Abstract
We studied cytokine proteins and mRNAs in mice with two forms of Toxoplasma gondii pneumonia resulting from reactivation of infection. In the first form, mice were infected with T. gondii, developed and recovered from systemic disease, and then developed pneumonia 3 weeks later. As pulmonary inflammation developed, levels of cytokine mRNAs for gamma interferon (IFN-γ), interleukin-2 (IL-2), IL-4, and IL-10 increased in bronchoalveolar lavage (BAL) cells or lung tissue, and the level of IFN-γ protein increased in BAL fluid. The second form of pneumonia occurred as a complication of primary cytomegalovirus (CMV) disease in mice with dormant T. gondii infection. During CMV disease, IL-2 mRNA levels decreased in lung tissue, IL-10 protein levels increased in lung tissue, and IL-10 protein levels increased in BAL fluid. As the mice recovered from CMV disease, T. gondii infection was reactivated in the lungs and was manifested as T. gondii pneumonia. During CMV-induced T. gondii pneumonia, IFN-γ, IL-2, IL-4, and IL-10 mRNA levels increased in BAL cells or lung tissue, and both IFN-γ and IL-2 protein levels increased in BAL fluid. We concluded that both forms of T. gondii pneumonia are accompanied by increases in both type 1 T-helper and type 2 T-helper cytokine levels in lungs. The mechanism of CMV-induced reactivation of T. gondii infection in lungs may involve local decreases in IL-2 levels and/or increases in IL-10 levels.
Severe postnatal toxoplasmosis is often the consequence of reactivation of infection in people with compromised immunity (4). Its frequency has increased dramatically with the spread of AIDS. Although much has been learned about host defenses against primary Toxoplasma gondii disease, less is known about the pathogenesis of reactivation. Pneumonia is the second most common manifestation of toxoplasmosis in persons with AIDS (27), and pneumonia is present in some cogenitally infected infants (2, 33). During local inflammatory events, lung cytokine and cellular responses are often different from systemic processes (7, 24). We studied lung cytokine responses in mice with T. gondii pneumonia due to reactivation of T. gondii infection. In order to compare local cytokine responses to systemic ones, we studied cytokine concentrations in serum and cytokine mRNA levels in spleens.
Two forms of pneumonia from the reactivation of dormant T. gondii infection have been extensively characterized (3, 13, 28–30). The first occurs approximately 3 weeks after mice recover from primary infection (29). The second occurs after mice with dormant T. gondii infection are infected with murine cytomegalovirus (CMV). CMV disease occurs during the week after CMV infection, followed within days by severe T. gondii pneumonia (29). T. gondii disease and CMV disease are each immunosuppressive in mice and humans, and these pathogens frequently coexist in lesions of human disease (29). Immunosuppression by either T. gondii or CMV disease may sufficiently depress host defenses to allow reactivation of latent infection with the other pathogen, especially in a person in whom host defenses are already suppressed.
We have previously reported that both forms of T. gondii reactivation pneumonia are preceded by a decrease in lung CD4+ lymphocyte levels and that pneumonia is characterized by large increases in lung CD8+ and CD4+ lymphocyte levels (28, 30). When the severity of CMV disease is diminished with antiviral therapy, T-lymphocyte changes are attenuated and T. gondii reactivation is prevented (3, 13).
In both mice and humans, the type 1 T-helper (Th1) subset of CD4+ lymphocytes is associated with strong cell-mediated immunity and control of infectious diseases, while the Th2 subset is associated with strong antibody responses, especially immunoglobulin A and immunoglobulin E, allergic reactions, and diminished cell-mediated immunity (1). Th1 lymphocytes secrete primarily gamma interferon (IFN-γ), interleukin-2 (IL-2), and tumor necrosis factor; Th2 lymphocytes secrete primarily IL-4, IL-5, IL-6, and IL-13 (1). Analogous CD8+ lymphocyte subsets, termed Tc1 (for type 1 T cytotoxic) and Tc2, have similar cytokine patterns (26, 34). Based on these characteristics and our previous work, we hypothesized that reactivation of T. gondii infection in lungs results from a decrease in Th1 cytokine levels or a transient increase in Th2 cytokine levels and that control of T. gondii reactivation is due to cells secreting Th1 cytokines. We were especially interested in the role of IFN-γ, which is central to the control of this pathogen in both acute and chronic infections (10, 11, 38, 39, 41).
MATERIALS AND METHODS
Mice, T. gondii, and CMV infection.
Specific-pathogen-free BALB/cAnNHsd female mice (Harlan, Indianapolis, Ind.) were maintained in American Association for Accreditation of Laboratory Animal Care-accredited facilities as previously described (29). At 6 weeks of age, they were inoculated intraperitoneally with 3 × 105 T. gondii C56 tachyzoites (29). From days 2 to 21 after infection, sulfadiazine (400 mg/liter) was added to their drinking water to keep the mice from dying during primary disease. As previously described (29), mice developed systemic T. gondii disease and recovered during the first 10 days.
There is no histologically detectable pneumonia during systemic infection (29). After recovery, mice appear well until 5 weeks after infection, when they develop toxoplasma pneumonia (termed primary reactivation pneumonia). These mice do not have detectable disease elsewhere in their bodies. Most mice recover without treatment, and survivors remain well for months.
