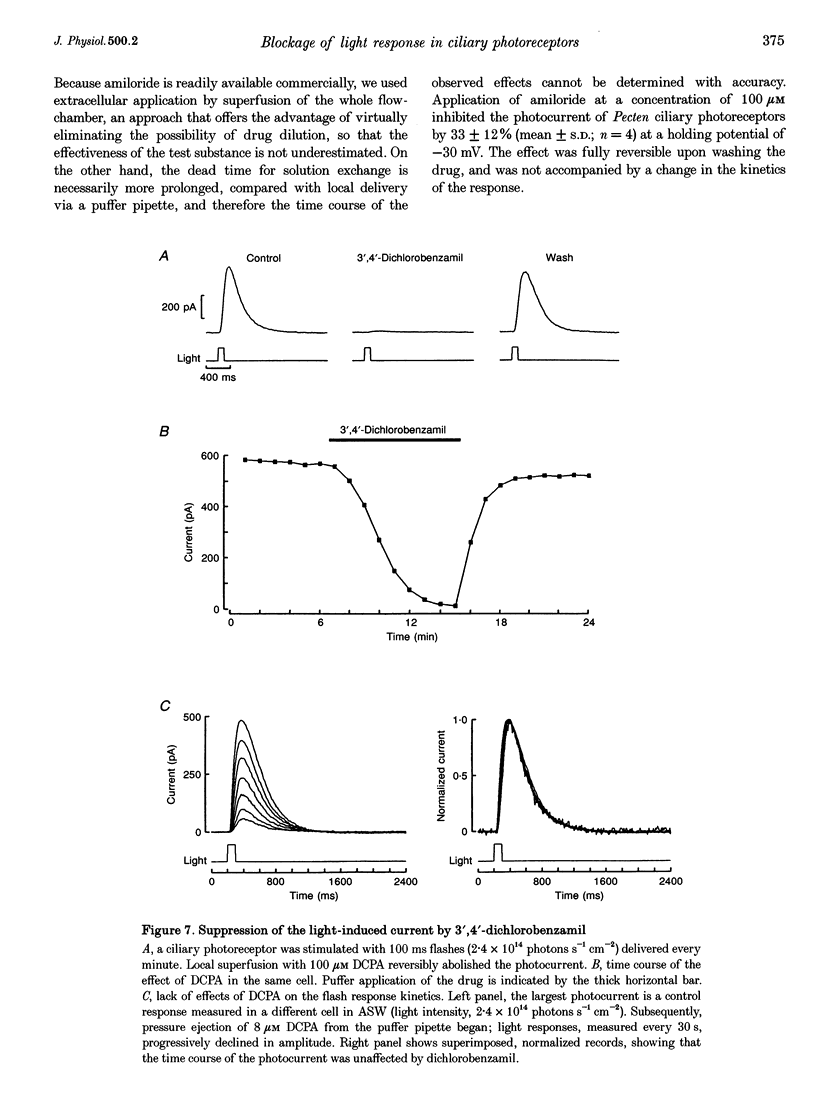
Abstract
1. Hyperpolarizing scallop photoreceptors, like vertebrate rods, use cGMP as an internal messenger and their light-sensing structure is also of ciliary origin. To ascertain possible functional similarities between the light-sensitive conductances in the two classes of visual cells, we examined in scallop photoreceptors the effects of several antagonists of the photocurrent of rods. 2. Extracellular application of L-cis-diltiazem rapidly and reversibly suppressed the photocurrent. The effect was stereospecific and dose dependent, with a K1/2 of approximately 400 microM. Intracellular dialysis at lower doses (100-200 microM) also induced a substantial inhibition. 3. L-cis-Diltiazem reduced the light-activated conductance without shifting the intensity-response curve. Furthermore, the drug also blocked the current directly evoked by application of cGMP. These observations indicate that the inhibitory effects result from blockage of the conductance, rather than from impairment of the activating cascade. 4. The fractional blockage increased e-fold per approximately 55 mV depolarization, regardless of the side of drug application, as if the charged form of L-cis-diltiazem can only access the blocking site from the intracellular compartment. 5. The amiloride derivative 3',4'-dichlorobenzamil potently suppressed the photocurrent (K1/2 approximately 5 microM), without affecting its kinetics or operating range. Amiloride itself was also effective at higher concentrations. 6. The pharmacological resemblance of these light-dependent channels to those of rods and cones indicates that significant aspects of the transduction cascade are conserved across disparate sensory cells of ciliary origin.
