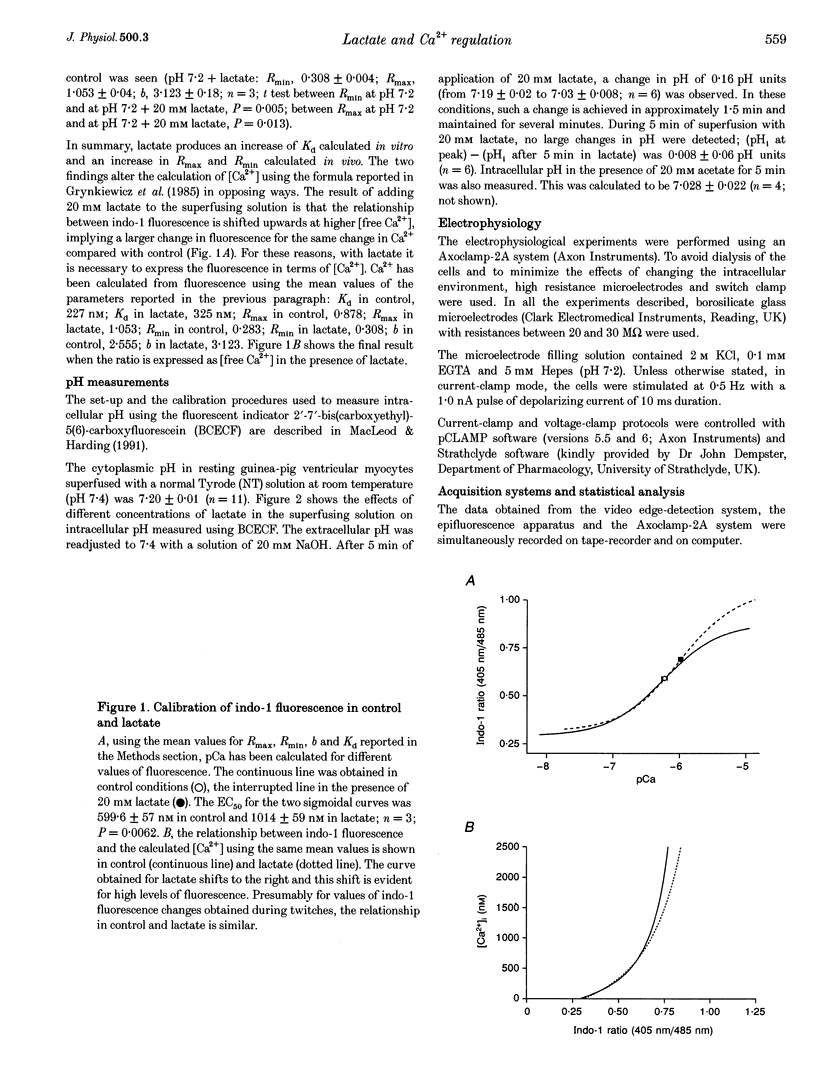
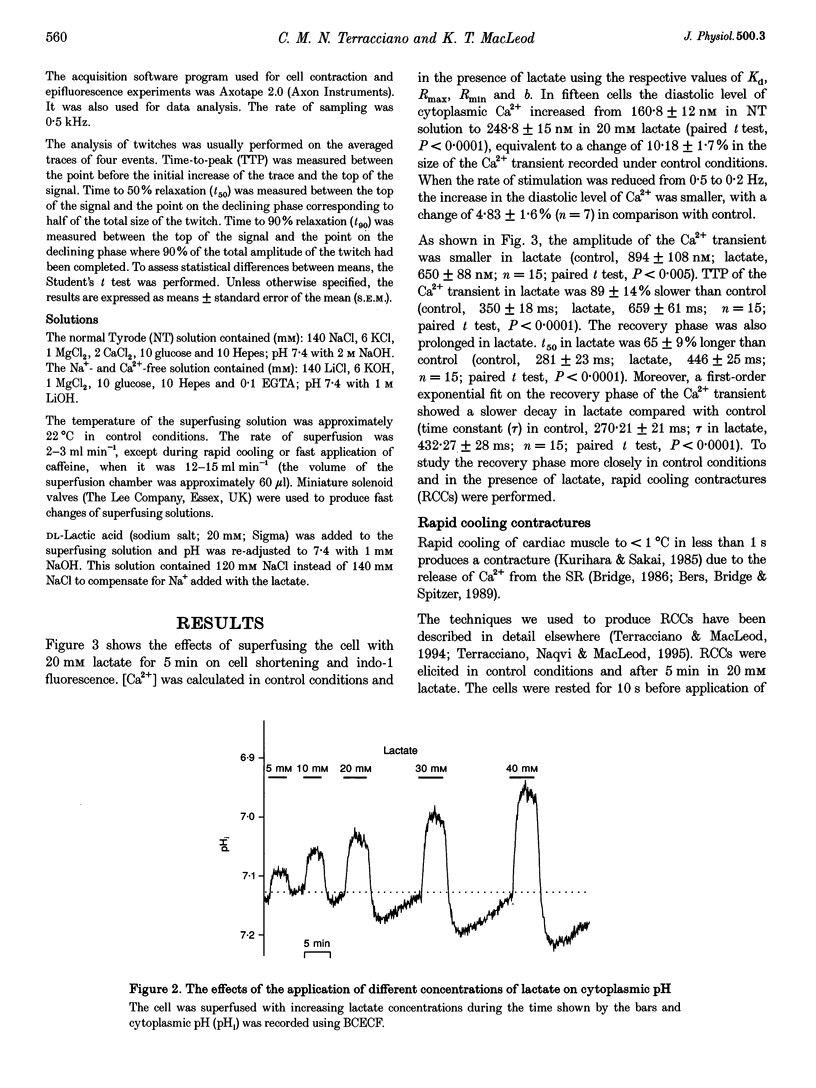
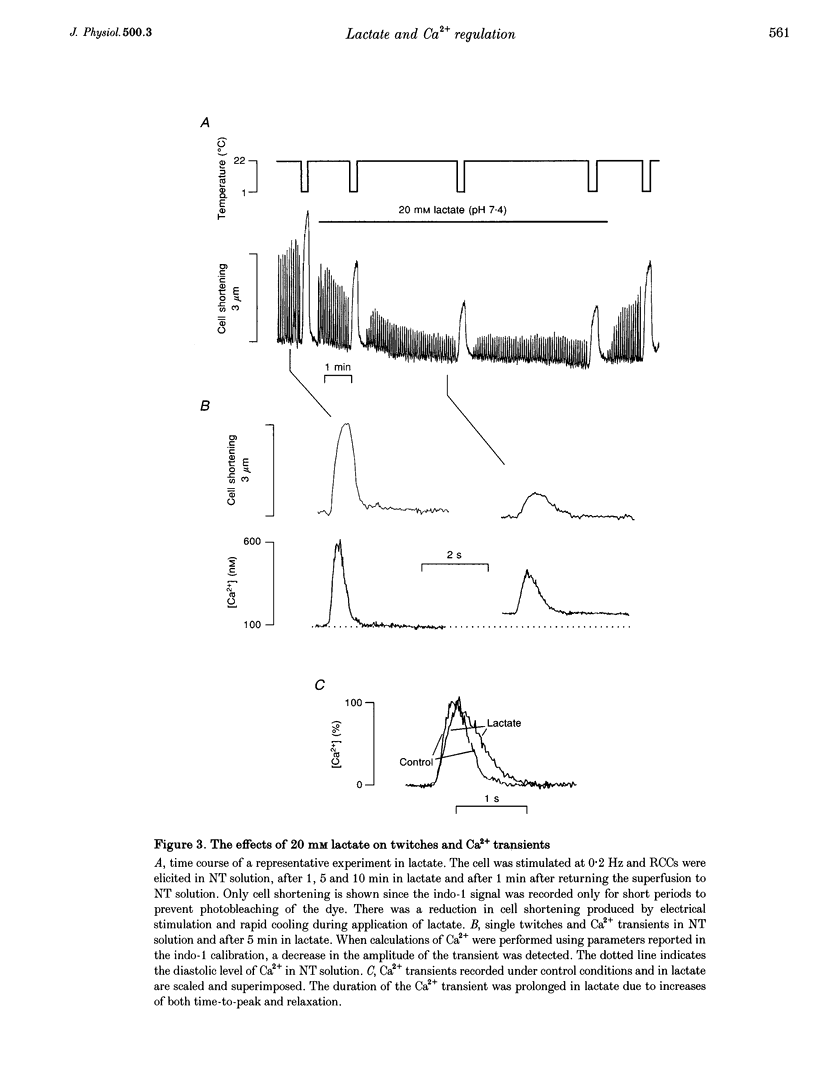
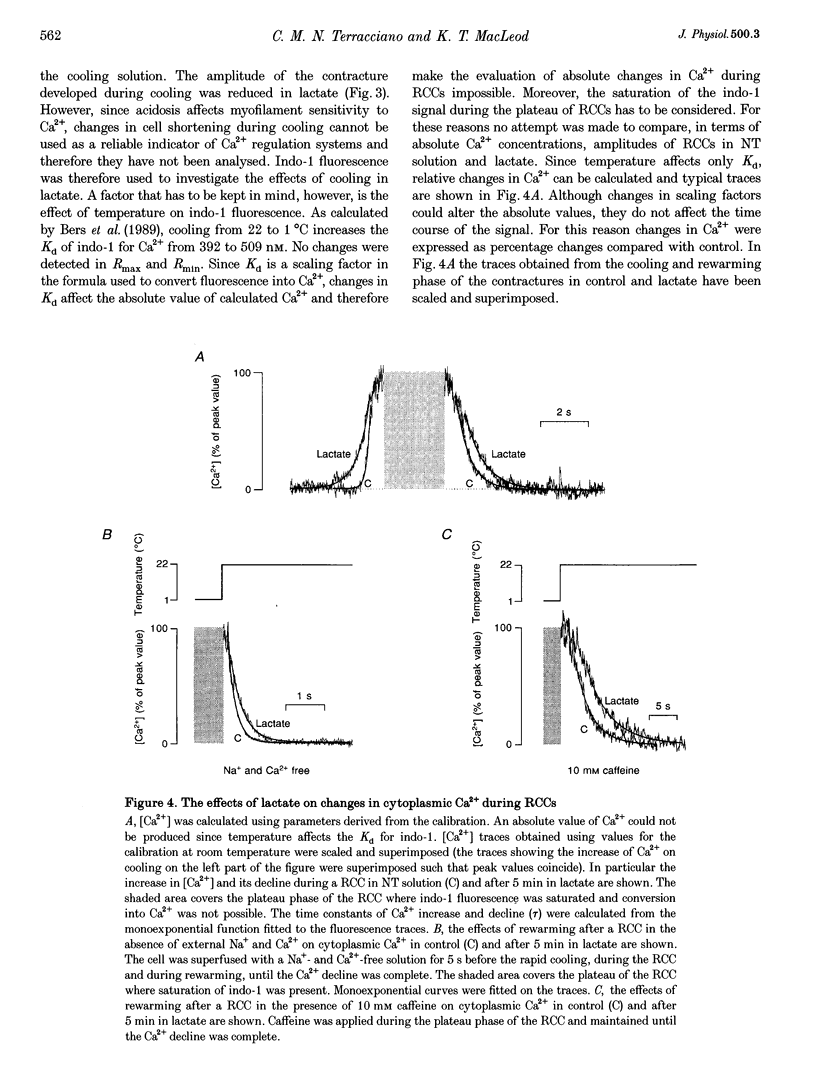
Abstract
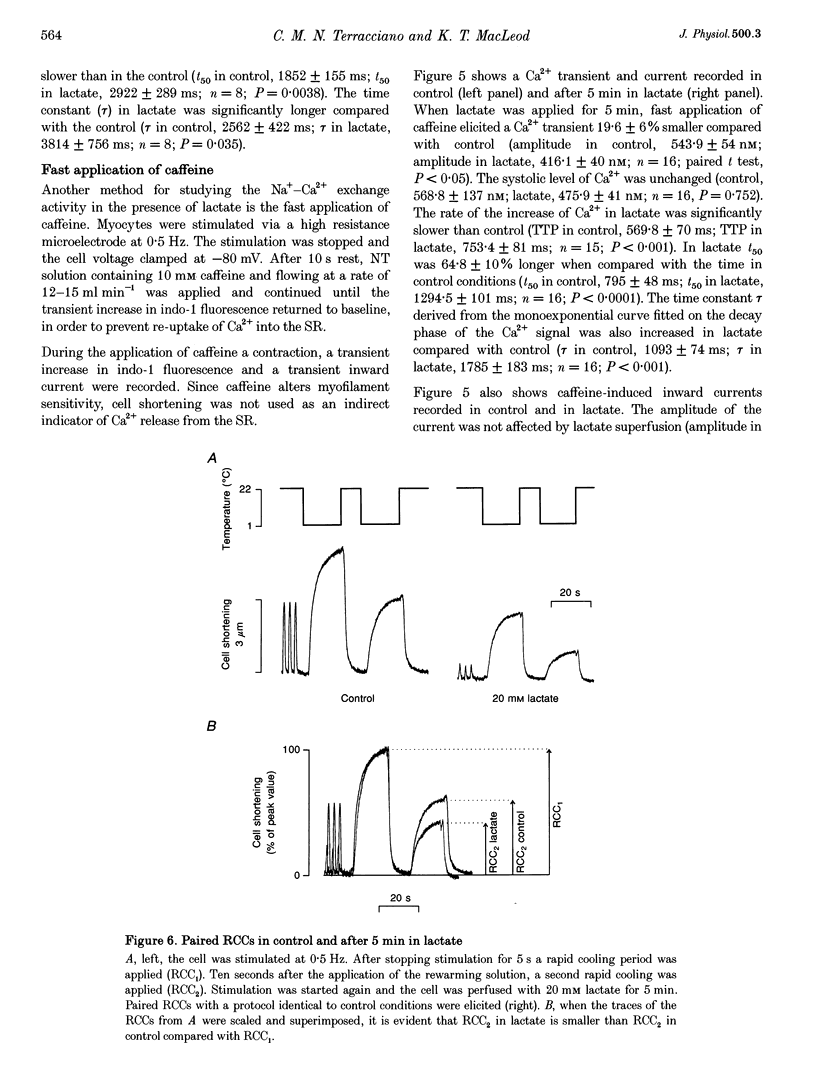
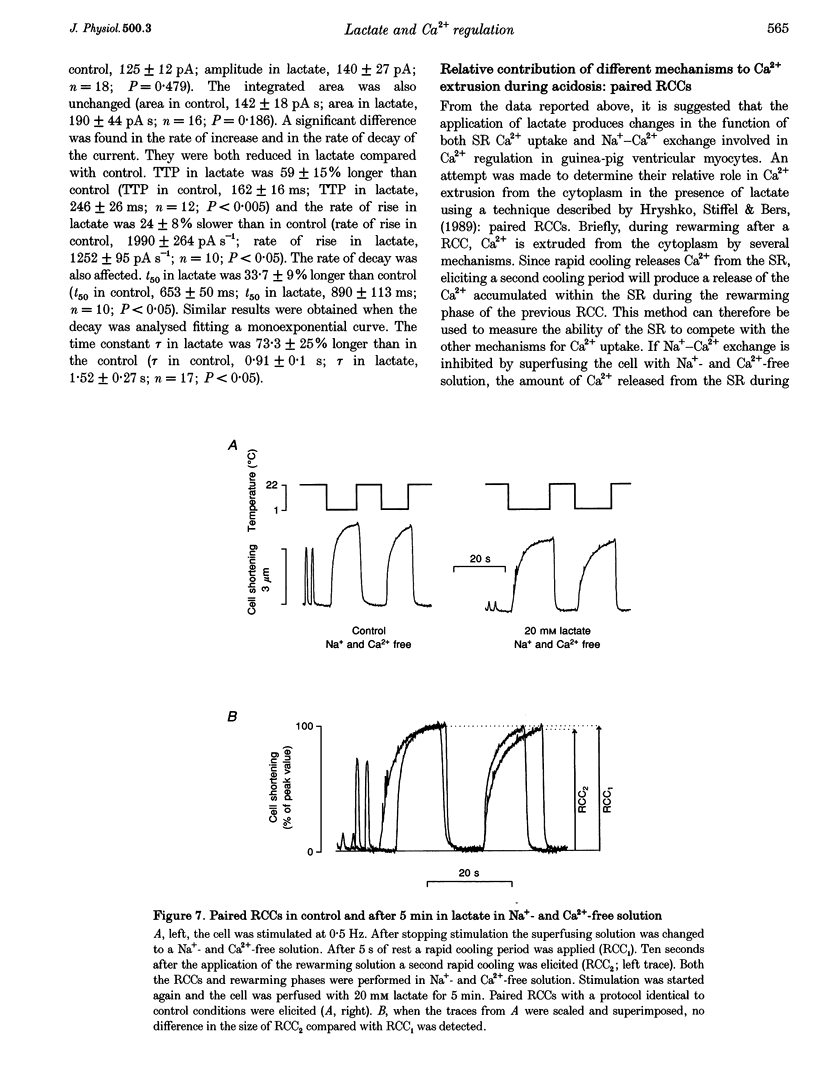
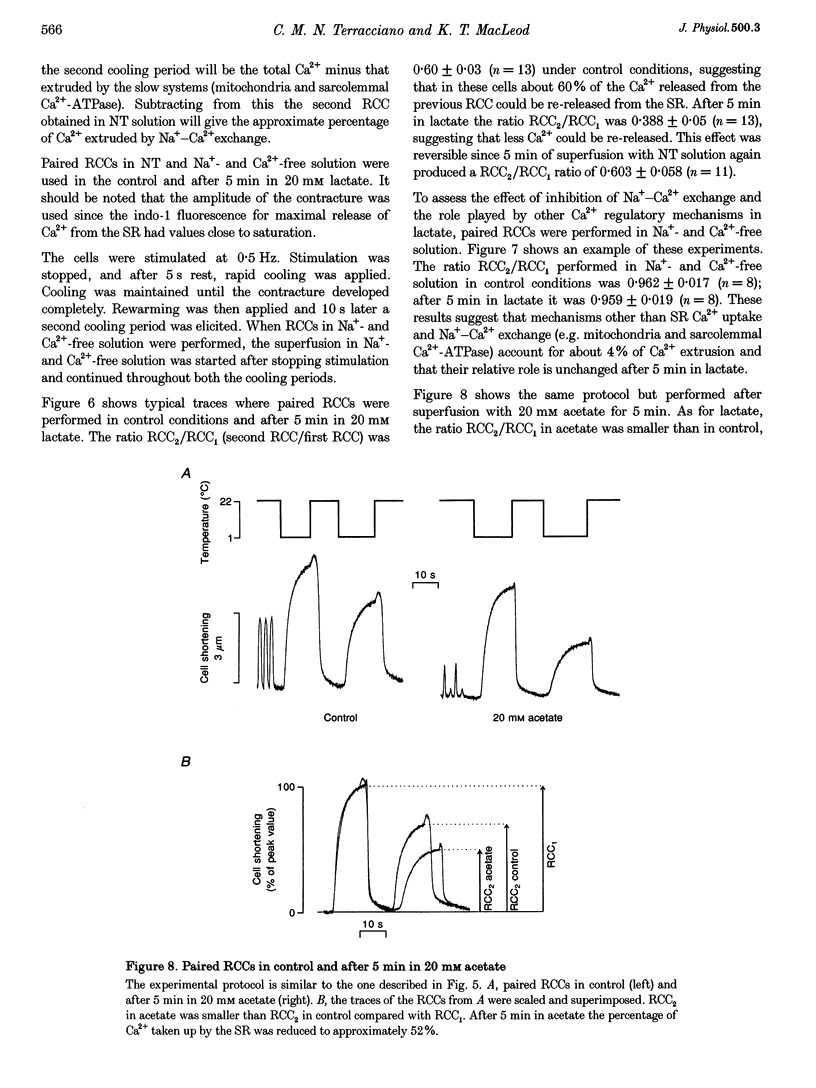
1. The aim of this study was to investigate the effects of 20 mM extracellular lactate on Ca2+ regulation mechanisms in enzymatically isolated single guinea-pig cardiac myocytes. 