The Smith strain of murine CMV was maintained by salivary gland passage in BALB/c mice (29). T. gondii-infected mice and controls were infected intraperitoneally with a sublethal quantity of CMV (∼2 × 104 PFU). This inoculum of CMV is slightly smaller than the amount that would kill BALB/c mice with dormant T. gondii infection and two- to threefold larger than lethal doses for uninfected BALB/c mice (29). We have demonstrated that mice that have dormant T. gondii infection and are then inoculated with CMV have a systemic illness lasting 7 to 10 days with no evidence of pneumonia (29). However, 14 days after CMV inoculation, mice reproducibly develop tachypnea and cyanosis and are found to have T. gondii pneumonia without evidence of T. gondii disease elsewhere (termed CMV-induced pneumonia [29]).
Cell, fluid, and tissue collection.
Serum was prepared from blood samples obtained by orbital plexus puncture. Bronchoalveolar lavage (BAL) was performed as previously described (29). For each mouse, the inferior vena cava was severed to drain the lungs of blood. Ten 1-ml volumes of phosphate-buffered saline (PBS) with 0.6 mM EDTA (pH 7.2) (PBS-EDTA) was used for lavage of the bronchi. Cell counts were obtained as previously described (30) and used to estimate the amount of RNA in samples. The supernatant from the first milliliter of fluid was collected for measurements of cytokine concentrations in order to minimize dilution with PBS-EDTA. Lung tissue was obtained after BAL to represent cells and tissues not easily removed by lavage. BAL pellets and spleen and lung tissues were snap frozen in liquid nitrogen in RNase-free microcentrifuge tubes.
RNA extraction, cDNA, and PCR.
RNA was extracted with phenol and chloroform after inactivation and dissolution of proteins with guanidinium thiocyanate (6). Briefly, frozen tissue was homogenized in lysis solution (3.2 M guanidinium thiocyanate, 20 mM sodium citrate, 100 mM sodium acetate, 0.05 M 2-mercaptoethanol, 0.4% Sarkosyl). RNA was extracted in a solution containing phenol-chloroform-isoamyl alcohol (25:25:1), precipitated in isopropanol, washed with 75% ethanol, and quantified by optical density measurements.
RNA was converted to cDNA with Moloney murine leukemia virus reverse transcriptase (32). Briefly, 0.05 mg of random hexamer primers (Promega, Madison, Wis.) per ml, 1,000 U of RNasin (Promega) per ml, and 4 μg of RNA were heated for 5 min at 65°C (32). Then, 104 U of Moloney murine leukemia virus reverse transcriptase (Gibco/BRL, Grand Island, N.Y.) per ml was added, and the solution was incubated at 37°C for 1.5 h. The reaction was stopped with heat (95°C, 5 min), and the samples were diluted fivefold.
PCR was performed as previously described (18). Briefly, the basic reaction mixture contained 1 to 5% cDNA transcribed from 4 μg of total RNA, 10 mM Tris (pH 8.3), 1.5 mM MgCl2, 50 mM KCl, 0.1% Triton X-100 (Fisher Biotech, Itasca, Ill.), 0.2 M deoxynucleoside triphosphates, 0.4 μM each sense and antisense primers (18), and 0.625 U of Bacillus thermoaquaticus DNA polymerase (Fisher Biotech). The reaction was carried through 32 cycles, each consisting of 93°C for 40 s, 55°C for 20 s, and 72°C for 40 s; the samples then were incubated at 72°C for 10 min for complete extension.
PCR products were separated by polyacrylamide gel electrophoresis, stained with ethidium bromide, and quantitated by densitometry (UV Max Technologies, Inc., Fremont, Calif.) by comparison to standards run in parallel. In each experiment, serial dilutions of a plasmid containing cytokine DNA sequences of interest, each containing a spacer, were run in parallel for quantitation (32). Quantitation was confirmed for selected samples by amplification in a competitive PCR with serial dilutions of the circular plasmid DNA containing cytokine DNA sequences with spacers (32). The housekeeping gene encoding hypoxanthine phosphoribosyltransferase was amplified in parallel, and results were corrected for the amount of hypoxanthine phosphoribosyltransferase mRNA present in each sample. To control for day-to-day variations, all samples from each experiment were extracted, converted to cDNA, amplified, electrophoresed, and analyzed by densitometry in parallel.
Cytokine quantitation.
Cytokines in serum and BAL fluid were quantified as previously described (14). Precise conditions for reagents for each cytokine were determined by block titration. Generally, primary monoclonal antibodies to mouse cytokines (PharMingen, San Diego, Calif.) were diluted to 0.5 to 4 μg/ml in 0.1 M NaHCO3 (pH 8.2) coating buffer, 50 μl was added to wells of enhanced protein binding enzyme-linked immunosorbent assay plates (25805-96; Corning Glass Inc., Corning, N.Y.), and the plates were sealed and incubated overnight at 4°C. The plates were washed with PBS containing 0.05% Tween 20 (PBS-T) and then blocked with PBS–10% fetal calf serum for 2 h at room temperature. The plates were washed, and cytokine standards (PharMingen) and samples were added to wells and incubated overnight at 4°C. The plates were washed with PBS-T, and then 100 μl of biotinylated anticytokine monoclonal antibody (0.5 to 4 μg/ml) in PBS–10% fetal calf serum was added and incubated at room temperature for 45 min. The plates were washed with PBS-T, and 100 μl of Extravidin-peroxidase (dilution, 1:400 to 1:1,000; Sigma, St. Louis, Mo.) was added and incubated for 30 min at room temperature. The plates were washed, 200 μl of substrate solution (3,3′,5,5′-tetramethylbenzidine system; Sigma) was added and allowed to develop at room temperature for 30 min, and reactions were stopped with 100 μl of 1 N H2SO4. The plates were examined at 450 nm in an enzyme-linked immunosorbent assay reader (Molecular Devices, Sunnyvale, Calif.).
