Abstract
1. Whole-cell and amphotericin-perforated patch-clamp techniques have been used to study the effects of hydrogen peroxide (H2O2) on action potentials and underlying ionic currents in single myocytes from the ventricles of adult rat hearts. 2. The results obtained differed markedly depending on the recording method utilized. Conventional whole-cell recordings, in which the myoplasm is dialysed with the contents of the pipette, failed to show any significant effects of H2O2 on the action potential or cell shortening. In contrast, when action potentials were recorded with the amphotericin-perforated patch method, H2O2 (50-200 microM) produced a marked prolongation of the action potential and an increase in cell shortening. 3. Voltage-clamp recordings with the amphotericin-perforated patch method showed that H2O2 caused no significant changes in either the Ca(2+)-independent transient outward K+ current (Ito) or the inwardly rectifying K+ current (IK1). 4. Application of tetrodotoxin (TTX; 8 x 10(-6) M), a Na+ channel blocker, largely inhibited the effects of H2O2 on the action potential. Moreover, anthopleurin A (4 x 10 (-7) M), which augments Na+ current (INa) by slowing its inactivation, mimicked the effects of H2O2 on the action potential of ventricular myocytes. These effects on INa were also blocked almost completely by TTX. 5. The hypothesis that H2O2 can augment INa by slowing its kinetics of inactivation was tested directly using ensemble recordings from cell-attached macropatches. These results demonstrated a significant enhancement of late opening events when H2O2 (200 microM) was included in the recording pipette. A corresponding slowing of inactivation of the ensemble INa was observed. 