Abstract
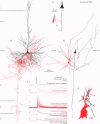
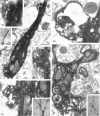
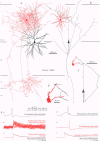
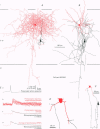
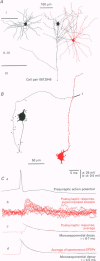
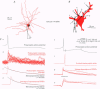
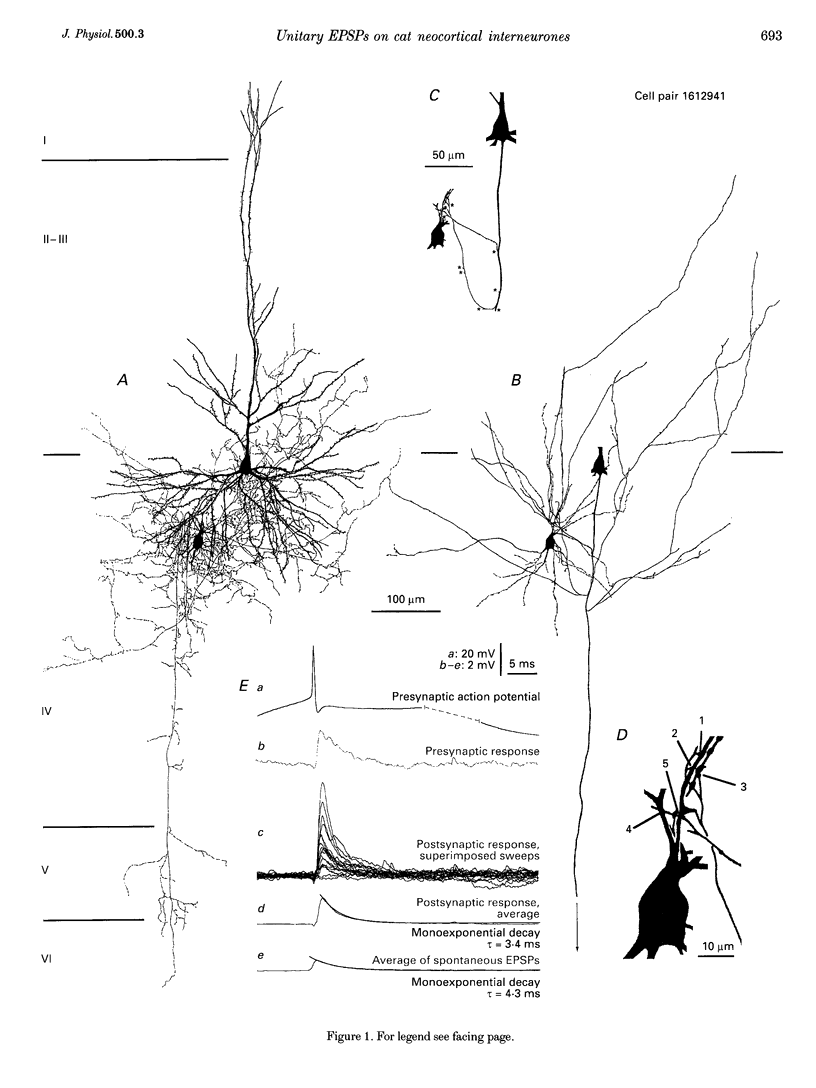
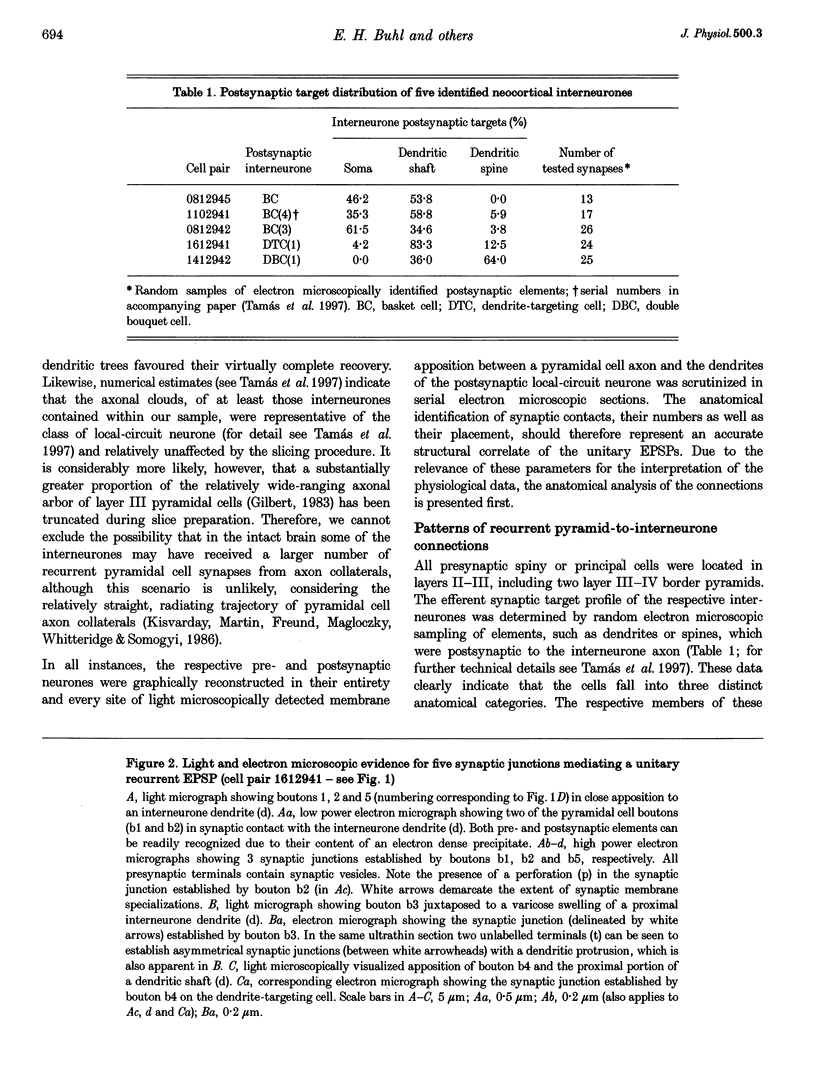
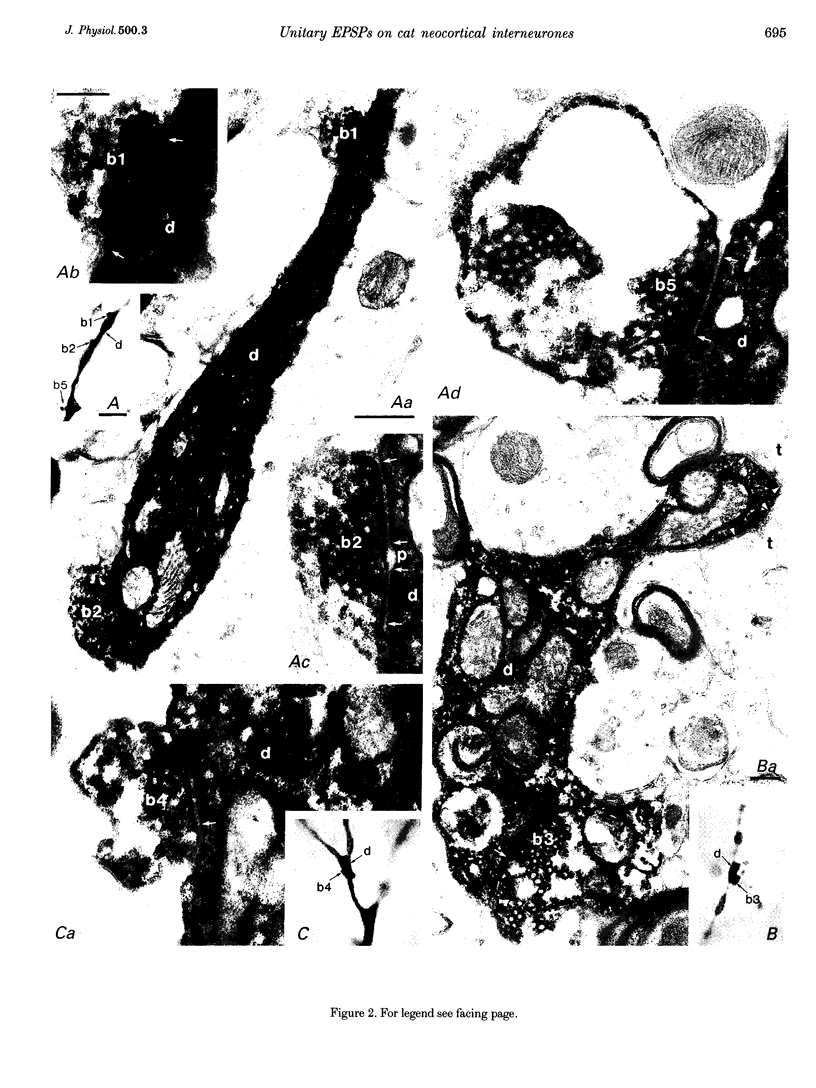
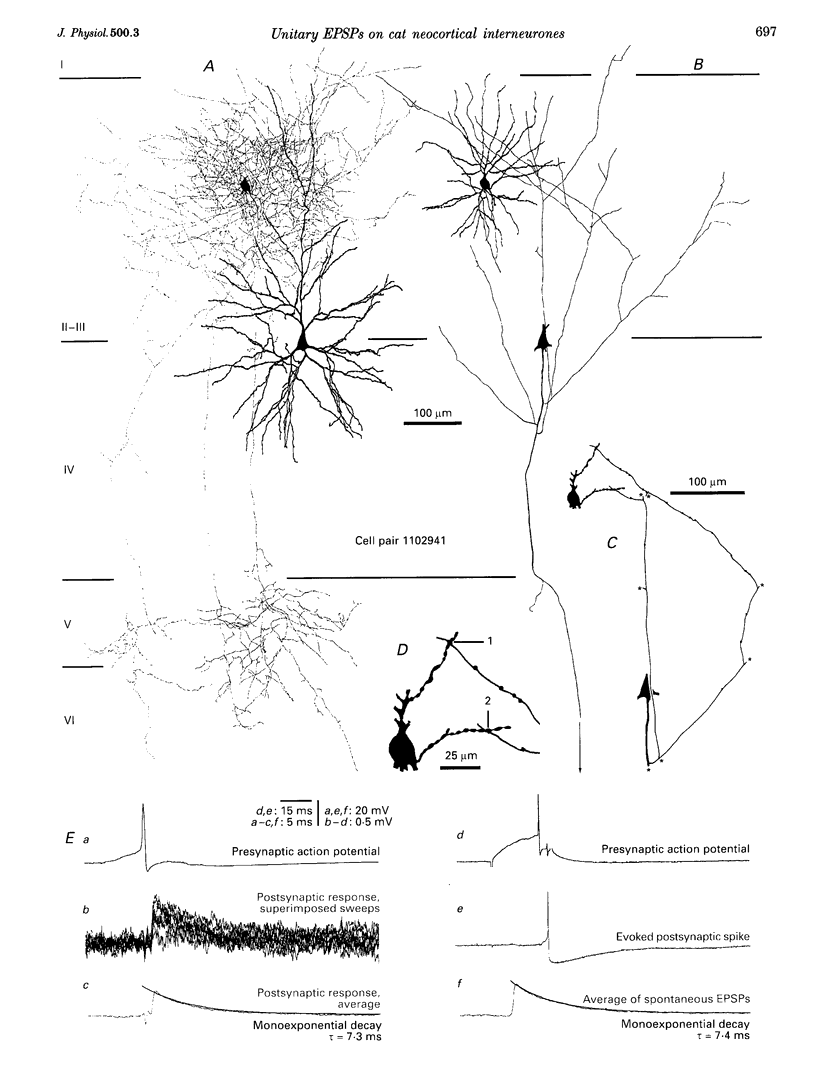
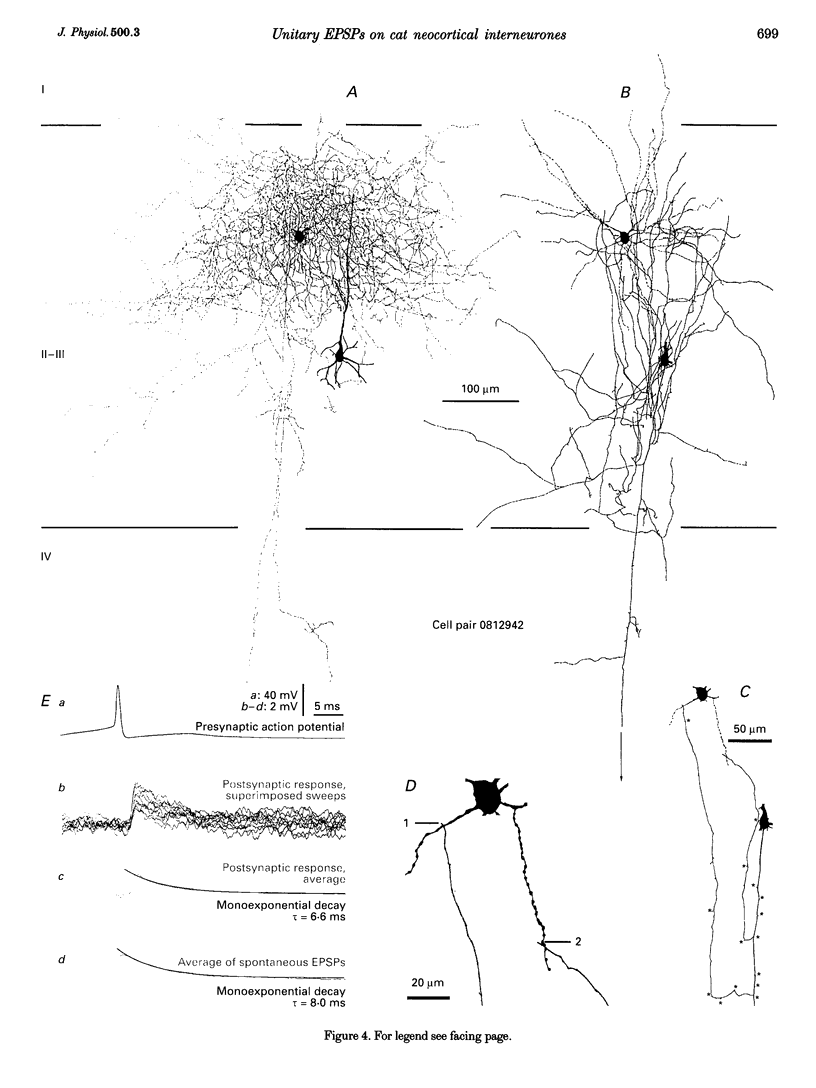
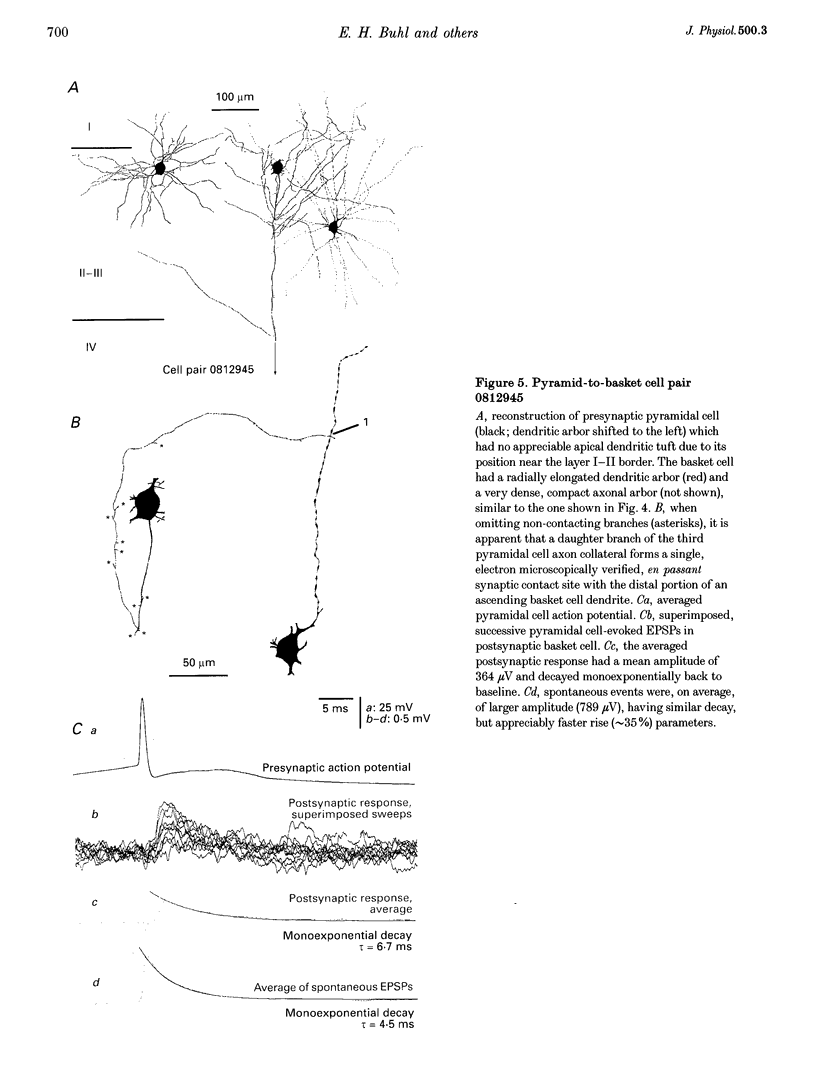
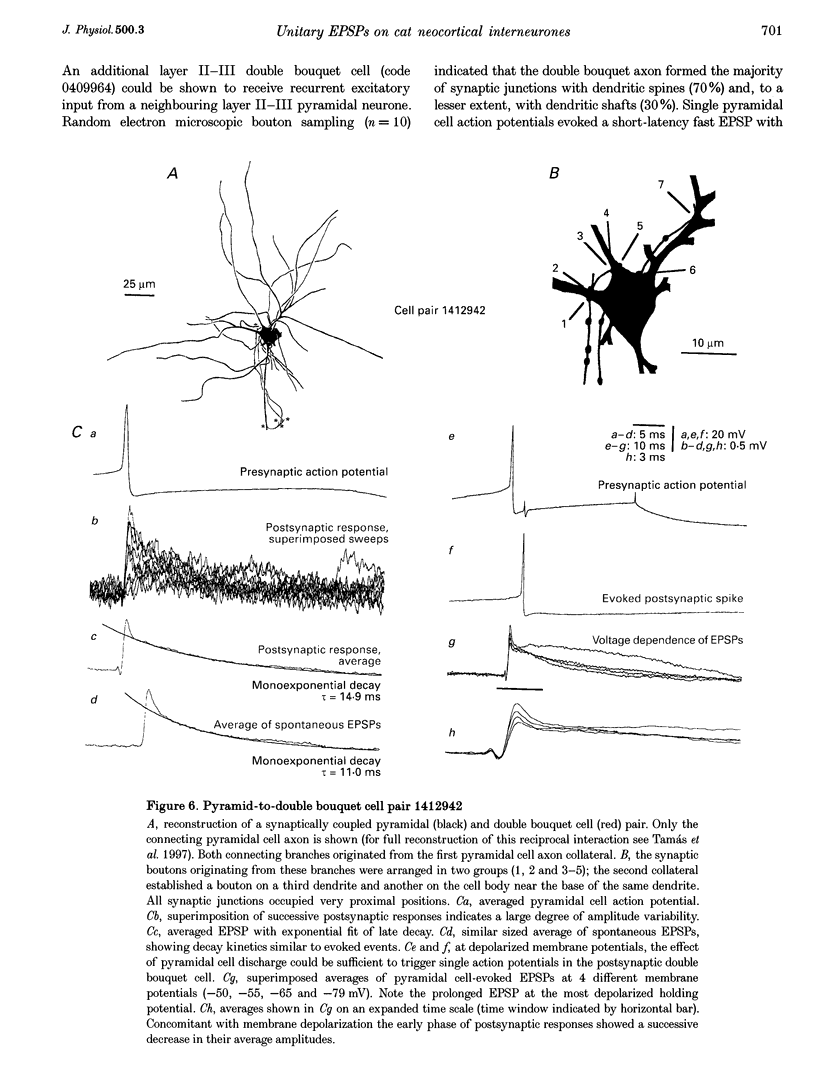
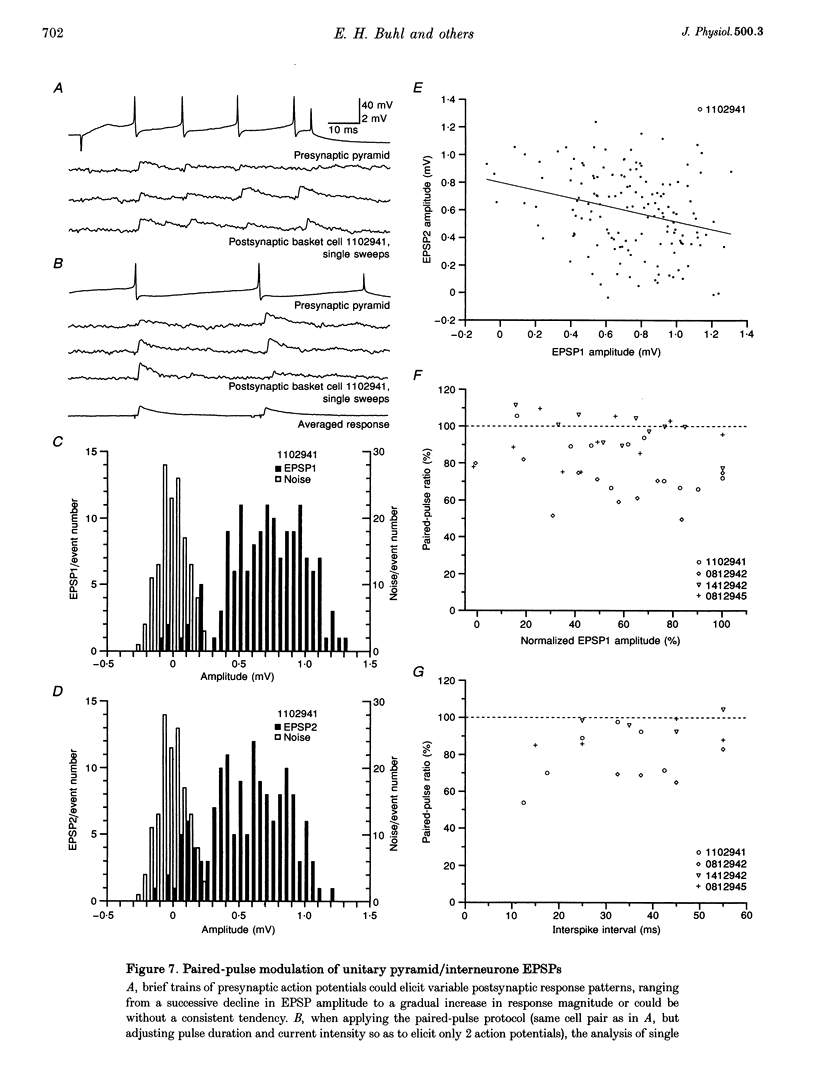
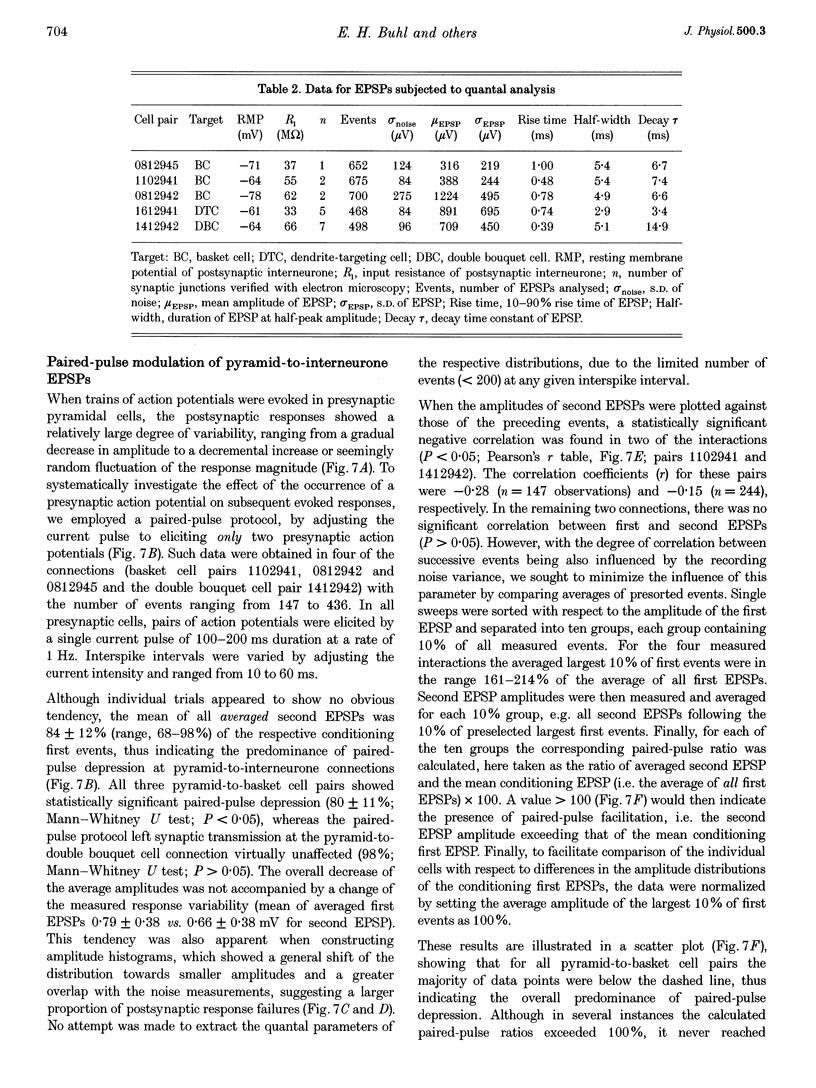
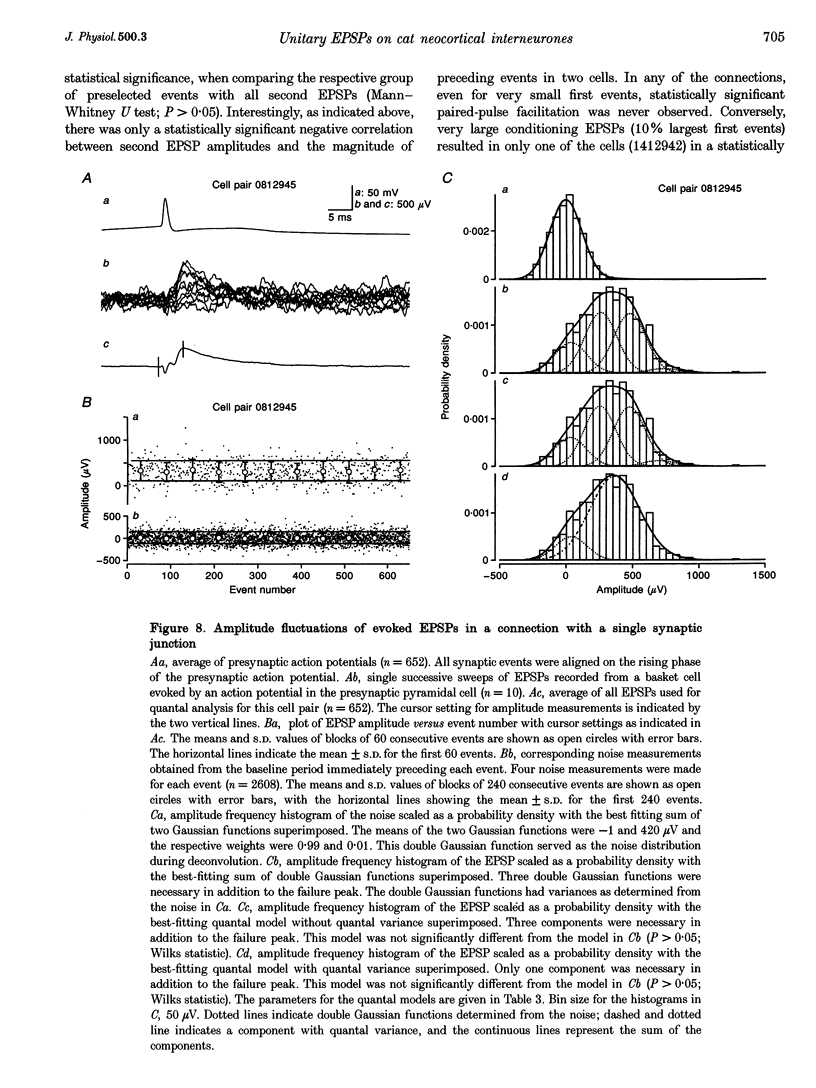
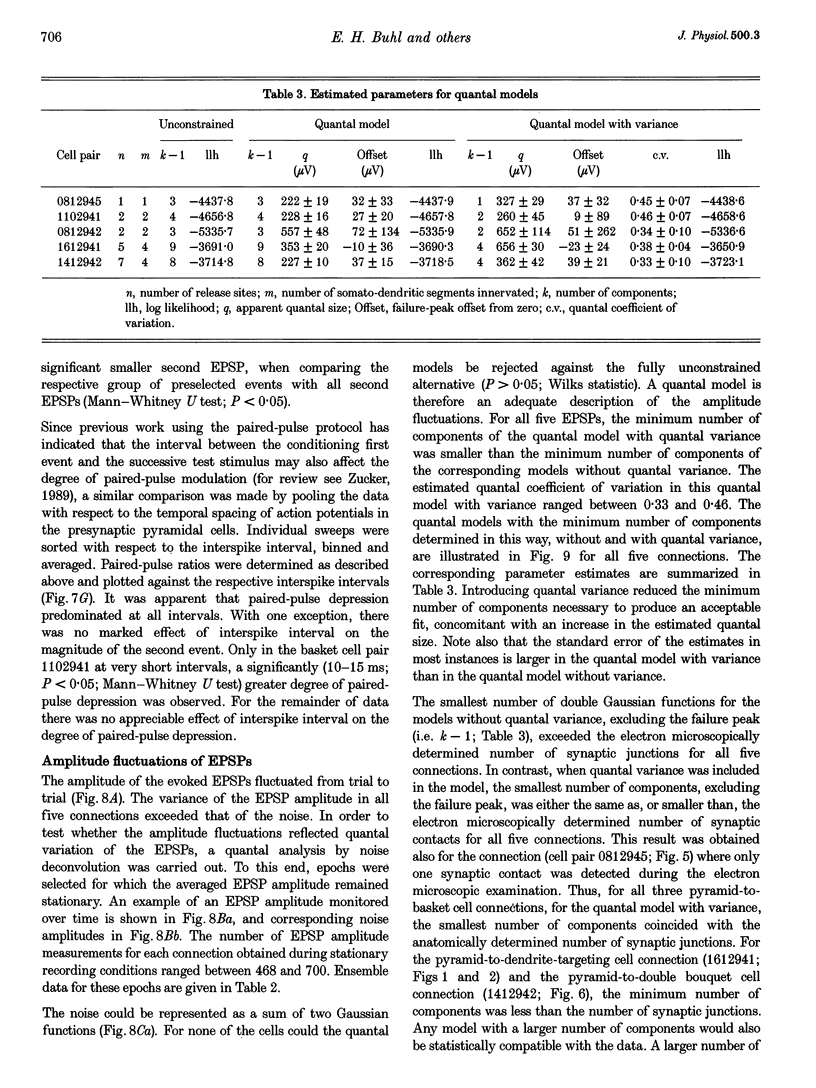
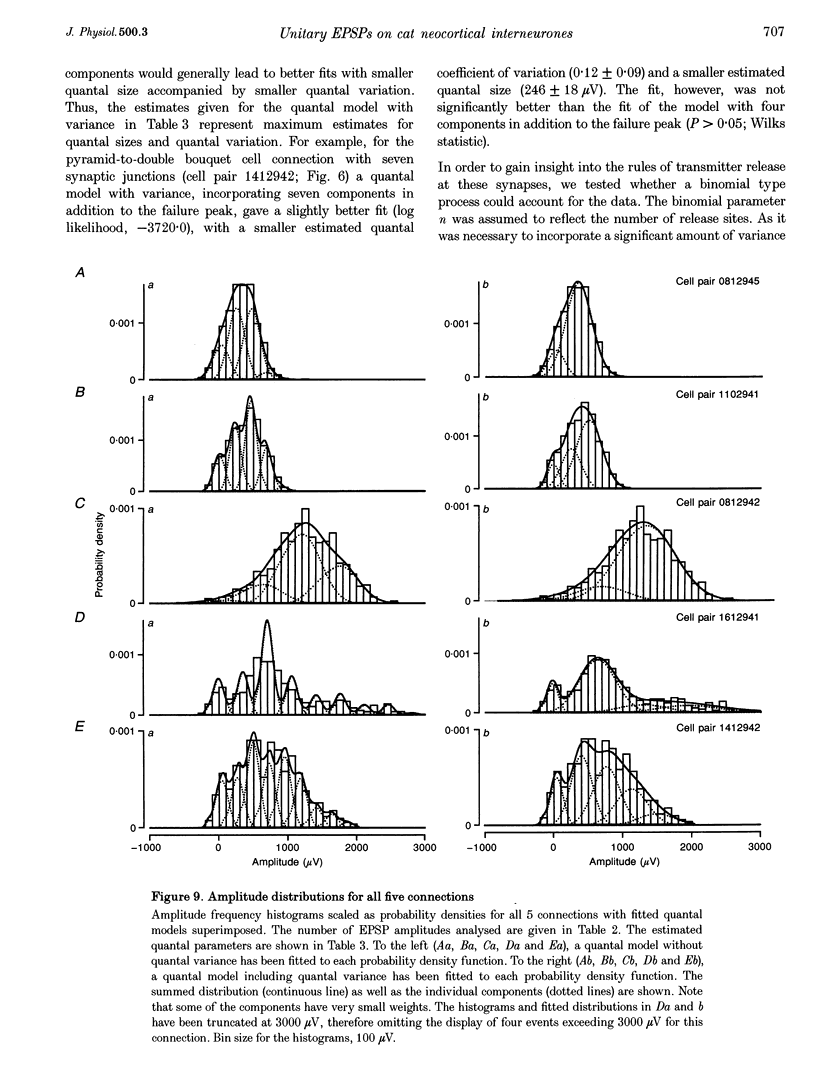
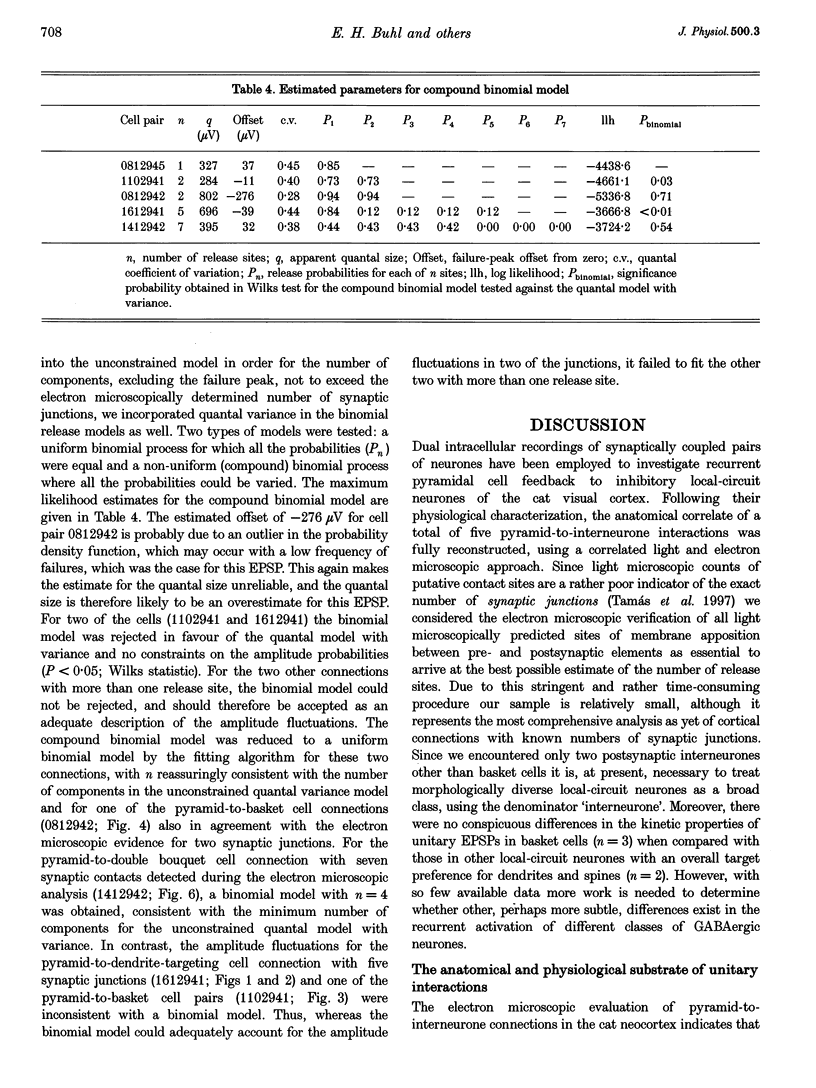
1. Dual intracellular recordings were made from synaptically coupled pyramidal cell-to-interneurone pairs (n = 5) of the cat visual cortex in vitro. Pre- and postsynaptic neurones were labelled with biocytin, followed by correlated light and electron microscopic analysis to determine all sites of synaptic interaction. 2. Pyramidal neurones in layers II-III elicited monosynaptic EPSPs in three distinct classes of smooth dendritic local-circuit neurones, namely basket cells (n = 3), a dendrite-targeting cell (n = 1) and a double bouquet cell (n = 1). Unitary EPSPs in basket cells were mediated by one, two, and two synaptic junctions, whereas the pyramid-to-dendrite-targeting cell and pyramid-to-double bouquet cell interaction were mediated by five