Statistical analysis.
Cytokine measurements were determined in duplicate with samples from three mice for each time point. Measurements of mRNA in BAL cell RNA were determined with cells pooled from 10 mice. Spleen and lung tissues were obtained from single mice. All experiments were repeated at least twice. Differences between groups were tested by the Student t test.
RESULTS
Primary reactivation pneumonia.
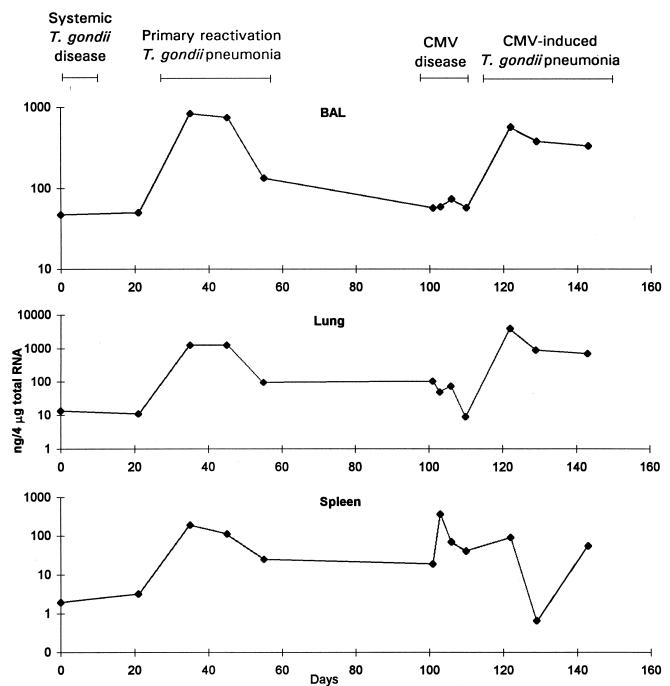
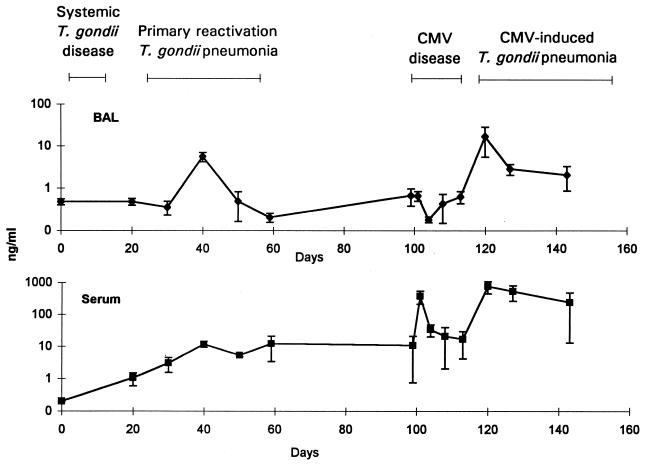
Expressed as a proportion of total RNA, the amount of IFN-γ mRNA increased substantially in BAL cell, lung, and spleen samples during primary reactivation pneumonia (Fig. 1). The amount of IFN-γ protein increased substantially in BAL fluid during the peak of pulmonary infiltration, 40 days after T. gondii inoculation (Fig. 2). Both the amounts of IFN-γ mRNA or protein and the relative increases in the amounts of IFN-γ mRNA or protein were greater than those for other cytokines. There was a gradual, small increase in serum IFN-γ levels (Fig. 2).
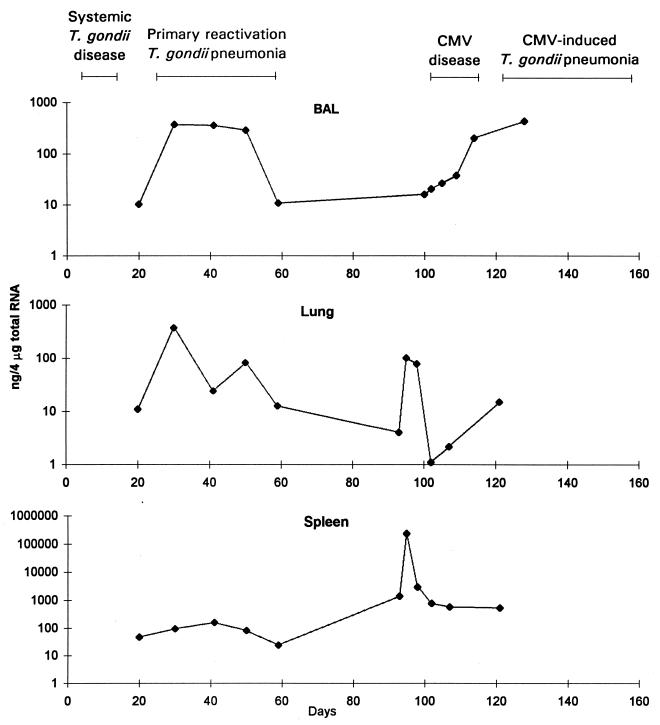
FIG. 1.
IFN-γ mRNA concentrations in BAL cells, lung tissue, and spleen tissue. Mice were inoculated with T. gondii on day 0 and with CMV on day 101. The data are representative of three separate experiments.
