Abstract
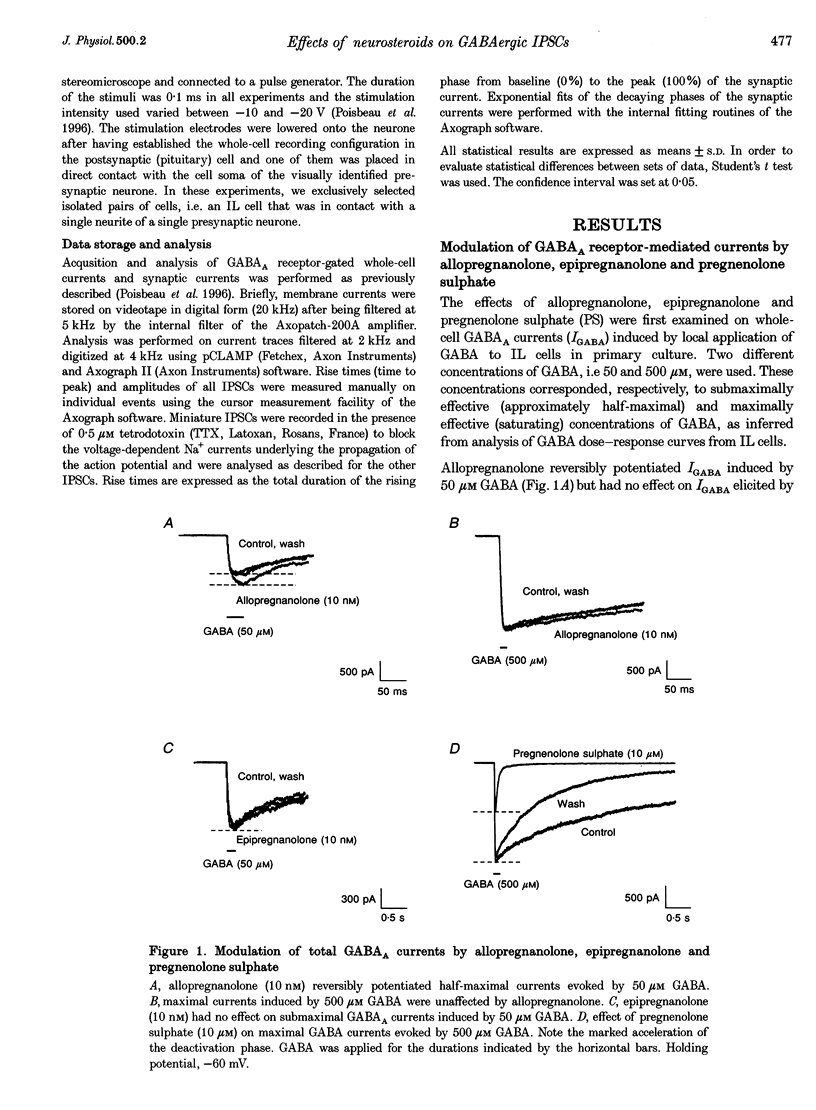
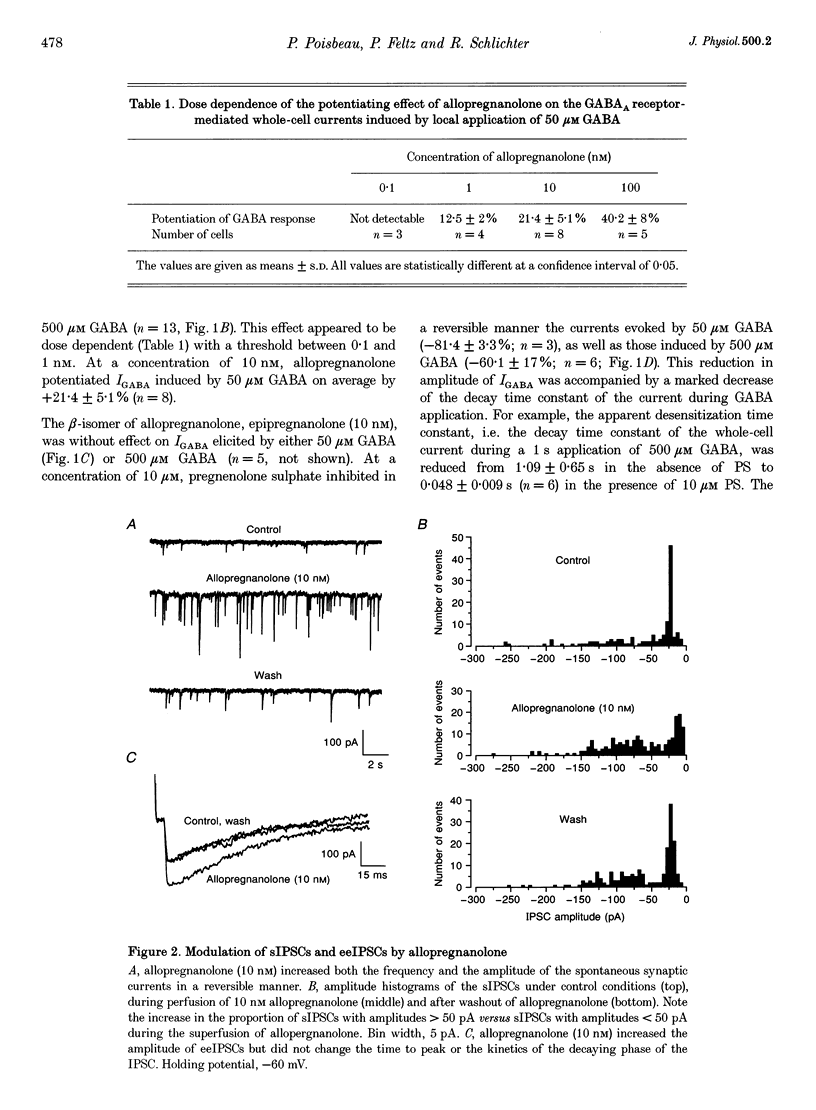
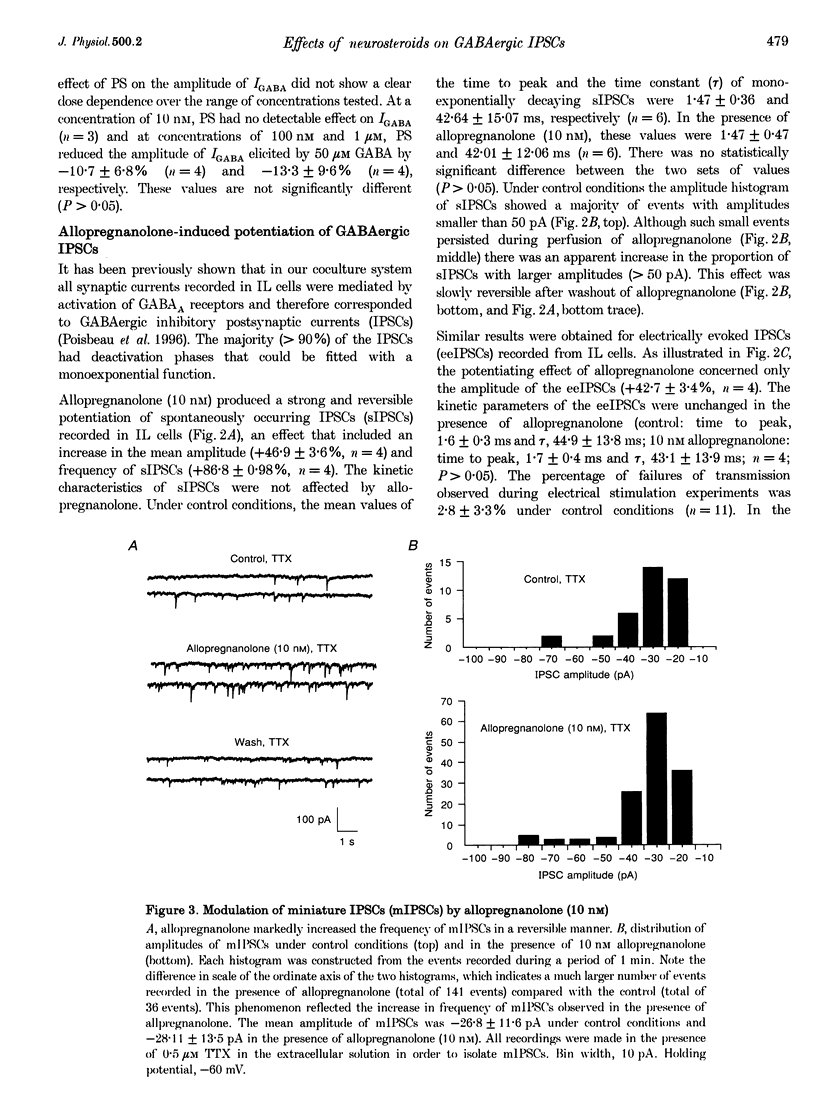
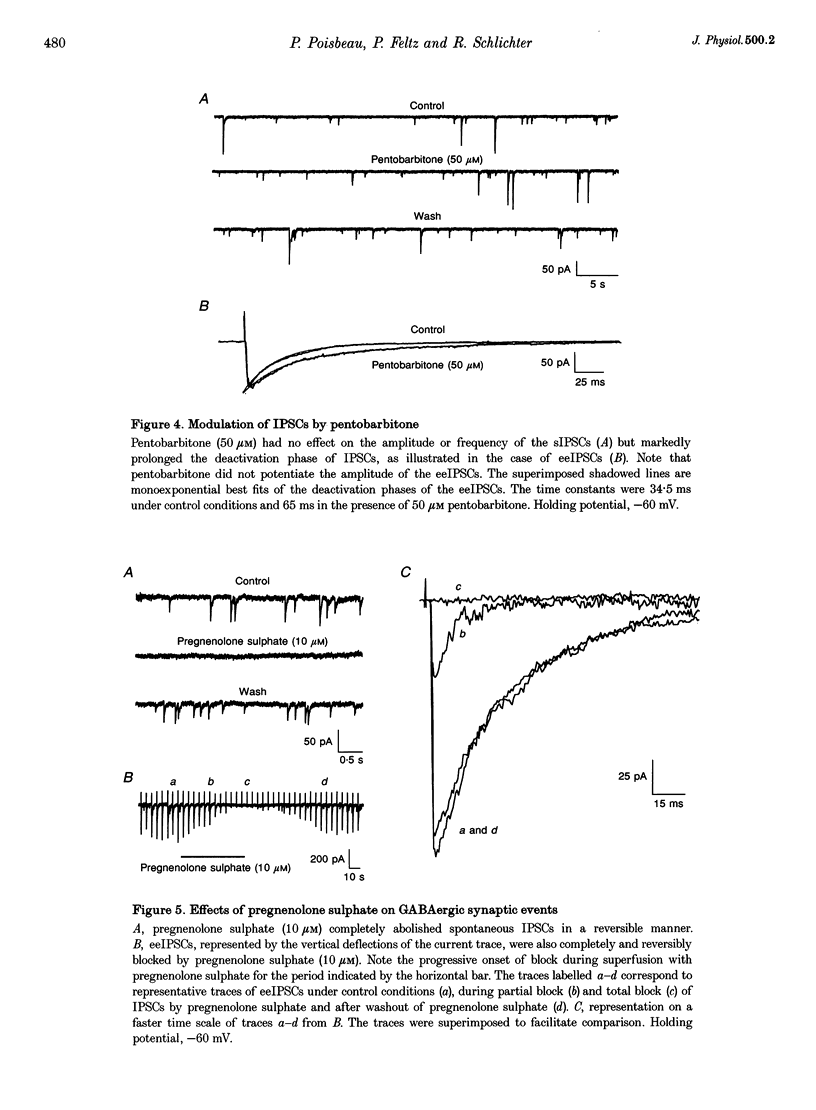
1. We have used the whole-cell configuration of the patch-clamp technique to investigate the effects of neuroactive steroids on GABAA receptor-mediated synaptic transmission between rat hypothalamic neurones and pituitary intermediate lobe (IL) cells grown in coculture. In order to discriminate between possible pre- and postsynaptic sites of action, the effects of neurosteroids on GABAA receptor-mediated synaptic currents (IPSCs) were compared with those of GABAA currents (IGABA) triggered by local application of 50 or 500 microM GABA, which yielded approximately half-maximal and maximal responses, respectively. 2. In primary cultures of rat pituitary IL cells, allopregnanolone (5 alpha-pregnan-3 alpha-ol-20-one) reversibly potentiated IGABA in a dose-dependent manner with a threshold between 0.1 and 1 nM. At a concentration of 10 nM, allopregnanolone increased the response evoked by 50 microM GABA by +21.4 +/- 5.1% (n = 8), but had no effect on IGABA induced by 500 microM GABA. The beta-isomer of allopregnanolone, epipregnanolone (5 beta-pregnan-3 beta-ol-20-one, 10 nM), had no effect on IGABA at any concentration of GABA tested. 3. At concentrations lower than 10 microM, pregnenolone sulphate (5-pregnen-3 alpha-ol-20-one sulphate) did not significantly inhibit IGABA. However, at 10 microM, a systematic reduction of IGABA evoked by 50 and 500 microM GABA was observed, with mean values of -80 and -60%, respectively. This blocking effect was reversible and accompanied by a marked acceleration of decay of GABAA currents during the application of GABA. 4. In isolated pairs of synaptically connected hypothalamic neurones and IL cells, allopregnanolone (10 nM) augmented the mean amplitude of spontaneous IPSCs (sIPSCs) and electrically