and seven synaptic junctions, respectively. Recurrent synaptic junctions were found on all somato-dendritic compartments, with a tendency to be clustered close to the soma on the double bouquet and dendrite-targeting cells. The latter interneurones were reciprocally connected with pyramidal cells. 3. Unitary EPSPs had an average peak amplitude of 1005 +/- 518 microV, fast rise times (10-90%; 0.67 +/- 0.25 ms) and were of short duration (at half-amplitude, 4.7 +/- 1.0 ms). Their decay was monoexponential (tau = 7.8 +/- 4.3 ms) at hyperpolarized membrane potentials and appeared to be shaped by passive membrane properties (tau = 9.2 +/- 8.5 ms). All parameters of concomitantly recorded spontaneous EPSPs were remarkably similar (mean amplitude, 981 +/- 433 microV; mean rise time, 0.68 +/- 0.18 ms; mean duration, 4.7 +/- 1.7 ms). 4. In all three pyramidal-to-basket cell pairs, closely timed (10-50 ms) pairs of presynaptic action potentials resulted in statistically significant paired-pulse depression, the mean of the averaged second EPSPs being 80 +/- 11% of the averaged conditioning event. The overall degree of paired-pulse modulation was relatively little affected by either the amplitude of the preceding event or the inter-event interval. 5. The probability density function of the peak amplitudes of the unitary EPSPs could be adequately fitted with a quantal model. Without quantal variance, however, the minimum number of components in the model, excluding the failures, exceeded the number of electron microscopically