FIG. 2.
IFN-γ concentrations in BAL and serum expressed logarithmically. Mice were inoculated with T. gondii on day 0 and with CMV on day 99. Error bars represent standard deviations. Data are representative of three separate experiments.
There was a severalfold increase in the amount of IL-2 mRNA in BAL cell samples during primary reactivation pneumonia, an earlier, more prolonged increase in lung samples, and an increase in spleen samples (Fig. 3). The amount of IL-2 mRNA was the lowest of the four cytokines that were studied, but the results were reproducible. IL-2 protein concentrations in BAL fluid or serum samples obtained during primary reactivation pneumonia were inconsistent (data not shown).
FIG. 3.
IL-2 mRNA concentrations in BAL cells, lung tissue, and spleen tissue. Mice were inoculated with T. gondii on day 0 and with CMV on day 93. The data are representative of three separate experiments.
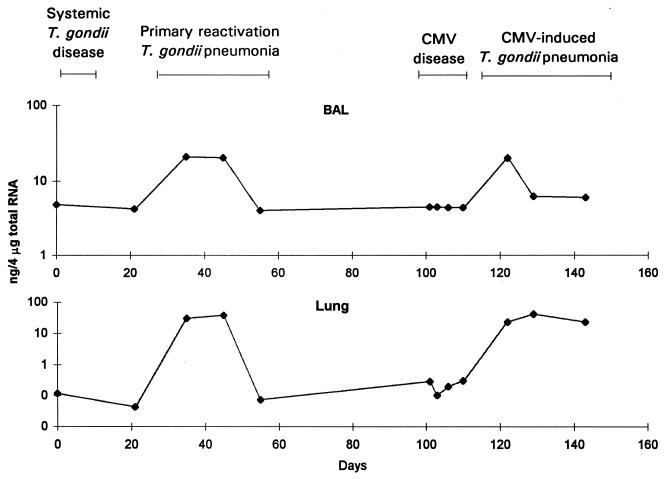
There were substantial increases in the amounts of IL-4 mRNA in BAL cell and lung samples during primary reactivation pneumonia (Fig. 4). The concentrations of IL-4 mRNA in spleen samples were mostly below concentrations that could be reliably measured, and these data are not presented. IL-4 protein concentrations in BAL fluid or serum samples were inconsistent during primary reactivation pneumonia (data not shown).
FIG. 4.
IL-4 mRNA concentrations in BAL cells and lung tissue. Mice were inoculated with T. gondii on day 0 and with CMV on day 101. The data are representative of three separate experiments. IL-4 mRNA concentrations in spleen tissue were low and not reproducible and are not presented.
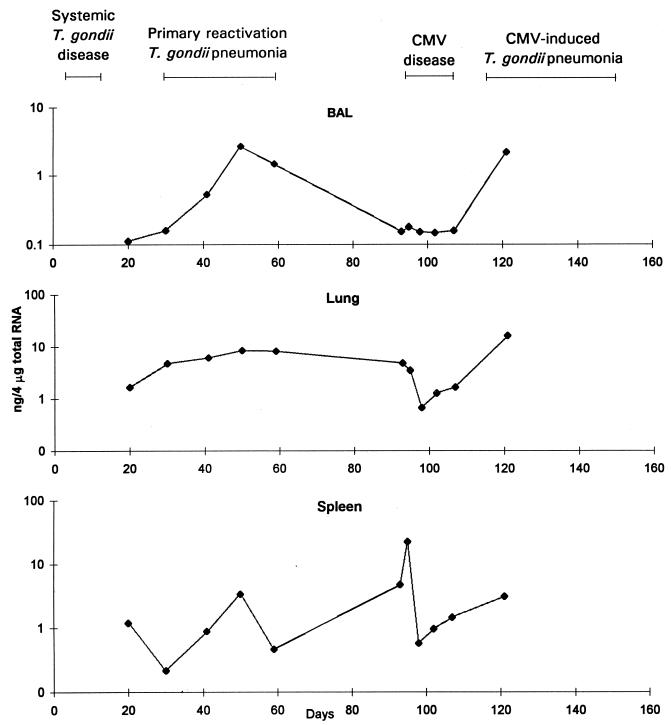
There was a striking increase in the amounts of IL-10 mRNA in BAL cell and lung samples during primary reactivation pneumonia (Fig. 5). The amount of IL-10 mRNA increased slightly in spleen samples in the experiment shown in Fig. 5, and there were greater increases in the other two experiments. Concentrations of IL-10 protein were between 20 and 100 pg/ml and did not change consistently in BAL fluid or serum samples obtained during primary reactivation pneumonia (data not shown).
FIG. 5.
IL-10 mRNA concentrations in BAL cells, lung tissue, and spleen tissue. Mice were inoculated with T. gondii on day 0 and with CMV on day 93. The data are representative of three separate experiments.
CMV disease.
For IFN-γ, IL-2, and IL-10, there were increases in cytokine proteins in serum and/or in cytokine mRNA in spleens during CMV disease. IFN-γ protein concentrations increased in serum (Fig. 2), and concentrations of IFN-γ mRNA increased substantially in spleens (Fig. 1). There were no substantial changes for IFN-γ in lungs.