6. The possibility that protein kinase C (PKC) is an intracellular second messenger for the observed effects of H2O2 was examined using the blocker bisindolylmaelimide (BIS; 10(-7) M). Bath application of BIS prior to H2O2 exposure significantly delayed and also attenuated the development of the action potential prolongation. 7. These results demonstrate marked electrophysiological effects of H2O2 in rat ventricle. The dependence of these effects on recording methods suggests involvement of an intracellular second messenger, and the results with the PKC inhibitor, BIS, support this possibility. The most prominent effect of H2O2 on the ionic currents which underlie the action potential is a slowing of inactivation of the TTX-sensitive INa. Recent molecular studies have demonstrated a PKC phosphorylation site on the rat cardiac Na+ channel isoform and have also shown that PKC activation can slow inactivation of INa.
Full text
PDF


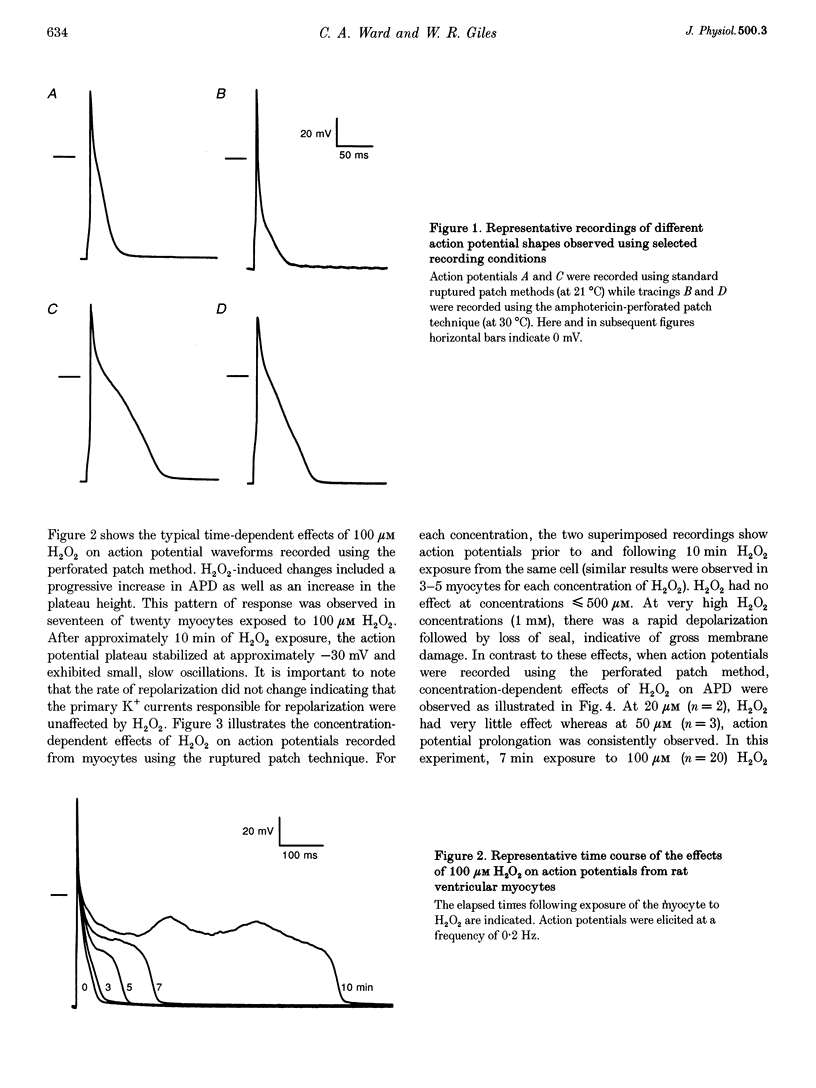
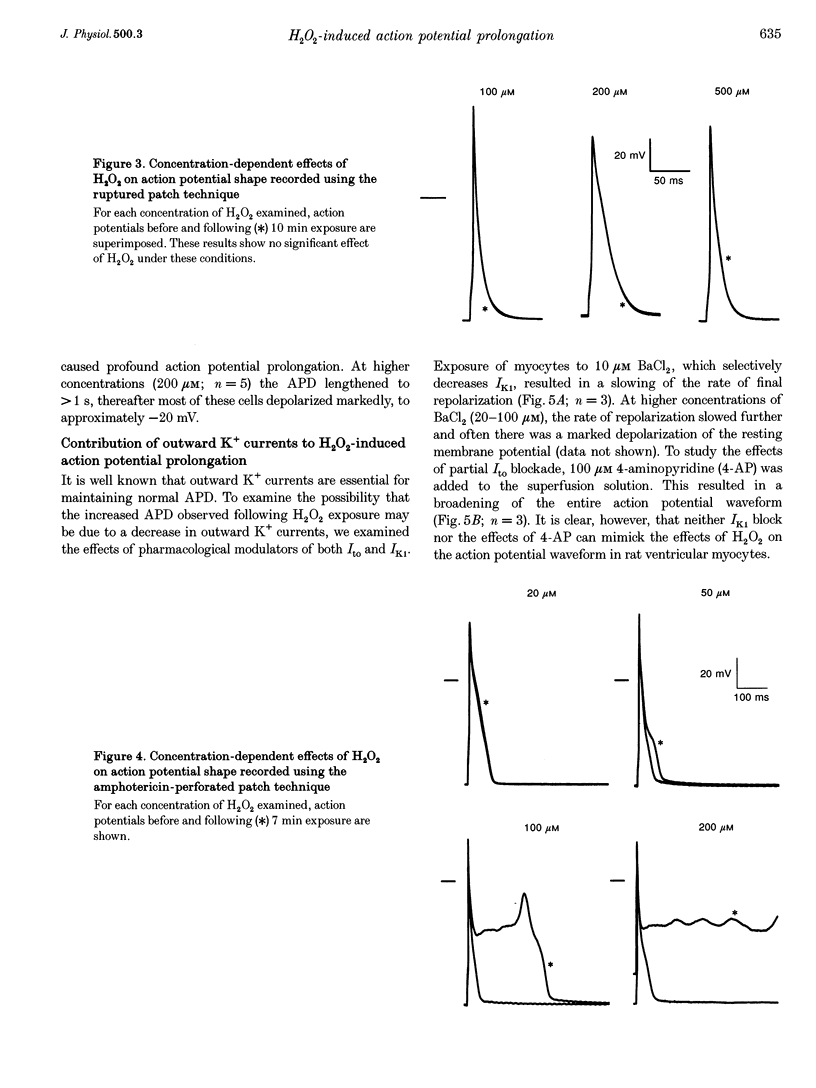
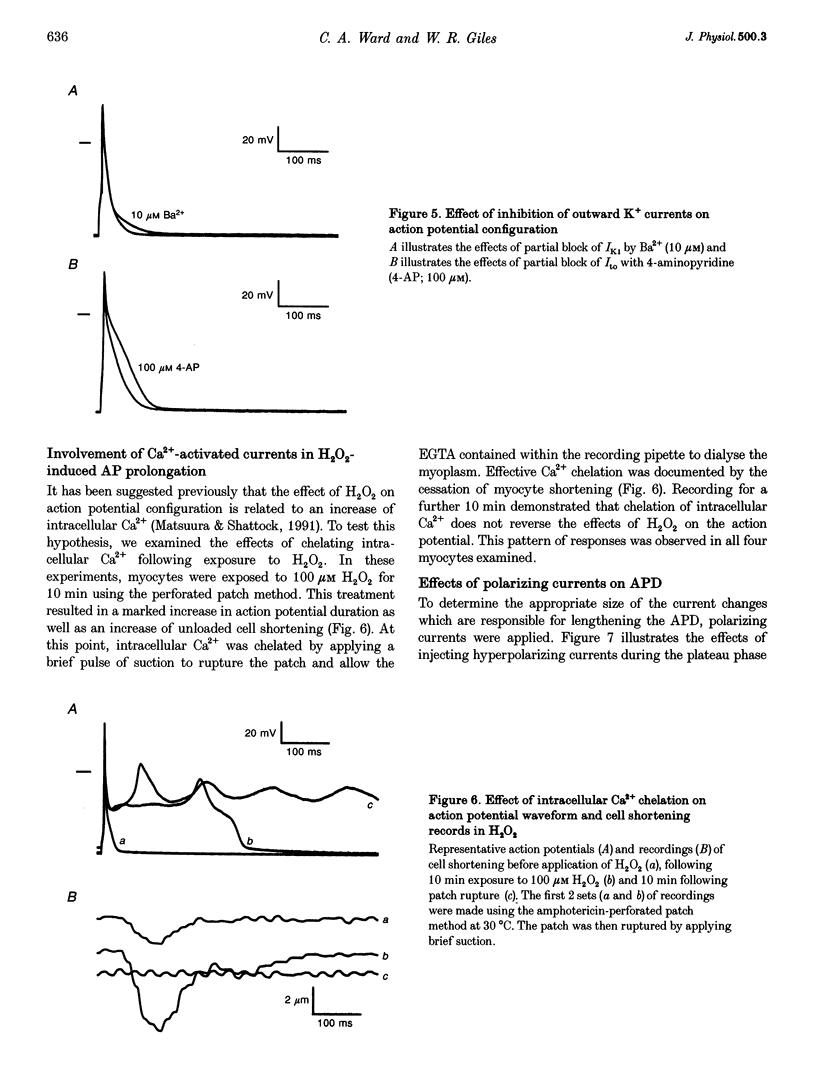
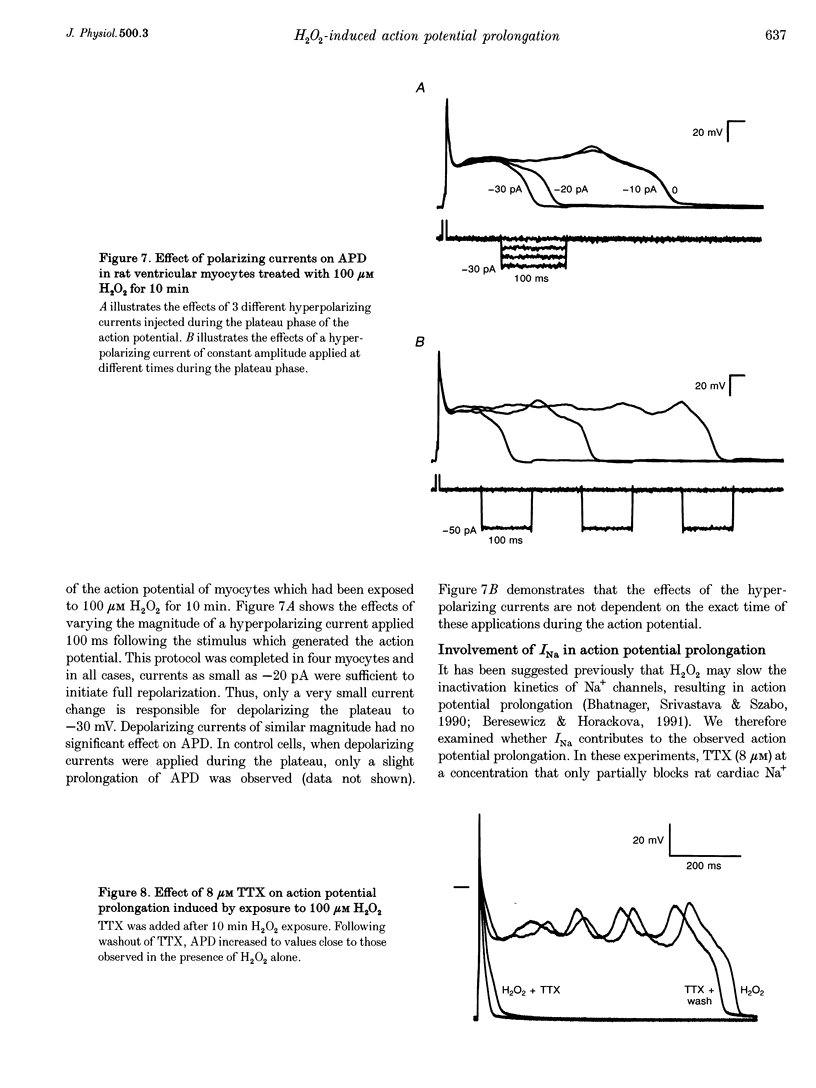
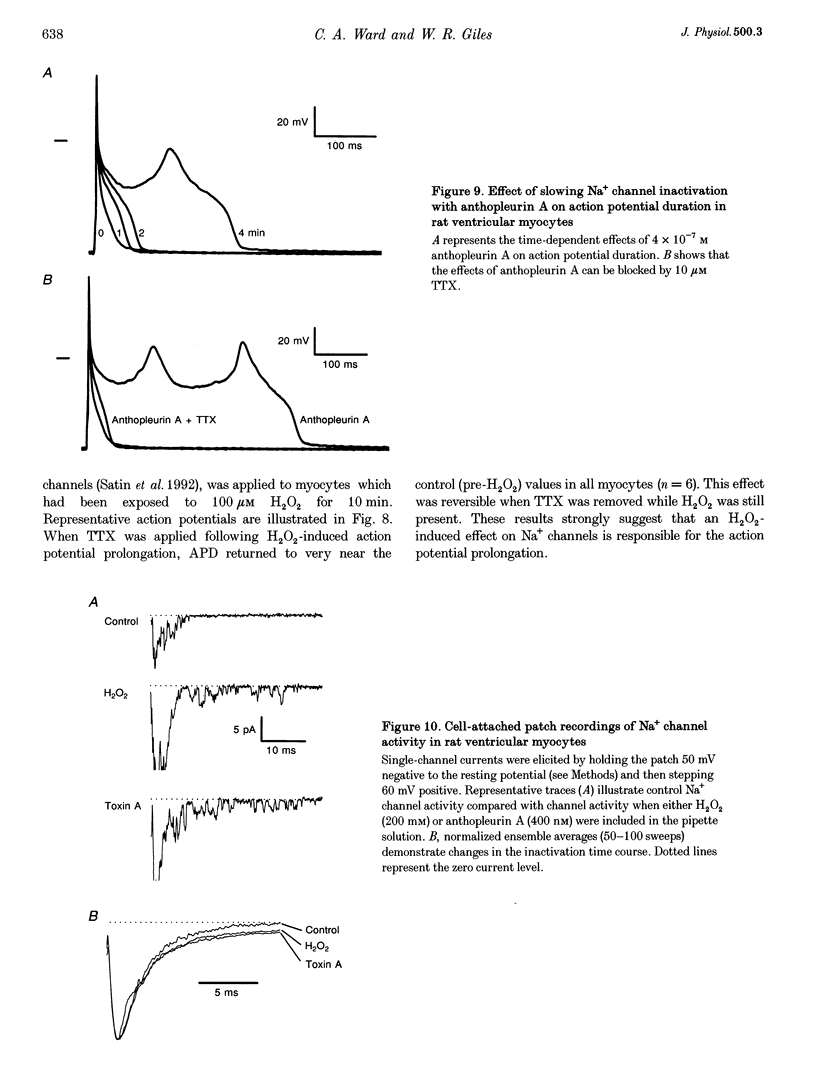
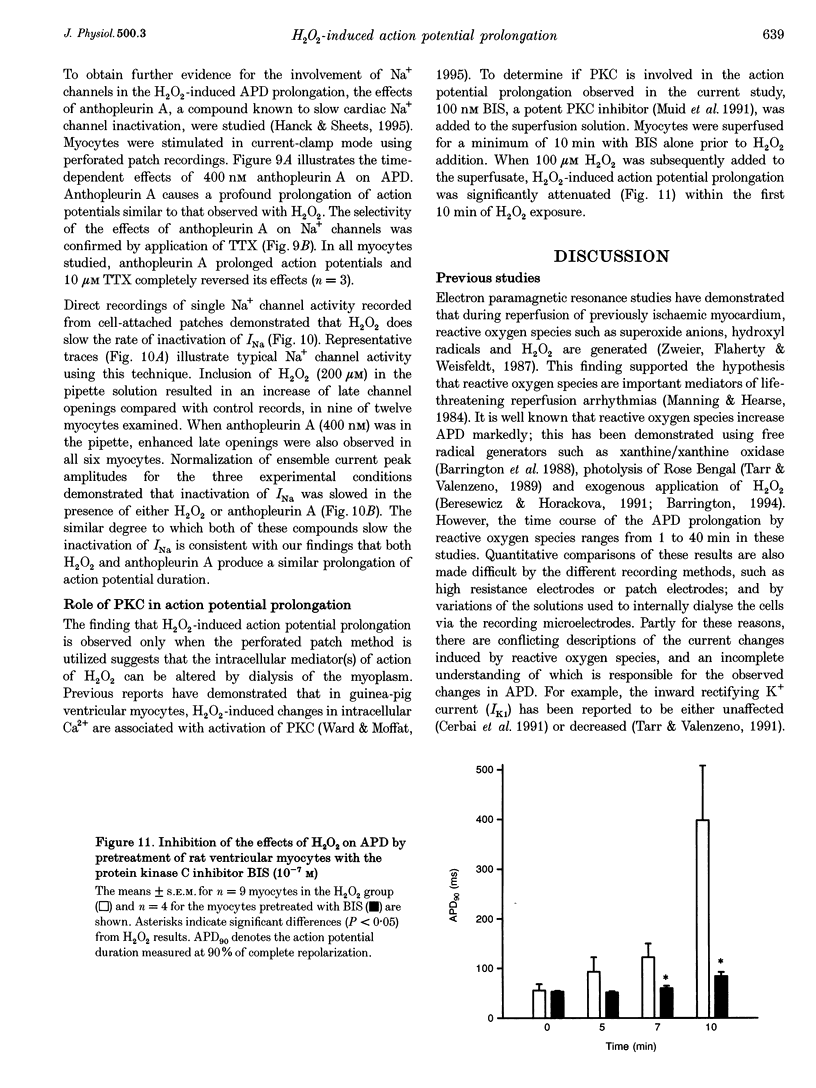



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrington P. L. Interactions of H2O2, EGTA and patch pipette recording methods in feline ventricular myocytes. J Mol Cell Cardiol. 1994 May;26(5):557–568. doi: 10.1006/jmcc.1994.1068. [DOI] [PubMed] [Google Scholar]