determined synaptic junctions for all five connections. In contrast, incorporating quantal variance gave a minimum number of components which was compatible with the number of synaptic junctions, and which fitted the data equally well as models incorporating additional components but no quantal variance. For this model with quantal variance with the minimum number of components the estimate of the quantal coefficient of variation ranged between 0.33 and 0.46, and the corresponding quantal sizes ranged between 260 and 657 microV. The peak EPSP amplitudes in two of the four connections with more than one synaptic junction could be adequately described by a uniform binomial model for transmitter release. 6. In conclusion, at least three distinct interneurone classes receive local excitatory pyramidal cell input which they relay to different compartments on their postsynaptic target neurones. The reliability of transmission is high, but the fast time course of the EPSPs constrains their temporal summation. Due to the relatively small amplitude of unitary EPSPs several convergent inputs will therefore be required to elicit suprathreshold responses.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baude A., Nusser Z., Molnár E., McIlhinney R. A., Somogyi P. High-resolution immunogold localization of AMPA type glutamate receptor subunits at synaptic and non-synaptic sites in rat hippocampus. Neuroscience. 1995 Dec;69(4):1031–1055. doi: 10.1016/0306-4522(95)00350-r. [DOI] [PubMed] [Google Scholar]
- Bernander O., Douglas R. J., Martin K. A., Koch C. Synaptic background activity influences spatiotemporal integration in single pyramidal cells. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11569–11573. doi: 10.1073/pnas.88.24.11569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhl E. H., Halasy K., Somogyi P. Diverse sources of hippocampal unitary inhibitory postsynaptic potentials and the number of synaptic release sites. Nature. 1994 Apr 28;368(6474):823–828. doi: 10.1038/368823a0. [DOI] [PubMed] [Google Scholar]
- Cobb S. R., Buhl E. H., Halasy K., Paulsen O., Somogyi P. Synchronization of neuronal activity in hippocampus by individual GABAergic interneurons. Nature. 1995 Nov 2;378(6552):75–78. doi: 10.1038/378075a0. [DOI] [PubMed] [Google Scholar]
- Deuchars J., Thomson A. M. Innervation of burst firing spiny interneurons by pyramidal cells in deep layers of rat somatomotor cortex: paired intracellular recordings with biocytin filling. Neuroscience. 1995 Dec;69(3):739–755. doi: 10.1016/0306-4522(95)00288-t. [DOI] [PubMed] [Google Scholar]
- Erwin E., Obermayer K., Schulten K. Models of orientation and ocular dominance columns in the visual cortex: a critical comparison. Neural Comput. 1995 May;7(3):425–468. doi: 10.1162/neco.1995.7.3.425. [DOI] [PubMed] [Google Scholar]
- Eysel U. T., Crook J. M., Machemer H. F. GABA-induced remote inactivation reveals cross-orientation inhibition in the cat striate cortex. Exp Brain Res. 1990;80(3):626–630. doi: 10.1007/BF00228003. [DOI] [PubMed] [Google Scholar]