By 5 days after CMV inoculation, IL-2 protein concentrations in serum increased an average of sixfold (from 12 to 84 pg/ml; P, 0.03). IL-2 mRNA concentrations in spleen samples increased substantially but briefly during CMV disease in all experiments (Fig. 3). There was little change in BAL cell IL-2 mRNA concentrations, but IL-2 mRNA concentrations decreased substantially in lung samples in two of three experiments (Fig. 3).
IL-4 protein concentrations in serum and BAL fluid did not change during CMV disease. IL-4 mRNA concentrations did not change substantially during CMV disease in BAL cell or lung samples (Fig. 4).
IL-10 protein concentrations increased in BAL fluid during the first 5 days of CMV disease from 58 to 210 pg/ml, but the difference was not significant (P, 0.07). IL-10 protein concentrations increased in serum during the first 5 days of CMV disease from 50 to 70 pg/ml, but the difference was not significant (P, 0.10). During this 5-day interval, there were increases in IL-10 mRNA concentrations in lungs in two of three experiments and in spleens in three of three experiments (Fig. 5).
CMV-induced pneumonia.
There was evidence from measurements of mRNA, protein, or both that the amounts of all four cytokines increased in lungs during CMV-induced pneumonia, similar to the increases seen during primary reactivation pneumonia. IFN-γ protein levels increased substantially in BAL fluid during the early phase of the pulmonary inflammatory response, 14 days after CMV inoculation (Fig. 2). There was a corresponding increase in serum IFN-γ protein levels (Fig. 2). IFN-γ mRNA levels also increased substantially in BAL cell and lung but not spleen samples (Fig. 1).
IL-2 protein levels increased in BAL fluid during the first 14 days of CMV-induced pneumonia, from 11 to 52 pg/ml (P, 0.02). IL-2 protein levels also increased in serum during this time period, from 12 to 113 pg/ml (P, 0.02). IL-2 mRNA levels increased in BAL cell and lung samples during CMV-induced pneumonia but remained near the baseline in spleen samples (Fig. 3).
IL-4 protein levels did not change substantially in BAL fluid or serum samples during CMV-induced pneumonia. IL-4 mRNA levels increased in BAL cell and lung samples during CMV-induced pneumonia (Fig. 4).
IL-10 protein levels did not change much in BAL fluid or serum samples during CMV-induced pneumonia. IL-10 mRNA levels increased in BAL cell samples during CMV-induced pneumonia but not consistently in other samples (Fig. 5).
DISCUSSION
There was evidence for substantial increases in the levels of all four cytokines, IFN-γ, IL-2, IL-4, and IL-10, in lungs during both forms of T. gondii pneumonia. The most striking increases were observed for IFN-γ. IFN-γ protein levels in BAL fluid and IFN-γ mRNA levels in BAL cell and lung samples increased more than 10-fold during pneumonia. Increases in IFN-γ mRNA levels in lungs during primary reactivation pneumonia were accompanied by increases in spleen IFN-γ mRNA levels (Fig. 1). Later, during CMV disease, increases in spleen IFN-γ mRNA levels were substantially greater than the increases observed during either primary or CMV-induced pneumonia. This maximal spleen IFN-γ mRNA activity probably reflected the systemic nature of CMV disease. IFN-γ protein levels increased substantially in serum during CMV disease as well (Fig. 2). From the magnitude of increases of IFN-γ protein and mRNA levels, it appeared that inflammation occurring during the two forms of pneumonia was driven predominantly by Th1 cytokines. IFN-γ is known to be a key cytokine in models of primary T. gondii infection (35, 36, 40–42).
IL-2 mRNA levels also increased substantially in lung tissue and BAL cells during pneumonia. The amounts of spleen IL-2 mRNA did not parallel the changes in lungs, emphasizing the localized nature of the inflammatory response. A sharp increase in the amounts of spleen IL-2 mRNA during CMV disease (Fig. 3) coincided with the increase in the amounts of spleen IFN-γ mRNA (Fig. 1). The main physiological activities of IL-2 are to stimulate clonal T-cell proliferation, natural killer cell differentiation into lymphocyte-activated killer cells, and activation of B lymphocytes, cytotoxic T lymphocytes, and macrophages (21). Previous observations suggest that IL-2 is important for effective control of primary toxoplasma infection (8, 15, 22, 37).
Interestingly, during CMV disease, IL-2 mRNA levels decreased in lung samples. A decrease in IL-2 and perhaps Th1 or Tc1 (26, 34) lymphocyte activity might have been responsible for the subsequent reactivation of T. gondii infection in lungs. The decreased IL-2 mRNA levels in lungs during CMV disease are consistent with our earlier observations that there is a consistent decrease in the levels of CD4+ cells in lung lavage populations just before either primary reactivation pneumonia or CMV-induced pneumonia (28, 30). Others have reported that mouse splenic lymphocytes are unresponsive to T. gondii antigens during the first week of primary T. gondii infection and that this finding is associated with diminished IL-2 production by these lymphocytes (17).
Although the increases in the levels of the Th1 cytokines IFN-γ and IL-2 were most impressive, increases in the levels of IL-10 and IL-4 suggested that these cytokines may have modulated the inflammation driven primarily by IFN-γ and IL-2. IL-10 mRNA levels increased in BAL cell and lung samples during primary reactivation pneumonia and in BAL cell samples during CMV-induced pneumonia. IL-10 is closely associated with Th2 activity, but IL-10 is also secreted by other T cells, B cells, macrophages, keratinocytes, and mast cells (9, 25). The source of IL-10 in our experiments was not known.