evoked IPSCs (eeIPSCs) by about 40% and increased the mean frequency of sIPSCs. Allopregnanolone (10 nM) also markedly increased the frequency of miniature IPSCs (mIPSCs) recorded in the presence of TTX (0.5 microM), but without modifying their mean amplitude. Epipregnanolone had no effect on the amplitude or frequency of sIPSCs. Neither epipregnanolone nor allopregnanolone modified the time to peak and decay time constants of GABAergic IPSCs. 5. Pentobarbitone (50 microM), a positive allosteric modulator of GABAA receptors, did not affect the amplitude of sIPSCs or eeIPSCs, but significantly increased the decay time constants of both types of IPSCs. Pentobarbitone had no effect on the frequency of sIPSCs. 6. Pregnenolone sulphate (10 microM) completely and reversibly blocked sIPSCs and eeIPSCs. Progressive block of IPSCs was correlated with a gradual decrease of the mean decay time constant. 7. Our results suggest that, under physiological conditions, allopregnanolone might be a potent modulator of GABAergic synaptic transmission, acting at both pre- and postsynaptic sites. The involvement of pregnenolone sulphate as a modulator of GABAergic IPSCs under physiological conditions is, however, more questionable. The mechanisms of action of both types of neurosteroids are discussed.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berman J. A., Roberts J. L., Pritchett D. B. Molecular and pharmacological characterization of GABAA receptors in the rat pituitary. J Neurochem. 1994 Nov;63(5):1948–1954. doi: 10.1046/j.1471-4159.1994.63051948.x. [DOI] [PubMed] [Google Scholar]
- Bottenstein J. E., Sato G. H. Growth of a rat neuroblastoma cell line in serum-free supplemented medium. Proc Natl Acad Sci U S A. 1979 Jan;76(1):514–517. doi: 10.1073/pnas.76.1.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayanithi G., Tapia-Arancibia L. Rise in intracellular calcium via a nongenomic effect of allopregnanolone in fetal rat hypothalamic neurons. J Neurosci. 