- Gilbert C. D. Microcircuitry of the visual cortex. Annu Rev Neurosci. 1983;6:217–247. doi: 10.1146/annurev.ne.06.030183.001245. [DOI] [PubMed] [Google Scholar]
- Gulyás A. I., Miles R., Sík A., Tóth K., Tamamaki N., Freund T. F. Hippocampal pyramidal cells excite inhibitory neurons through a single release site. Nature. 1993 Dec 16;366(6456):683–687. doi: 10.1038/366683a0. [DOI] [PubMed] [Google Scholar]
- Han Z. S., Buhl E. H., Lörinczi Z., Somogyi P. A high degree of spatial selectivity in the axonal and dendritic domains of physiologically identified local-circuit neurons in the dentate gyrus of the rat hippocampus. Eur J Neurosci. 1993 May 1;5(5):395–410. doi: 10.1111/j.1460-9568.1993.tb00507.x. [DOI] [PubMed] [Google Scholar]
- Jack J. J., Redman S. J., Wong K. The components of synaptic potentials evoked in cat spinal motoneurones by impulses in single group Ia afferents. J Physiol. 1981 Dec;321:65–96. doi: 10.1113/jphysiol.1981.sp013972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisvárday Z. F., Martin K. A., Freund T. F., Maglóczky Z., Whitteridge D., Somogyi P. Synaptic targets of HRP-filled layer III pyramidal cells in the cat striate cortex. Exp Brain Res. 1986;64(3):541–552. doi: 10.1007/BF00340492. [DOI] [PubMed] [Google Scholar]
- Kisvárday Z. F., Martin K. A., Whitteridge D., Somogyi P. Synaptic connections of intracellularly filled clutch cells: a type of small basket cell in the visual cortex of the cat. J Comp Neurol. 1985 Nov 8;241(2):111–137. doi: 10.1002/cne.902410202. [DOI] [PubMed] [Google Scholar]