Full text
PDF


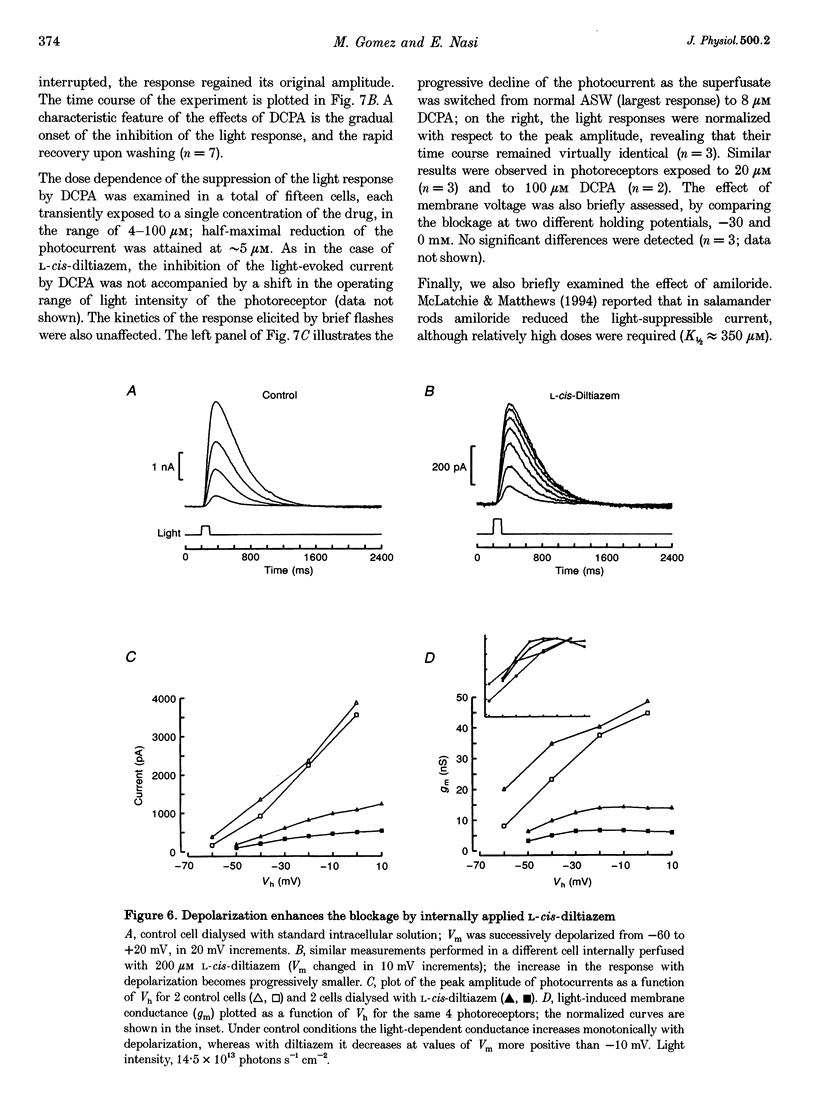



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson K. E. Preclinical pharmacology of diltiazem. Acta Pharmacol Toxicol (Copenh) 1985;57 (Suppl 2):1–9. doi: 10.1111/j.1600-0773.1985.tb03569.x. [DOI] [PubMed] [Google Scholar]
- Bacigalupo J., Johnson E. C., Vergara C., Lisman J. E. Light-dependent channels from excised patches of Limulus ventral photoreceptors are opened by cGMP. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):7938–7942. doi: 10.1073/pnas.88.18.7938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann A., Frings S., Godde M., Seifert R., Kaupp U. B. Primary structure and functional expression of a Drosophila cyclic nucleotide-gated channel present in eyes and antennae. EMBO J. 1994 Nov 1;13(21):5040–5050. doi: 10.1002/j.1460-2075.1994.tb06833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benos D. J. Amiloride: a molecular probe of sodium transport in tissues and cells. Am J Physiol. 1982 Mar;242(3):C131–C145. doi: 10.1152/ajpcell.1982.242.3.C131. [DOI] [PubMed] [Google Scholar]
- Cornwall M. C., Gorman A. L. Contribution of calcium and potassium permeability changes to the off response of scallop hyperpolarizing photoreceptors. J Physiol. 1979 Jun;291:207–232. doi: 10.1113/jphysiol.1979.sp012808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornwall M. C., Gorman A. L. The cation selectivity and voltage dependence of the light-activated potassium conductance in scallop distal photoreceptor. J Physiol. 1983 Jul;340:287–305. doi: 10.1113/jphysiol.1983.sp014763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eakin R. M. Evolution of photoreceptors. Cold Spring Harb Symp Quant Biol. 1965;30:363–370. doi: 10.1101/sqb.1965.030.01.036. [DOI] [PubMed] [Google Scholar]
- Fesenko E. E., Kolesnikov S. S., Lyubarsky A. L. Induction by cyclic GMP of cationic conductance in plasma membrane of retinal rod outer segment. Nature. 1985 Jan 24;313(6000):310–313. doi: 10.1038/313310a0. [DOI] [PubMed] [Google Scholar]
- Frings S., Lynch J. W., Lindemann B. Properties of cyclic nucleotide-gated channels mediating olfactory transduction. Activation, selectivity, and blockage. J Gen Physiol. 1992 Jul;100(1):45–67. doi: 10.1085/jgp.100.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia M. L., King V. F., Shevell J. L., Slaughter R. S., Suarez-Kurtz G., Winquist R. J., Kaczorowski G. J. Amiloride analogs inhibit L-type calcium channels and display calcium entry blocker activity. J Biol Chem. 1990 Mar 5;265(7):3763–3771. [PubMed] [Google Scholar]