- Barrington P. L., Meier C. F., Jr, Weglicki W. B. Abnormal electrical activity induced by free radical generating systems in isolated cardiocytes. J Mol Cell Cardiol. 1988 Dec;20(12):1163–1178. doi: 10.1016/0022-2828(88)90596-2. [DOI] [PubMed] [Google Scholar]
- Benz I., Herzig J. W., Kohlhardt M. Opposite effects of angiotensin II and the protein kinase C activator OAG on cardiac Na+ channels. J Membr Biol. 1992 Nov;130(2):183–190. doi: 10.1007/BF00231895. [DOI] [PubMed] [Google Scholar]
- Beresewicz A., Horackova M. Alterations in electrical and contractile behavior of isolated cardiomyocytes by hydrogen peroxide: possible ionic mechanisms. J Mol Cell Cardiol. 1991 Aug;23(8):899–918. doi: 10.1016/0022-2828(91)90133-7. [DOI] [PubMed] [Google Scholar]
- Bhatnagar A., Srivastava S. K., Szabo G. Oxidative stress alters specific membrane currents in isolated cardiac myocytes. Circ Res. 1990 Sep;67(3):535–549. doi: 10.1161/01.res.67.3.535. [DOI] [PubMed] [Google Scholar]
- Bouchard R. A., Clark R. B., Giles W. R. Effects of action potential duration on excitation-contraction coupling in rat ventricular myocytes. Action potential voltage-clamp measurements. Circ Res. 1995 May;76(5):790–801. doi: 10.1161/01.res.76.5.790. [DOI] [PubMed] [Google Scholar]
- Cerbai E., Ambrosio G., Porciatti F., Chiariello M., Giotti A., Mugelli A. Cellular electrophysiological basis for oxygen radical-induced arrhythmias. A patch-clamp study in guinea pig ventricular myocytes. Circulation. 1991 Oct;84(4):1773–1782. doi: 10.1161/01.cir.84.4.1773. [DOI] [PubMed] [Google Scholar]