- Koh D. S., Geiger J. R., Jonas P., Sakmann B. Ca(2+)-permeable AMPA and NMDA receptor channels in basket cells of rat hippocampal dentate gyrus. J Physiol. 1995 Jun 1;485(Pt 2):383–402. doi: 10.1113/jphysiol.1995.sp020737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- König P., Engel A. K., Singer W. Integrator or coincidence detector? The role of the cortical neuron revisited. Trends Neurosci. 1996 Apr;19(4):130–137. doi: 10.1016/s0166-2236(96)80019-1. [DOI] [PubMed] [Google Scholar]
- Maccaferri G., McBain C. J. Passive propagation of LTD to stratum oriens-alveus inhibitory neurons modulates the temporoammonic input to the hippocampal CA1 region. Neuron. 1995 Jul;15(1):137–145. doi: 10.1016/0896-6273(95)90071-3. [DOI] [PubMed] [Google Scholar]
- Mason A., Nicoll A., Stratford K. Synaptic transmission between individual pyramidal neurons of the rat visual cortex in vitro. J Neurosci. 1991 Jan;11(1):72–84. doi: 10.1523/JNEUROSCI.11-01-00072.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redman S. Quantal analysis of synaptic potentials in neurons of the central nervous system. Physiol Rev. 1990 Jan;70(1):165–198. doi: 10.1152/physrev.1990.70.1.165. [DOI] [PubMed] [Google Scholar]
- Sillito A. M. GABA mediated inhibitory processes in the function of the geniculo-striate system. Prog Brain Res. 1992;90:349–384. doi: 10.1016/s0079-6123(08)63622-5. [DOI] [PubMed] [Google Scholar]
- Singer W., Gray C. M. Visual feature integration and the temporal correlation hypothesis. Annu Rev Neurosci. 1995;18:555–586. doi: 10.1146/annurev.ne.18.030195.003011. [DOI] [PubMed] [Google Scholar]