- Gomez M. D., Nasi E. Blockage of the light-sensitive conductance in hyperpolarizing photoreceptors of the scallop. Effects of tetraethylammonium and 4-aminopyridine. J Gen Physiol. 1994 Sep;104(3):487–505. doi: 10.1085/jgp.104.3.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez M. D., Nasi E. Ion permeation through light-activated channels in rhabdomeric photoreceptors. Role of divalent cations. J Gen Physiol. 1996 Jun;107(6):715–730. doi: 10.1085/jgp.107.6.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez M. P., Nasi E. The light-sensitive conductance of hyperpolarizing invertebrate photoreceptors: a patch-clamp study. J Gen Physiol. 1994 Jun;103(6):939–956. doi: 10.1085/jgp.103.6.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman A. L., McReynolds J. S., Barnes S. N. Photoreceptors in primitive chordates: fine structure, hyperpolarizing receptor potentials, and evolution. Science. 1971 Jun 4;172(3987):1052–1054. doi: 10.1126/science.172.3987.1052. [DOI] [PubMed] [Google Scholar]
- Gorman A. L., McReynolds J. S. Hyperpolarizing and depolarizing receptor potentials in the scallop eye. Science. 1969 Jul 18;165(3890):309–310. doi: 10.1126/science.165.3890.309. [DOI] [PubMed] [Google Scholar]
- Gorman A. L., McReynolds J. S. Ionic effects on the membrane potential of hyperpolarizing photoreceptors in scallop retina. J Physiol. 1978 Feb;275:345–355. doi: 10.1113/jphysiol.1978.sp012193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy H. R., Durell S. R., Warmke J., Drysdale R., Ganetzky B. Similarities in amino acid sequences of Drosophila eag and cyclic nucleotide-gated channels. Science. 1991 Nov 1;254(5032):730–730. doi: 10.1126/science.1658932. [DOI] [PubMed] [Google Scholar]
- Haynes L. W. Block of the cyclic GMP-gated channel of vertebrate rod and cone photoreceptors by l-cis-diltiazem. J Gen Physiol. 1992 Nov;100(5):783–801. doi: 10.1085/jgp.100.5.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ildefonse M., Bennett N. Single-channel study of the cGMP-dependent conductance of retinal rods from incorporation of native vesicles into planar lipid bilayers. J Membr Biol. 1991 Aug;123(2):133–147. doi: 10.1007/BF01998084. [DOI] [PubMed] [Google Scholar]
- Kaczorowski G. J., Barros F., Dethmers J. K., Trumble M. J., Cragoe E. J., Jr Inhibition of Na+/Ca2+ exchange in pituitary plasma membrane vesicles by analogues of amiloride. Biochemistry. 1985 Mar 12;24(6):1394–1403. doi: 10.1021/bi00327a017. [DOI] [PubMed] [Google Scholar]
- Kaupp U. B. The cyclic nucleotide-gated channels of vertebrate photoreceptors and olfactory epithelium. Trends Neurosci. 1991 Apr;14(4):150–157. doi: 10.1016/0166-2236(91)90087-b. [DOI] [PubMed] [Google Scholar]
- Koch K. W., Kaupp U. B. Cyclic GMP directly regulates a cation conductance in membranes of bovine rods by a cooperative mechanism. J Biol Chem. 1985 Jun 10;260(11):6788–6800. [PubMed] [Google Scholar]
- Kolesnikov S. S., Zhainazarov A. B., Kosolapov A. V. Cyclic nucleotide-activated channels in the frog olfactory receptor plasma membrane. FEBS Lett. 1990 Jun 18;266(1-2):96–98. doi: 10.1016/0014-5793(90)81515-p. [DOI] [PubMed] [Google Scholar]