- Clark R. B., Bouchard R. A., Salinas-Stefanon E., Sanchez-Chapula J., Giles W. R. Heterogeneity of action potential waveforms and potassium currents in rat ventricle. Cardiovasc Res. 1993 Oct;27(10):1795–1799. doi: 10.1093/cvr/27.10.1795. [DOI] [PubMed] [Google Scholar]
- Duan J., Moffat M. P. Potential cellular mechanisms of hydrogen peroxide-induced cardiac arrhythmias. J Cardiovasc Pharmacol. 1992 Apr;19(4):593–601. doi: 10.1097/00005344-199204000-00017. [DOI] [PubMed] [Google Scholar]
- Duprat F., Guillemare E., Romey G., Fink M., Lesage F., Lazdunski M., Honore E. Susceptibility of cloned K+ channels to reactive oxygen species. Proc Natl Acad Sci U S A. 1995 Dec 5;92(25):11796–11800. doi: 10.1073/pnas.92.25.11796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari R. Oxygen-free radicals at myocardial level: effects of ischaemia and reperfusion. Adv Exp Med Biol. 1994;366:99–111. doi: 10.1007/978-1-4615-1833-4_8. [DOI] [PubMed] [Google Scholar]
- Giles W. R., Imaizumi Y. Comparison of potassium currents in rabbit atrial and ventricular cells. J Physiol. 1988 Nov;405:123–145. doi: 10.1113/jphysiol.1988.sp017325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldhaber J. I., Ji S., Lamp S. T., Weiss J. N. Effects of exogenous free radicals on electromechanical function and metabolism in isolated rabbit and guinea pig ventricle. Implications for ischemia and reperfusion injury. J Clin Invest. 