2. The activities of the Ca2+ regulation mechanisms during application of lactate were studied using rapid cooling contractures (RCCs) and fast application of caffeine. Cytoplasmic Ca2+ was monitored using the fluorescent indicator indo-1. 3. After application of 20 mM lactate for 5 min, the diastolic level of Ca2+ was increased. The change in cytoplasmic Ca2+ elicited by stimulation (Ca2+ transient) was also changed. With lactate, the amplitude of the Ca2+ transient was smaller, and its time course was slower compared with control. 4. The recovery of cytoplasmic Ca2+ during rewarming after rapid cooling in lactate was slower than under control conditions. When the rewarming was performed either in Na(+)- and Ca(2+)-free solution or in the presence of 10 mM caffeine, the rate of recovery of cytoplasmic Ca2+ in lactate was slower than under control conditions, suggesting that the activity of both SR Ca2+ uptake and Na(+)-Ca2+ exchange is affected by lactate. 5. Cytoplasmic Ca2+ recovery during application of 10 mM caffeine in lactate was slower than in the control. The rate of recovery of the caffeine-induced transient inward current was also slower supporting the hypothesis of a slower Ca2+ extrusion brought about by Na(+)-Ca2+ exchange. 6. The relative contribution of the Ca2+ extrusion mechanisms in the presence of lactate was investigated using paired RCCs. In lactate, a second RCC (RCC2) induced immediately after recovery from the first (RCC1) was greatly reduced compared with the control. RCC2/RCC1 x 100 in lactate was 39% and RCC2/RCC1 x 100 in control conditions was 60%, suggesting that the net sarcoplasmic reticulum Ca2+ uptake is smaller in the presence of lactate. 7. When Na(+)-free Ca2+ solution was used during the paired RCCs and rewarming, RCC2/RCC1 x 100 was increased to 96 and 95% in lactate and control conditions, respectively, implying that Ca2+ efflux from the cell can be maintained by the Na(+)-Ca2+ exchanger and that other Ca2+ removal mechanisms (mitochondria and sarcolemmal Ca(2+)-ATPase) remain largely unchanged in the presence of lactate.