1996 Jan;16(1):130–136. doi: 10.1523/JNEUROSCI.16-01-00130.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Koninck Y., Mody I. Noise analysis of miniature IPSCs in adult rat brain slices: properties and modulation of synaptic GABAA receptor channels. J Neurophysiol. 1994 Apr;71(4):1318–1335. doi: 10.1152/jn.1994.71.4.1318. [DOI] [PubMed] [Google Scholar]
- Fenwick E. M., Marty A., Neher E. A patch-clamp study of bovine chromaffin cells and of their sensitivity to acetylcholine. J Physiol. 1982 Oct;331:577–597. doi: 10.1113/jphysiol.1982.sp014393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Harrison N. L., Majewska M. D., Harrington J. W., Barker J. L. Structure-activity relationships for steroid interaction with the gamma-aminobutyric acidA receptor complex. J Pharmacol Exp Ther. 1987 Apr;241(1):346–353. [PubMed] [Google Scholar]
- Lambert J. J., Belelli D., Hill-Venning C., Peters J. A. Neurosteroids and GABAA receptor function. Trends Pharmacol Sci. 1995 Sep;16(9):295–303. doi: 10.1016/s0165-6147(00)89058-6. [DOI] [PubMed] [Google Scholar]
- Lambert J. J., Peters J. A., Sturgess N. C., Hales T. G. Steroid modulation of the GABAA receptor complex: electrophysiological studies. Ciba Found Symp. 1990;153:56–82. doi: 10.1002/9780470513989.ch4. [DOI] [PubMed] [Google Scholar]
- Macdonald R. L., Olsen R. W. GABAA receptor channels. Annu Rev Neurosci. 1994;17:569–602. doi: 10.1146/annurev.ne.17.030194.003033. [DOI] [PubMed] [Google Scholar]
- Majewska M. D. Neurosteroids: endogenous bimodal modulators of the GABAA receptor. Mechanism of action and physiological significance. Prog Neurobiol. 1992;38(4):379–395. doi: 10.1016/0301-0082(92)90025-a. [DOI] [PubMed] [Google Scholar]
- Mienville J. M., Vicini S. Pregnenolone sulfate antagonizes GABAA receptor-mediated currents via a reduction of channel opening frequency. Brain Res. 1989 Jun 5;489(1):190–194. doi: 10.1016/0006-8993(89)90024-3. [DOI] [PubMed] [Google Scholar]