- Softky W. Sub-millisecond coincidence detection in active dendritic trees. Neuroscience. 1994 Jan;58(1):13–41. doi: 10.1016/0306-4522(94)90154-6. [DOI] [PubMed] [Google Scholar]
- Spruston N., Jaffe D. B., Johnston D. Dendritic attenuation of synaptic potentials and currents: the role of passive membrane properties. Trends Neurosci. 1994 Apr;17(4):161–166. doi: 10.1016/0166-2236(94)90094-9. [DOI] [PubMed] [Google Scholar]
- Stern P., Edwards F. A., Sakmann B. Fast and slow components of unitary EPSCs on stellate cells elicited by focal stimulation in slices of rat visual cortex. J Physiol. 1992 Apr;449:247–278. doi: 10.1113/jphysiol.1992.sp019085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stricker C., Field A. C., Redman S. J. Statistical analysis of amplitude fluctuations in EPSCs evoked in rat CA1 pyramidal neurones in vitro. J Physiol. 1996 Jan 15;490(Pt 2):419–441. doi: 10.1113/jphysiol.1996.sp021155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stricker C., Redman S., Daley D. Statistical analysis of synaptic transmission: model discrimination and confidence limits. Biophys J. 1994 Aug;67(2):532–547. doi: 10.1016/S0006-3495(94)80513-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamás G., Buhl E. H., Somogyi P. Fast IPSPs elicited via multiple synaptic release sites by different types of GABAergic neurone in the cat visual cortex. J Physiol. 