1989 Jun;83(6):1800–1809. doi: 10.1172/JCI114085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldhaber J. I., Liu E. Excitation-contraction coupling in single guinea-pig ventricular myocytes exposed to hydrogen peroxide. J Physiol. 1994 May 15;477(Pt 1):135–147. doi: 10.1113/jphysiol.1994.sp020178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopalakrishna R., Anderson W. B. Ca2+- and phospholipid-independent activation of protein kinase C by selective oxidative modification of the regulatory domain. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6758–6762. doi: 10.1073/pnas.86.17.6758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanck D. A., Sheets M. F. Modification of inactivation in cardiac sodium channels: ionic current studies with Anthopleurin-A toxin. J Gen Physiol. 1995 Oct;106(4):601–616. doi: 10.1085/jgp.106.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyslop P. A., Hinshaw D. B., Schraufstätter I. U., Sklar L. A., Spragg R. G., Cochrane C. G. Intracellular calcium homeostasis during hydrogen peroxide injury to cultured P388D1 cells. J Cell Physiol. 1986 Dec;129(3):356–366. doi: 10.1002/jcp.1041290314. [DOI] [PubMed] [Google Scholar]
- Larsson R., Cerutti P. Translocation and enhancement of phosphotransferase activity of protein kinase C following exposure in mouse epidermal cells to oxidants. Cancer Res. 1989 Oct 15;49(20):5627–5632. [PubMed] [Google Scholar]
- Manning A. S., Hearse D. J. Reperfusion-induced arrhythmias: mechanisms and prevention. J Mol Cell Cardiol. 1984 Jun;16(6):497–518. doi: 10.1016/s0022-2828(84)80638-0. [DOI] [PubMed] [Google Scholar]