Full text
PDF

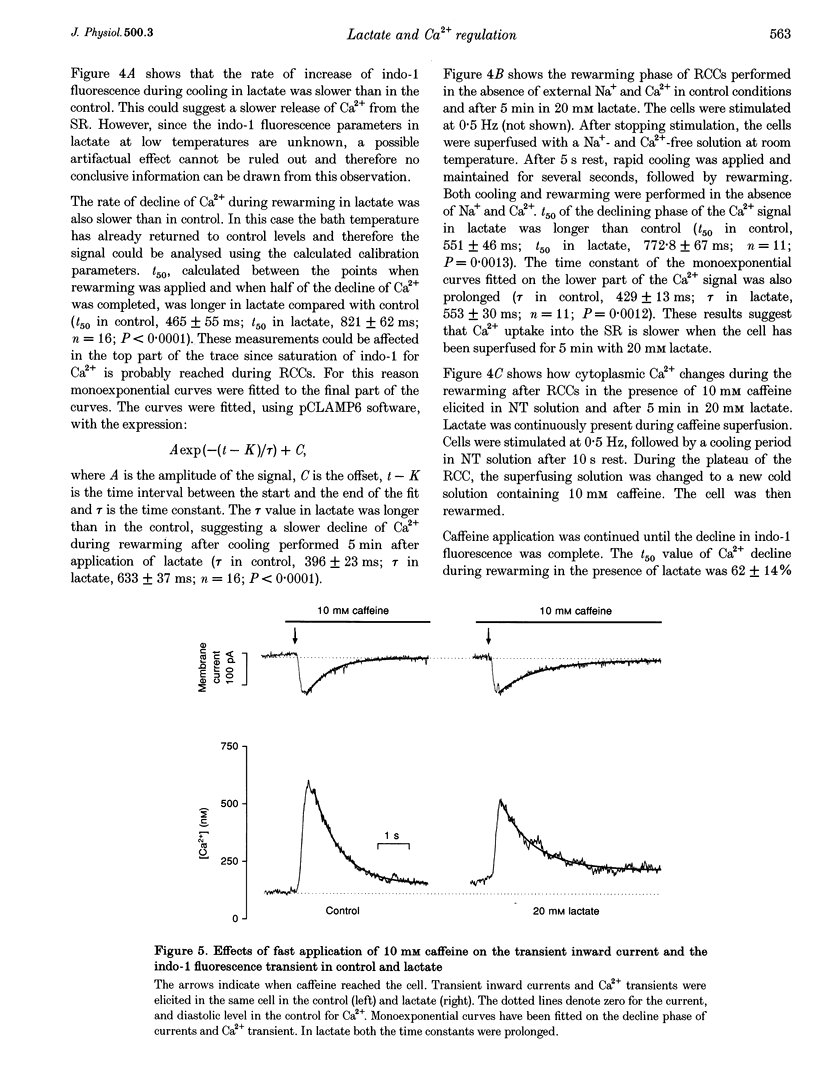




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen D. G., Lee J. A., Smith G. L. The consequences of simulated ischaemia on intracellular Ca2+ and tension in isolated ferret ventricular muscle. J Physiol. 1989 Mar;410:297–323. doi: 10.1113/jphysiol.1989.sp017534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen D. G., Orchard C. H. The effects of changes of pH on intracellular calcium transients in mammalian cardiac muscle. J Physiol. 1983 Feb;335:555–567. doi: 10.1113/jphysiol.1983.sp014550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassani J. W., Bassani R. A., Bers D. M. Relaxation in rabbit and rat cardiac cells: species-dependent differences in cellular mechanisms. J Physiol. 1994 Apr 15;476(2):279–293. doi: 10.1113/jphysiol.1994.sp020130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bers D. M., Bridge J. H. Relaxation of rabbit ventricular muscle by Na-Ca exchange and sarcoplasmic reticulum calcium pump. Ryanodine and voltage sensitivity. Circ Res. 1989 Aug;65(2):334–342. doi: 10.1161/01.res.65.2.334. [DOI] [PubMed] [Google Scholar]
- Bers D. M., Bridge J. H., Spitzer K. W. Intracellular Ca2+ transients during rapid cooling contractures in guinea-pig ventricular myocytes. J Physiol. 1989 Oct;417:537–553. doi: 10.1113/jphysiol.1989.sp017817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bers D. M., Ellis D. Intracellular calcium and sodium activity in sheep heart Purkinje fibres. Effect of changes of external sodium and intracellular pH. Pflugers Arch. 1982 Apr;393(2):171–178. doi: 10.1007/BF00582941. [DOI] [PubMed] [Google Scholar]
- Boron W. F., De Weer P. Intracellular pH transients in squid giant axons caused by CO2, NH3, and metabolic inhibitors. J Gen Physiol. 1976 Jan;67(1):91–112. doi: 10.1085/jgp.67.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bountra C., Vaughan-Jones R. D. Effect of intracellular and extracellular pH on contraction in isolated, mammalian cardiac muscle. J Physiol. 1989 Nov;418:163–187. doi: 10.1113/jphysiol.1989.sp017833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridge J. H. Relationships between the sarcoplasmic reticulum and sarcolemmal calcium transport revealed by rapidly cooling rabbit ventricular muscle. J Gen Physiol. 1986 Oct;88(4):437–473. doi: 10.1085/jgp.88.4.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns S. P., Westerblad H., Allen D. G. Changes in myoplasmic pH and calcium concentration during exposure to lactate in isolated rat ventricular myocytes. J Physiol. 1993 May;464:561–574. doi: 10.1113/jphysiol.1993.sp019651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callewaert G., Cleemann L., Morad M. Caffeine-induced Ca2+ release activates Ca2+ extrusion via Na+-Ca2+ exchanger in cardiac myocytes. Am J Physiol. 1989 Jul;257(1 Pt 1):C147–C152. doi: 10.1152/ajpcell.1989.257.1.C147. [DOI] [PubMed] [Google Scholar]