- Mody I., De Koninck Y., Otis T. S., Soltesz I. Bridging the cleft at GABA synapses in the brain. Trends Neurosci. 1994 Dec;17(12):517–525. doi: 10.1016/0166-2236(94)90155-4. [DOI] [PubMed] [Google Scholar]
- Oertel W. H., Mugnaini E., Tappaz M. L., Weise V. K., Dahl A. L., Schmechel D. E., Kopin I. J. Central GABAergic innervation of neurointermediate pituitary lobe: biochemical and immunocytochemical study in the rat. Proc Natl Acad Sci U S A. 1982 Jan;79(2):675–679. doi: 10.1073/pnas.79.2.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul S. M., Purdy R. H. Neuroactive steroids. FASEB J. 1992 Mar;6(6):2311–2322. [PubMed] [Google Scholar]
- Poisbeau P., René F., Egles C., Félix J. M., Feltz P., Schlichter R. Characterization of functional GABAergic synapses formed between rat hypothalamic neurons and pituitary intermediate lobe cells in coculture: Ca2+ dependence of spontaneous IPSCs. J Neurosci. 1996 Aug 15;16(16):4835–4845. doi: 10.1523/JNEUROSCI.16-16-04835.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puia G., Ducić I., Vicini S., Costa E. Does neurosteroid modulatory efficacy depend on GABAA receptor subunit composition? Receptors Channels. 1993;1(2):135–142. [PubMed] [Google Scholar]
- Puia G., Santi M. R., Vicini S., Pritchett D. B., Purdy R. H., Paul S. M., Seeburg P. H., Costa E. Neurosteroids act on recombinant human GABAA receptors. Neuron. 1990 May;4(5):759–765. doi: 10.1016/0896-6273(90)90202-q. [DOI] [PubMed] [Google Scholar]
- Schneggenburger R., Konnerth A. GABA-mediated synaptic transmission in neuroendocrine cells: a patch-clamp study in a pituitary slice preparation. Pflugers Arch. 1992 Jul;421(4):364–373. doi: 10.1007/BF00374225. [DOI] [PubMed] [Google Scholar]
- Sieghart W. Structure and pharmacology of gamma-aminobutyric acidA receptor subtypes. Pharmacol Rev. 1995 Jun;47(2):181–234. [PubMed] [Google Scholar]