- MILLER W. H. Derivatives of cilia in the distal sense cells of the retina of Pecten. J Biophys Biochem Cytol. 1958 Mar 25;4(2):227–228. doi: 10.1083/jcb.4.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLatchie L. M., Matthews H. R. The effect of pH on the block by L-cis-diltiazem and amiloride of the cyclic GMP-activated conductance of salamander rods. Proc Biol Sci. 1994 Mar 22;255(1344):231–236. doi: 10.1098/rspb.1994.0033. [DOI] [PubMed] [Google Scholar]
- McLatchie L. M., Matthews H. R. Voltage-dependent block by L-cis-diltiazem of the cyclic GMP-activated conductance of salamander rods. Proc Biol Sci. 1992 Feb 22;247(1319):113–119. doi: 10.1098/rspb.1992.0016. [DOI] [PubMed] [Google Scholar]
- Mpitosos G. J. Physiology of vision in the mollusk Lima scabra. J Neurophysiol. 1973 Mar;36(2):371–383. doi: 10.1152/jn.1973.36.2.371. [DOI] [PubMed] [Google Scholar]
- Nasi E. Electrophysiological properties of isolated photoreceptors from the eye of Lima scabra. J Gen Physiol. 1991 Jan;97(1):17–34. doi: 10.1085/jgp.97.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicol G. D., Schnetkamp P. P., Saimi Y., Cragoe E. J., Jr, Bownds M. D. A derivative of amiloride blocks both the light-regulated and cyclic GMP-regulated conductances in rod photoreceptors. J Gen Physiol. 1987 Nov;90(5):651–669. doi: 10.1085/jgp.90.5.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rispoli G., Menini A. The blocking effect of l-cis-diltiazem on the light-sensitive current of isolated rods of the tiger salamander. Eur Biophys J. 1988;16(2):65–71. doi: 10.1007/BF00255515. [DOI] [PubMed] [Google Scholar]
- Stern J. H., Kaupp U. B., MacLeish P. R. Control of the light-regulated current in rod photoreceptors by cyclic GMP, calcium, and l-cis-diltiazem. Proc Natl Acad Sci U S A. 1986 Feb;83(4):1163–1167. doi: 10.1073/pnas.83.4.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez-Kurtz G., Kaczorowski G. J. Effects of dichlorobenzamil on calcium currents in clonal GH3 pituitary cells. J Pharmacol Exp Ther. 1988 Oct;247(1):248–253. [PubMed] [Google Scholar]
- TOKUYASU K., YAMADA E. The fine structure of the retina studied with the electron microscope. IV. Morphogenesis of outer segments of retinal rods. J Biophys Biochem Cytol. 1959 Oct;6:225–230. doi: 10.1083/jcb.6.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang C. M., Presser F., Morad M. Amiloride selectively blocks the low threshold (T) calcium channel. Science. 1988 Apr 8;240(4849):213–215. doi: 10.1126/science.2451291. [DOI] [PubMed] [Google Scholar]
- Yau K. W. Cyclic nucleotide-gated channels: an expanding new family of ion channels. Proc Natl Acad Sci U S A. 1994 Apr 26;91(9):3481–3483. doi: 10.1073/pnas.91.9.3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Pilar Gomez M., Nasi E. Activation of light-dependent K+ channels in ciliary invertebrate photoreceptors involves cGMP but not the IP3/Ca2+ cascade. Neuron. 1995 Sep;15(3):607–618. doi: 10.1016/0896-6273(95)90149-3. [DOI] [PubMed] [Google Scholar]