The main physiological activities of IL-10 are to inhibit cytokine secretion by Th1 lymphocytes, Th2 lymphocytes, and mononuclear phagocytes. IL-10 suppresses the synthesis of IFN-γ and inhibits macrophage effector and antigen-presenting activities (9, 25). IL-10 has been associated with immunodepression during acute T. gondii infection (12, 16, 19, 20, 23) and appears to protect mice from excessive, potentially lethal inflammation during T. gondii disease (12). It is likely that one effect of increased IL-10 levels in mouse lungs during primary reactivation pneumonia and CMV-induced pneumonia was to limit the inflammation that occurred in response to local T. gondii replication. In support of this notion, recent unpublished data from our laboratory indicate that depletion of IL-10 allows inflammation to worsen.
During CMV disease, IL-10 mRNA levels increased in lung samples in two of three experiments, and there was a nearly fourfold increase in BAL fluid IL-10 protein levels in all experiments. The increases in IL-10 protein levels in BAL fluid were not quite statistically significant; there was substantial variability from mouse to mouse. In view of the effects of IL-10 on Th1 cell function, it is of interest that IL-2 mRNA levels decreased in lungs during CMV disease. Our results suggest the intriguing possibility that IL-10 produced during CMV disease may have led to reactivation of T. gondii infection in lungs. Experiments to answer this question are ongoing. Spleen IL-10 mRNA levels also increased and may have been involved in modulating the systemic inflammatory response during CMV disease.
IL-4 is secreted by Th2 cells and shifts immune responses toward Th2 domination (5). Specifically, IL-4 stimulates B cells and increases their ability to process antigens and to produce immunoglobulins, especially IgE (5). In our experiments, the amounts of IL-4 mRNA increased in BAL cells and lung tissue during primary reactivation pneumonia and CMV-induced pneumonia, but the amounts of IL-4 mRNA and the degree of the increase were smaller than those for IFN-γ or IL-10. It appeared that IL-4-producing cells were present in the lungs during times of intense lung inflammation but that the amounts produced were more limited than for IFN-γ. Since only small amounts of IL-4 were produced and since IL-10 has so many other potential sources, it seems likely that Th2 cells were present in the lungs but that they did not predominate in the inflammatory response, as has been observed for some other infections (31). It did not appear that a predominance of Th2 cells was responsible for the reactivation of T. gondii infection, as we had initially hypothesized.
In mice with reactivation of T. gondii infection, disease was limited to the lungs, and pulmonary inflammatory responses often differed from those elsewhere in the body (7, 24). We used two complementary approaches to describe cytokines present in mice before, during, and after toxoplasma pneumonia. One was to measure the levels of cytokines directly in BAL fluid and serum. BAL fluid reflected lung cytokines that diffused intact into the bronchi. Serum reflected cytokine secretion throughout the body. Since neither fluid was taken directly from lung parenchyma, we also measured the levels of cytokine mRNA in BAL cells and lung tissue. The advantage of measuring mRNA levels directly was that we could be confident that the mRNA was produced in vivo. On the other hand, posttranslational events may have altered the relationship between mRNA production and secretion. Another approach sometimes used is to remove cells and measure cytokine production in vitro in response to mitogens or antigens. This approach measures the types of cells that can be cultured and what their capabilities are but not what the cells are doing in the complex milieu of inflammatory sites. The strength of our experimental approach was that it provided information about pathophysiological events occurring in vivo during the disease process in the relevant organ and in the whole mouse.
Increased cytokine mRNA concentrations were not always reflected in BAL fluid cytokine protein concentrations for IL-2, IL-4, and IL-10. Cytokine protein concentrations were variable between experiments and more difficult to interpret. It was likely that cytokines secreted in focal areas of inflammation in the lungs were degraded locally or removed in the circulation before they diffused into BAL fluid. There is no feasible way to measure cytokine protein concentrations bathing individual cells within inflammatory foci.
Our results indicated that both Th1 and Th2 cytokines were present in mouse lungs during T. gondii pneumonia and that decreases in IL-2 and/or increases in IL-10 levels during CMV disease may have induced the reactivation of T. gondii infection. If true for humans, these observations may be critical for understanding the reactivation of T. gondii infection. They would also be important for the development of rational approaches to prevent or control this or other opportunistic infections in humans by modulation of immune responses.
ACKNOWLEDGMENTS
This work was supported by Department of Veterans Affairs merit review grants.
