Abstract
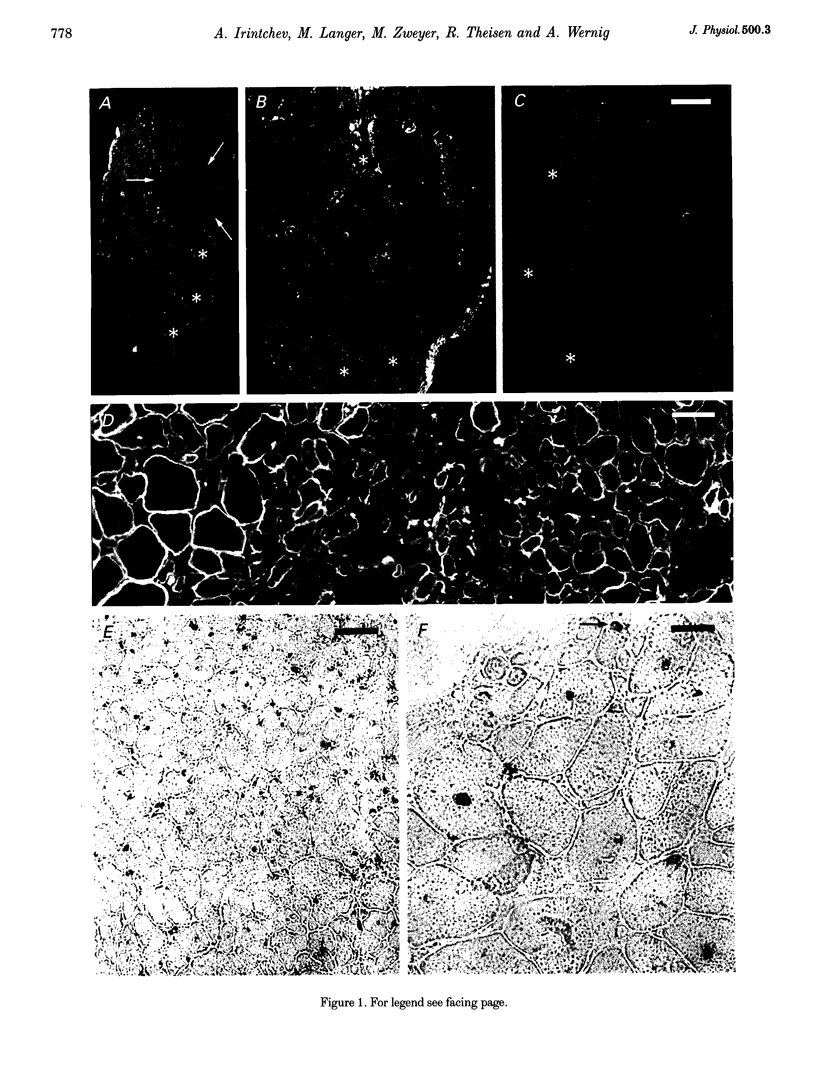
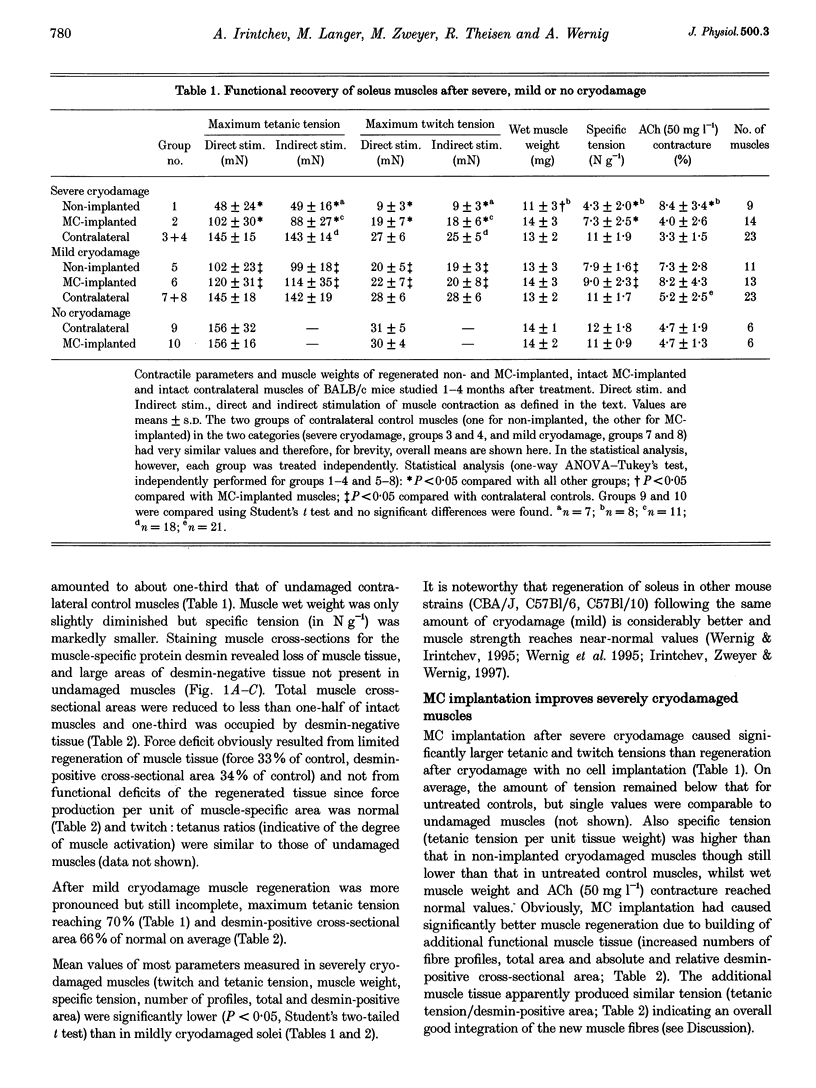
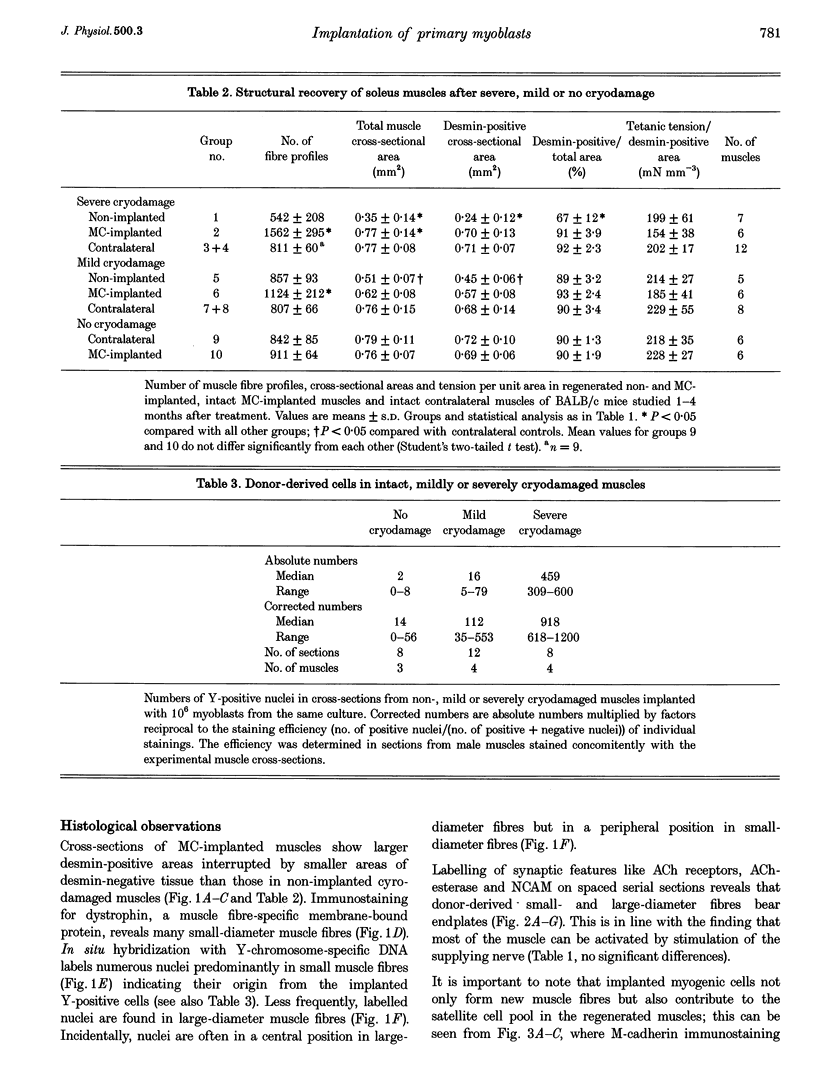
1. Myoblasts from expanded primary cultures were implanted into cryodamaged soleus muscles of adult BALB/c mice. One to four months later isometric tension recordings were performed in vitro, and the male donor cells implanted into female hosts were traced on histological sections using a Y-chromosome-specific probe. The muscles were either mildly or severely cryodamaged, which led to reductions in tetanic muscle force to 33% (n = 9 muscles, 9 animals) and 70% (n = 11) of normal, respectively. Reduced forces resulted from deficits in regeneration of muscle tissue as judged from the reduced desmin-positive cross-sectional areas (34 and 66% of control, respectively). 2. Implantation of 10(6) myogenic cells into severely cryodamaged muscles more than doubled muscle tetanic force (to 70% of normal, n = 14), as well as specific force (to 66% of