- Taraskevich P. S., Douglas W. W. Pharmacological and ionic features of gamma-aminobutyric acid receptors influencing electrical properties of melanotrophs isolated from the rat pars intermedia. Neuroscience. 1985 Jan;14(1):301–308. doi: 10.1016/0306-4522(85)90179-4. [DOI] [PubMed] [Google Scholar]
- Tomiko S. A., Taraskevich P. S., Douglas W. W. GABA acts directly on cells of pituitary pars intermedia to alter hormone output. Nature. 1983 Feb 24;301(5902):706–707. doi: 10.1038/301706a0. [DOI] [PubMed] [Google Scholar]
- Twyman R. E., Macdonald R. L. Neurosteroid regulation of GABAA receptor single-channel kinetic properties of mouse spinal cord neurons in culture. J Physiol. 1992 Oct;456:215–245. doi: 10.1113/jphysiol.1992.sp019334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent S. R., Hökfelt T., Wu J. Y. GABA neuron systems in hypothalamus and the pituitary gland. Immunohistochemical demonstration using antibodies against glutamate decarboxylase. Neuroendocrinology. 1982 Feb;34(2):117–125. doi: 10.1159/000123288. [DOI] [PubMed] [Google Scholar]
- Wisden W., Laurie D. J., Monyer H., Seeburg P. H. The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. I. Telencephalon, diencephalon, mesencephalon. J Neurosci. 1992 Mar;12(3):1040–1062. doi: 10.1523/JNEUROSCI.12-03-01040.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W. J., Wang J. F., Krueger K. E., Vicini S. Delta subunit inhibits neurosteroid modulation of GABAA receptors. J Neurosci. 1996 Nov 1;16(21):6648–6656. doi: 10.1523/JNEUROSCI.16-21-06648.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ffrench-Mullen J. M., Danks P., Spence K. T. Neurosteroids modulate calcium currents in hippocampal CA1 neurons via a pertussis toxin-sensitive G-protein-coupled mechanism. J Neurosci. 1994 Apr;14(4):1963–1977. doi: 10.1523/JNEUROSCI.14-04-01963.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]