REFERENCES
- 1.Abbas A K, Murphy K M, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–793. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 2.Alford C J, Stagno S, Reynolds D W. Congenital toxoplasmosis: clinical, laboratory, and therapeutic considerations, with special reference to subclinical disease. Bull N Y Acad Med. 1974;50:160–181. [PMC free article] [PubMed] [Google Scholar]
- 3.Banister S, Pomeroy C. Effect of ganciclovir on murine cytomegalovirus-induced reactivation of toxoplasma pneumonia. J Lab Clin Med. 1993;122:576–580. [PubMed] [Google Scholar]
- 4.Beaman M H, McCabe R E, Wong S-Y, Remington J S. Toxoplasma gondii. In: Mandell G, Bennett J, Dolin R, editors. Principles and practice of infectious diseases. 4th ed. New York, N.Y: Churchill Livingstone; 1995. pp. 2455–2475. [Google Scholar]
- 5.Choi P, Reiser H. IL-4: role in disease and regulation of production. Clin Exp Immunol. 1998;113:317–319. doi: 10.1046/j.1365-2249.1998.00690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 7.Curtis J L, Kaltreider H B. Characterization of bronchoalveolar lymphocytes during a specific antibody-forming cell response in the lungs of mice. Am Rev Respir Dis. 1989;139:393–400. doi: 10.1164/ajrccm/139.2.393. [DOI] [PubMed] [Google Scholar]
- 8.Denkers E Y, Scharton-Kersten R, Barbieri S, Caspar P, Sher A. A role for CD4+NK1.1+ T lymphocytes as major histocompatibility complex class II independent helper cells in the generation of CD8+ effector function against intracellular infection. J Exp Med. 1996;184:131–139. doi: 10.1084/jem.184.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Vries J E. Immunosuppressive and anti-inflammatory properties of interleukin 10. Ann Med. 1995;27:537–541. doi: 10.3109/07853899509002465. [DOI] [PubMed] [Google Scholar]
- 10.Gazzinelli R T, Hakim F T, Hieny S, Shearer G M, Sher A. Synergistic role of CD4+ T lymphocytes in IFN-γ production and protective immunity induced by an attenuated Toxoplasma gondii vaccine. J Immunol. 1991;146:286–292. [PubMed] [Google Scholar]
- 11.Gazzinelli R T, Hartley J W, Fredrickson T N, Chattopadhyay S K, Sher A, Morse H C., III Opportunistic infections and retrovirus-induced immunodeficiency: studies of acute and chronic infections with Toxoplasma gondii in mice infected with LP-BM5 murine leukemia viruses. Infect Immun. 1992;60:4394–4401. doi: 10.1128/iai.60.10.4394-4401.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gazzinelli R T, Wysocka M, Hieny S, Scharton-Kersten T, Cheever A, Kuhn R, Muller W, Trinchieri G, Sher A. In the absence of endogenous IL-10, mice acutely infected with Toxoplasma gondii succumb to a lethal immune response dependent on CD4+ T cells and accompanied by overproduction of IL-12, IFN-γ and TNF-α. J Immunol. 1996;157:798–805. [PubMed] [Google Scholar]
- 13.Goetz L, Pomeroy C. Impact of prophylactic ganciclovir on bronchoalveolar lavage lymphocyte numbers and phenotypes in murine cytomegalovirus-induced reactivation of Toxoplasma pneumonia. J Lab Clin Med. 1996;128:384–391. doi: 10.1016/s0022-2143(96)80010-2. [DOI] [PubMed] [Google Scholar]
- 14.Graham M B, Braciale V L, Braciale T J. Influenza virus-specific CD4+ T helper type 2 T lymphocytes do not promote recovery from experimental virus infection. J Exp Med. 1994;180:1273–1282. doi: 10.1084/jem.180.4.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hakim F T, Gazzinelli R T, Denkers E, Hieny S, Shearer G M, Sher A. CD8+ T cells from mice vaccinated against Toxoplasma gondii are cytotoxic for parasite-infected or antigen-pulsed host cells. J Immunol. 1991;147:2310–2316. [PubMed] [Google Scholar]
- 16.Haque S, Haque A, Kasper L H. A Toxoplasma gondii-derived factor(s) stimulates immune downregulation: an in vitro model. Infect Immun. 1995;63:3442–3447. doi: 10.1128/iai.63.9.3442-3447.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haque S, Khan I, Haque A, Kasper L. Impairment of the cellular immune response in acute murine toxoplasmosis: regulation of interleukin 2 production and macrophage-mediated inhibitory effects. Infect Immun. 1994;62:2908–2916. doi: 10.1128/iai.62.7.2908-2916.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hitt J A, Filice G A. Detection of Toxoplasma gondii parasitemia by gene amplification cell culture and mouse inoculation. J Clin Microbiol. 1992;30:3181–3184. doi: 10.1128/jcm.30.12.3181-3184.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hunter C A, Abrams J S, Beaman M H, Remington J S. Cytokine mRNA in the central nervous system of SCID mice infected with Toxoplasma gondii: importance of T-cell-independent regulation of resistance to T. gondii. J Immunol. 1995;61:4038–4044. doi: 10.1128/iai.61.10.4038-4044.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hunter C A, Subauste C, Van Cleave V, Remington J S. Production of gamma interferon by natural killer cells from Toxoplasma gondii-infected SCID mice: regulation by interleukin-10, interleukin-12, and tumor necrosis factor alpha. Infect Immun. 1994;62:2818–2824. doi: 10.1128/iai.62.7.2818-2824.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnston J A, Bacon C M, Riedy M C, O’Shea J J. Signaling by IL-2 and related cytokines: JAKs, STATs, and relationship to immunodeficiency. J Leukoc Biol. 1996;60:441–452. doi: 10.1002/jlb.60.4.441. [DOI] [PubMed] [Google Scholar]