1997 May 1;500(Pt 3):715–738. doi: 10.1113/jphysiol.1997.sp022054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson A. M., Deuchars J., West D. C. Large, deep layer pyramid-pyramid single axon EPSPs in slices of rat motor cortex display paired pulse and frequency-dependent depression, mediated presynaptically and self-facilitation, mediated postsynaptically. J Neurophysiol. 1993 Dec;70(6):2354–2369. doi: 10.1152/jn.1993.70.6.2354. [DOI] [PubMed] [Google Scholar]
- Thomson A. M., Deuchars J., West D. C. Single axon excitatory postsynaptic potentials in neocortical interneurons exhibit pronounced paired pulse facilitation. Neuroscience. 1993 May;54(2):347–360. doi: 10.1016/0306-4522(93)90257-g. [DOI] [PubMed] [Google Scholar]
- Thomson A. M., West D. C., Deuchars J. Properties of single axon excitatory postsynaptic potentials elicited in spiny interneurons by action potentials in pyramidal neurons in slices of rat neocortex. Neuroscience. 1995 Dec;69(3):727–738. doi: 10.1016/0306-4522(95)00287-s. [DOI] [PubMed] [Google Scholar]
- Thurbon D., Field A., Redman S. Electrotonic profiles of interneurons in stratum pyramidale of the CA1 region of rat hippocampus. J Neurophysiol. 1994 May;71(5):1948–1958. doi: 10.1152/jn.1994.71.5.1948. [DOI] [PubMed] [Google Scholar]
- Yuste R., Tank D. W. Dendritic integration in mammalian neurons, a century after Cajal. Neuron. 1996 Apr;16(4):701–716. doi: 10.1016/s0896-6273(00)80091-4. [DOI] [PubMed] [Google Scholar]
- Zucker R. S. Short-term synaptic plasticity. Annu Rev Neurosci. 1989;12:13–31. doi: 10.1146/annurev.ne.12.030189.000305. [DOI] [PubMed] [Google Scholar]