- Matsuura H., Shattock M. J. Effects of oxidant stress on steady-state background currents in isolated ventricular myocytes. Am J Physiol. 1991 Nov;261(5 Pt 2):H1358–H1365. doi: 10.1152/ajpheart.1991.261.5.H1358. [DOI] [PubMed] [Google Scholar]
- Mekhfi H., Veksler V., Mateo P., Maupoil V., Rochette L., Ventura-Clapier R. Creatine kinase is the main target of reactive oxygen species in cardiac myofibrils. Circ Res. 1996 Jun;78(6):1016–1027. doi: 10.1161/01.res.78.6.1016. [DOI] [PubMed] [Google Scholar]
- Mitchell M. R., Powell T., Terrar D. A., Twist V. W. Strontium, nifedipine and 4-aminopyridine modify the time course of the action potential in cells from rat ventricular muscle. Br J Pharmacol. 1984 Mar;81(3):551–556. doi: 10.1111/j.1476-5381.1984.tb10108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muid R. E., Dale M. M., Davis P. D., Elliott L. H., Hill C. H., Kumar H., Lawton G., Twomey B. M., Wadsworth J., Wilkinson S. E. A novel conformationally restricted protein kinase C inhibitor, Ro 31-8425, inhibits human neutrophil superoxide generation by soluble, particulate and post-receptor stimuli. FEBS Lett. 1991 Nov 18;293(1-2):169–172. doi: 10.1016/0014-5793(91)81178-b. [DOI] [PubMed] [Google Scholar]
- Natarajan V., Taher M. M., Roehm B., Parinandi N. L., Schmid H. H., Kiss Z., Garcia J. G. Activation of endothelial cell phospholipase D by hydrogen peroxide and fatty acid hydroperoxide. J Biol Chem. 1993 Jan 15;268(2):930–937. [PubMed] [Google Scholar]
- Nishizuka Y. Protein kinase C and lipid signaling for sustained cellular responses. FASEB J. 1995 Apr;9(7):484–496. [PubMed] [Google Scholar]