- Cobbold P. H., Rink T. J. Fluorescence and bioluminescence measurement of cytoplasmic free calcium. Biochem J. 1987 Dec 1;248(2):313–328. doi: 10.1042/bj2480313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A. Calcium-induced release of calcium from the cardiac sarcoplasmic reticulum. Am J Physiol. 1983 Jul;245(1):C1–14. doi: 10.1152/ajpcell.1983.245.1.C1. [DOI] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Effects of pH on the myofilaments and the sarcoplasmic reticulum of skinned cells from cardiace and skeletal muscles. J Physiol. 1978 Mar;276:233–255. doi: 10.1113/jphysiol.1978.sp012231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A. Use of aequorin for the appraisal of the hypothesis of the release of calcium from the sarcoplasmic reticulum induced by a change of pH in skinned cardiac cells. Cell Calcium. 1985 Apr;6(1-2):95–108. doi: 10.1016/0143-4160(85)90037-5. [DOI] [PubMed] [Google Scholar]
- Gambassi G., Hansford R. G., Sollott S. J., Hogue B. A., Lakatta E. G., Capogrossi M. C. Effects of acidosis on resting cytosolic and mitochondrial Ca2+ in mammalian myocardium. J Gen Physiol. 1993 Sep;102(3):575–597. doi: 10.1085/jgp.102.3.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskell W. H. On the Tonicity of the Heart and Blood Vessels. J Physiol. 1880 Aug;3(1):48–92.16. doi: 10.1113/jphysiol.1880.sp000083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hryshko L. V., Stiffel V., Bers D. M. Rapid cooling contractures as an index of sarcoplasmic reticulum calcium content in rabbit ventricular myocytes. Am J Physiol. 1989 Nov;257(5 Pt 2):H1369–H1377. doi: 10.1152/ajpheart.1989.257.5.H1369. [DOI] [PubMed] [Google Scholar]
- Irisawa H., Sato R. Intra- and extracellular actions of proton on the calcium current of isolated guinea pig ventricular cells. Circ Res. 1986 Sep;59(3):348–355. doi: 10.1161/01.res.59.3.348. [DOI] [PubMed] [Google Scholar]
- Kao J. P. Practical aspects of measuring [Ca2+] with fluorescent indicators. Methods Cell Biol. 1994;40:155–181. doi: 10.1016/s0091-679x(08)61114-0. [DOI] [PubMed] [Google Scholar]
- Kurihara S., Sakai T. Effects of rapid cooling on mechanical and electrical responses in ventricular muscle of guinea-pig. J Physiol. 1985 Apr;361:361–378. doi: 10.1113/jphysiol.1985.sp015650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lattanzio F. A., Jr The effects of pH and temperature on fluorescent calcium indicators as determined with Chelex-100 and EDTA buffer systems. Biochem Biophys Res Commun. 1990 Aug 31;171(1):102–108. doi: 10.1016/0006-291x(90)91362-v. [DOI] [PubMed] [Google Scholar]
- MacLeod K. T., Harding S. E. Effects of phorbol ester on contraction, intracellular pH and intracellular Ca2+ in isolated mammalian ventricular myocytes. J Physiol. 1991 Dec;444:481–498. doi: 10.1113/jphysiol.1991.sp018889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neely J. R., Whitmer J. T., Rovetto M. J. Effect of coronary blood flow on glycolytic flux and intracellular pH in isolated rat hearts. Circ Res. 1975 Dec;37(6):733–741. doi: 10.1161/01.res.37.6.733. [DOI] [PubMed] [Google Scholar]