- 22.Khan I A, Matsuura T, Kasper L H. Activation-mediated CD4+ T cell unresponsiveness during acute Toxoplasma gondii infection in mice. Int Immunol. 1996;8:887–896. doi: 10.1093/intimm/8.6.887. [DOI] [PubMed] [Google Scholar]
- 23.Khan I A, Matsuura T, Kasper L H. IL-10 mediates immunosuppression following primary infection with Toxoplasma gondii in mice. Parasite Immunol. 1995;17:185–195. doi: 10.1111/j.1365-3024.1995.tb00888.x. [DOI] [PubMed] [Google Scholar]
- 24.Klemm A, Tschernig T, Krug N, Pabst R. Lymphocyte subsets in distinct lung compartments show a different ability to produce interferon-gamma (IFNγ) during a pulmonary immune response. Clin Exp Immunol. 1998;113:252–257. doi: 10.1046/j.1365-2249.1998.00657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moore K, O’Garra A, de Waal Malefyt R, Vieira P, Mosmann T R. Interleukin-10. Annu Rev Immunol. 1993;11:165–190. doi: 10.1146/annurev.iy.11.040193.001121. [DOI] [PubMed] [Google Scholar]
- 26.Mosmann T R, Li L, Hengartner H, Kagi D, Fu W, Sad S. Differentiation and functions of T cell subsets. Ciba Found Symp. 1997;204:148–158. doi: 10.1002/9780470515280.ch10. [DOI] [PubMed] [Google Scholar]
- 27.Pomeroy C, Filice G A. Pulmonary toxoplasmosis. Clin Infect Dis. 1992;14:863–870. doi: 10.1093/clinids/14.4.863. [DOI] [PubMed] [Google Scholar]
- 28.Pomeroy C, Filice G A, Hitt J, Jordan M C. Cytomegalovirus-induced reactivation of Toxoplasma gondii pneumonia in mice: lung lymphocyte phenotypes and suppressor function. J Infect Dis. 1992;166:677–681. doi: 10.1093/infdis/166.3.677. [DOI] [PubMed] [Google Scholar]
- 29.Pomeroy C, Kline S, Jordan M C, Filice G A. Reactivation of Toxoplasma gondii by cytomegalovirus disease in mice: antimicrobial activities of macrophages. J Infect Dis. 1989;160:305–311. doi: 10.1093/infdis/160.2.305. [DOI] [PubMed] [Google Scholar]
- 30.Pomeroy C, Miller L, McFarling L, Kennedy C, Filice G. Phenotypes, proliferative responses and suppressor function of lung lymphocytes during Toxoplasma gondii pneumonia in mice. J Infect Dis. 1991;164:1227–1232. doi: 10.1093/infdis/164.6.1227. [DOI] [PubMed] [Google Scholar]
- 31.Reiner S L, Locksley R M. The regulation of immunity to Leishmania major. Annu Rev Immunol. 1995;13:151–177. doi: 10.1146/annurev.iy.13.040195.001055. [DOI] [PubMed] [Google Scholar]
- 32.Reiner S L, Zhen S, Corry D B, Locksley R M. Constructing polycompetitor cDNAs for quantitative PCR. J Immunol Methods. 1993;165:37–46. doi: 10.1016/0022-1759(93)90104-f. [DOI] [PubMed] [Google Scholar]
- 33.Remington J S, McLeod R, Desmonts G. Toxoplasmosis. In: Remington J S, Klein J O, editors. Infectious diseases of the fetus and newborn infant. 4th ed. Philadelphia, Pa: The W. B. Saunders Co.; 1995. pp. 140–267. [Google Scholar]
- 34.Sad S, Marcotte R, Mosmann T R. Cytokine-induced differentiation of precursor mouse CD8+ cells into cytotoxic CD8+ T cells secreting Th1 or Th2 cytokines. Immunity. 1995;2:271–279. doi: 10.1016/1074-7613(95)90051-9. [DOI] [PubMed] [Google Scholar]
- 35.Scharton-Kersten T, Contursi C, Masumi A, Sher A, Ozato K. Interferon consensus sequence binding protein-deficient mice display impaired resistance to intracellular infection due to a primary defect in interleukin 12 p40 induction. J Exp Med. 1997;186:1523–1534. doi: 10.1084/jem.186.9.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scharton-Kersten T M, Wynn T A, Denkers E Y, Bala S, Grunvald E, Hieny S, Gazzinelli R T, Sher A. In the absence of endogenous IFN-γ, mice develop unimpaired IL-12 responses to Toxoplasma gondii while failing to control acute infection. J Immunol. 1996;157:4045–4054. [PubMed] [Google Scholar]
- 37.Sharma S D, Hofflin J M, Remington J S. In vivo recombinant interleukin 2 administration enhances survival against a lethal challenge with Toxoplasma gondii. J Immunol. 1985;135:4160–4163. [PubMed] [Google Scholar]
- 38.Subauste C S, Remington J S. Role of gamma interferon in Toxoplasma gondii infection. Eur J Clin Microbiol Infect Dis. 1991;10:58–67. doi: 10.1007/BF01964408. [DOI] [PubMed] [Google Scholar]
- 39.Suzuki Y, Conley F K, Remington J S. Importance of endogenous IFN-γ for prevention of toxoplasmic encephalitis in mice. J Immunol. 1989;143:2045–2050. [PubMed] [Google Scholar]
- 40.Suzuki Y, Conley F K, Remington J S. Treatment of toxoplasmic encephalitis in mice with recombinant gamma interferon. Infect Immun. 1990;58:3050–3055. doi: 10.1128/iai.58.9.3050-3055.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Suzuki Y, Orellana M A, Schreiber R D, Remington J S. Interferon-γ: the major mediator of resistance against Toxoplasma gondii. Science. 1988;240:516–518. doi: 10.1126/science.3128869. [DOI] [PubMed] [Google Scholar]
- 42.Yamamura M, Uyemura K, Deans R J, Weinberg K, Rea T H, Bloom B R, Modlin R L. Defining protective responses to pathogens: cytokine profiles in leprosy lesions. Science. 1991;254:277–279. doi: 10.1126/science.254.5029.277. [DOI] [PubMed] [Google Scholar]