- Palumbo E. J., Sweatt J. D., Chen S. J., Klann E. Oxidation-induced persistent activation of protein kinase C in hippocampal homogenates. Biochem Biophys Res Commun. 1992 Sep 30;187(3):1439–1445. doi: 10.1016/0006-291x(92)90463-u. [DOI] [PubMed] [Google Scholar]
- Qu Y., Rogers J. C., Tanada T. N., Catterall W. A., Scheuer T. Phosphorylation of S1505 in the cardiac Na+ channel inactivation gate is required for modulation by protein kinase C. J Gen Physiol. 1996 Nov;108(5):375–379. doi: 10.1085/jgp.108.5.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu Y., Rogers J., Tanada T., Scheuer T., Catterall W. A. Modulation of cardiac Na+ channels expressed in a mammalian cell line and in ventricular myocytes by protein kinase C. Proc Natl Acad Sci U S A. 1994 Apr 12;91(8):3289–3293. doi: 10.1073/pnas.91.8.3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin R., Farber J. L. Mechanisms of the killing of cultured hepatocytes by hydrogen peroxide. Arch Biochem Biophys. 1984 Feb 1;228(2):450–459. doi: 10.1016/0003-9861(84)90010-9. [DOI] [PubMed] [Google Scholar]
- Satin J., Kyle J. W., Chen M., Bell P., Cribbs L. L., Fozzard H. A., Rogart R. B. A mutant of TTX-resistant cardiac sodium channels with TTX-sensitive properties. Science. 1992 May 22;256(5060):1202–1205. doi: 10.1126/science.256.5060.1202. [DOI] [PubMed] [Google Scholar]