- Negretti N., O'Neill S. C., Eisner D. A. The relative contributions of different intracellular and sarcolemmal systems to relaxation in rat ventricular myocytes. Cardiovasc Res. 1993 Oct;27(10):1826–1830. doi: 10.1093/cvr/27.10.1826. [DOI] [PubMed] [Google Scholar]
- Orchard C. H., Cingolani H. E. Acidosis and arrhythmias in cardiac muscle. Cardiovasc Res. 1994 Sep;28(9):1312–1319. doi: 10.1093/cvr/28.9.1312. [DOI] [PubMed] [Google Scholar]
- Orchard C. H., Houser S. R., Kort A. A., Bahinski A., Capogrossi M. C., Lakatta E. G. Acidosis facilitates spontaneous sarcoplasmic reticulum Ca2+ release in rat myocardium. J Gen Physiol. 1987 Jul;90(1):145–165. doi: 10.1085/jgp.90.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orchard C. H., Kentish J. C. Effects of changes of pH on the contractile function of cardiac muscle. Am J Physiol. 1990 Jun;258(6 Pt 1):C967–C981. doi: 10.1152/ajpcell.1990.258.6.C967. [DOI] [PubMed] [Google Scholar]
- Orchard C. H. The role of the sarcoplasmic reticulum in the response of ferret and rat heart muscle to acidosis. J Physiol. 1987 Mar;384:431–449. doi: 10.1113/jphysiol.1987.sp016462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseau E., Pinkos J. pH modulates conducting and gating behaviour of single calcium release channels. Pflugers Arch. 1990 Feb;415(5):645–647. doi: 10.1007/BF02583520. [DOI] [PubMed] [Google Scholar]
- Solaro R. J., Lee J. A., Kentish J. C., Allen D. G. Effects of acidosis on ventricular muscle from adult and neonatal rats. Circ Res. 1988 Oct;63(4):779–787. doi: 10.1161/01.res.63.4.779. [DOI] [PubMed] [Google Scholar]
- Steadman B. W., Moore K. B., Spitzer K. W., Bridge J. H. A video system for measuring motion in contracting heart cells. IEEE Trans Biomed Eng. 1988 Apr;35(4):264–272. doi: 10.1109/10.1375. [DOI] [PubMed] [Google Scholar]
- Terracciano C. M., MacLeod K. T. Effects of acidosis on Na+/Ca2+ exchange and consequences for relaxation in guinea pig cardiac myocytes. Am J Physiol. 1994 Aug;267(2 Pt 2):H477–H487. doi: 10.1152/ajpheart.1994.267.2.H477. [DOI] [PubMed] [Google Scholar]
- Terracciano C. M., Naqvi R. U., MacLeod K. T. Effects of rest interval on the release of calcium from the sarcoplasmic reticulum in isolated guinea pig ventricular myocytes. Circ Res. 1995 Aug;77(2):354–360. doi: 10.1161/01.res.77.2.354. [DOI] [PubMed] [Google Scholar]
- Uto A., Arai H., Ogawa Y. Reassessment of Fura-2 and the ratio method for determination of intracellular Ca2+ concentrations. Cell Calcium. 1991 Jan;12(1):29–37. doi: 10.1016/0143-4160(91)90082-p. [DOI] [PubMed] [Google Scholar]
- Varro A., Negretti N., Hester S. B., Eisner D. A. An estimate of the calcium content of the sarcoplasmic reticulum in rat ventricular myocytes. Pflugers Arch. 1993 Apr;423(1-2):158–160. doi: 10.1007/BF00374975. [DOI] [PubMed] [Google Scholar]
- Vaughan-Jones R. D., Lederer W. J., Eisner D. A. Ca2+ ions can affect intracellular pH in mammalian cardiac muscle. Nature. 1983 Feb 10;301(5900):522–524. doi: 10.1038/301522a0. [DOI] [PubMed] [Google Scholar]