- Sato N., Nishimura M., Tanaka H., Homma N., Watanabe Y. Augmentation and subsequent attenuation of Ca2+ current due to lipid peroxidation of the membrane caused by t-butyl hydroperoxide in the rabbit sinoatrial node. Br J Pharmacol. 1989 Nov;98(3):721–723. doi: 10.1111/j.1476-5381.1989.tb14598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steadman B. W., Moore K. B., Spitzer K. W., Bridge J. H. A video system for measuring motion in contracting heart cells. IEEE Trans Biomed Eng. 1988 Apr;35(4):264–272. doi: 10.1109/10.1375. [DOI] [PubMed] [Google Scholar]
- Tarr M. T., Valenzeno D. P. Modification of cardiac action potential by photosensitizer-generated reactive oxygen. J Mol Cell Cardiol. 1989 Jun;21(6):539–543. doi: 10.1016/0022-2828(89)90819-5. [DOI] [PubMed] [Google Scholar]
- Tarr M., Valenzeno D. P. Modification of cardiac ionic currents by photosensitizer-generated reactive oxygen. J Mol Cell Cardiol. 1991 May;23(5):639–649. doi: 10.1016/0022-2828(91)90055-q. [DOI] [PubMed] [Google Scholar]
- Vega-Saenz de Miera E., Rudy B. Modulation of K+ channels by hydrogen peroxide. Biochem Biophys Res Commun. 1992 Aug 14;186(3):1681–1687. doi: 10.1016/s0006-291x(05)81602-x. [DOI] [PubMed] [Google Scholar]
- Ward C. A., Moffat M. P. Role of protein kinase C in mediating effects of hydrogen peroxide in guinea-pig ventricular myocytes. J Mol Cell Cardiol. 1995 Apr;27(4):1089–1097. doi: 10.1016/0022-2828(95)90077-2. [DOI] [PubMed] [Google Scholar]
- Zweier J. L., Flaherty J. T., Weisfeldt M. L. Direct measurement of free radical generation following reperfusion of ischemic myocardium. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1404–1407. doi: 10.1073/pnas.84.5.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]