normal). Absolute and relative amount of desmin-positive muscle cross-sectional areas were significantly increased indicating improved microarchitecture and less fibrosis. Newly formed muscle tissue was fully innervated since the tetanic forces resulting from direct and indirect (nerve-evoked) stimulation were equal. Endplates were found on numerous Y-positive muscle fibres. 3. As judged from their position under basal laminae of muscle fibres and the expression of M-cadherin, donor-derived cells contributed to the pool of satellite cells on small- and large-diameter muscle fibres. 4. Myoblast implantation after mild cryodamage and in undamaged muscles had little or no functional or structural effects; in both preparations only a few Y-positive muscle nuclei were detected. It is concluded that myoblasts from expanded primary cultures-unlike permanent cell lines-significantly contribute to muscle regeneration only when previous muscle damage is extensive and loss of host satellite cells is severe.
Full text
PDF



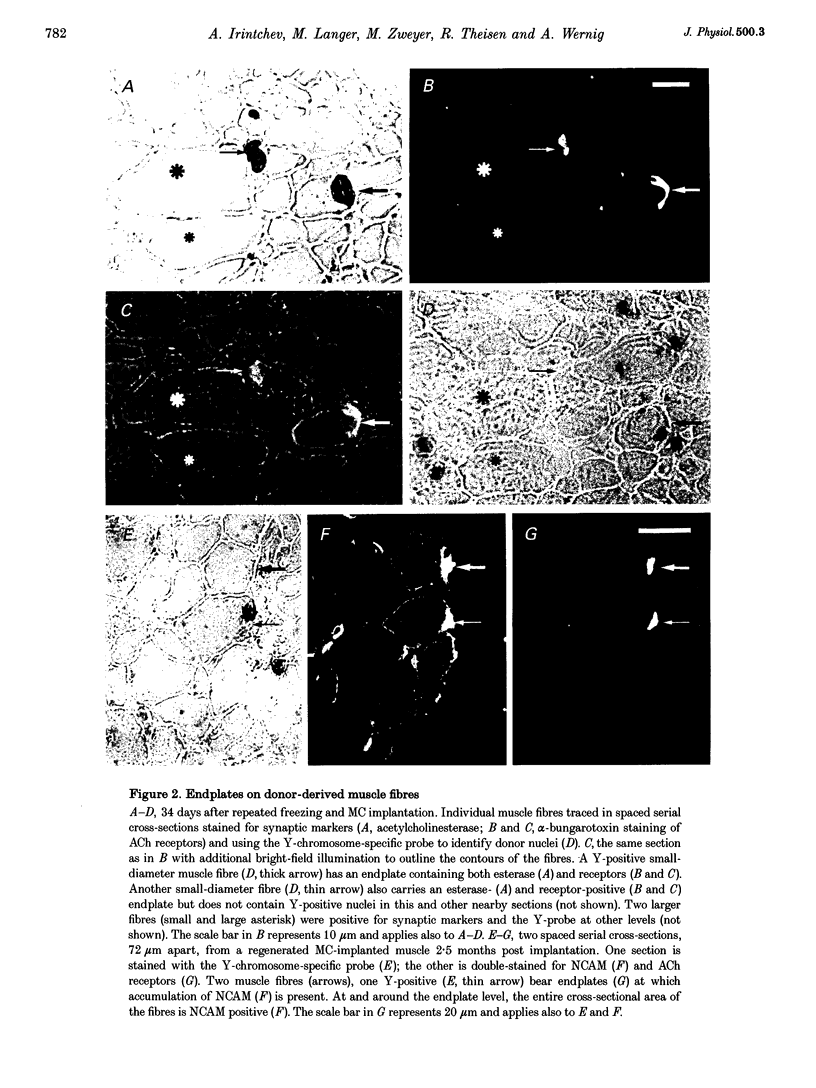



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alameddine H. S., Louboutin J. P., Dehaupas M., Sébille A., Fardeau M. Functional recovery induced by satellite cell grafts in irreversibly injured muscles. Cell Transplant. 1994 Jan-Feb;3(1):3–14. doi: 10.1177/096368979400300103. [DOI] [PubMed] [Google Scholar]
- Baroffio A., Aubry J. P., Kaelin A., Krause R. M., Hamann M., Bader C. R. Purification of human muscle satellite cells by flow cytometry. Muscle Nerve. 1993 May;16(5):498–505. doi: 10.1002/mus.880160511. [DOI] [PubMed] [Google Scholar]
- Brooks S. V., Faulkner J. A. Contractile properties of skeletal muscles from young, adult and aged mice. J Physiol. 1988 Oct;404:71–82. doi: 10.1113/jphysiol.1988.sp017279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantini M., Massimino M. L., Bruson A., Catani C., Dalla Libera L., Carraro U. Macrophages regulate proliferation and differentiation of satellite cells. Biochem Biophys Res Commun. 1994 Aug 15;202(3):1688–1696. doi: 10.1006/bbrc.1994.2129. [DOI] [PubMed] [Google Scholar]
- Carlson B. M. The regeneration of minced muscles. Monogr Dev Biol. 1972;4:1–128. [PubMed] [Google Scholar]
- Clarke M. S., Khakee R., McNeil P. L. Loss of cytoplasmic basic fibroblast growth factor from physiologically wounded myofibers of normal and dystrophic muscle. J Cell Sci. 1993 Sep;106(Pt 1):121–133. doi: 10.1242/jcs.106.1.121. [DOI] [PubMed] [Google Scholar]
- Fan Y., Beilharz M. W., Grounds M. D. A potential alternative strategy for myoblast transfer therapy: the use of sliced muscle grafts. Cell Transplant. 1996 May-Jun;5(3):421–429. doi: 10.1177/096368979600500309. [DOI] [PubMed] [Google Scholar]
- Grounds M. D., Lai M. C., Fan Y., Codling J. C., Beilharz M. W. Transplantation in the mouse model--the use of a Y-chromosome-specific DNA clone to identify donor cells in situ. Transplantation. 1991 Dec;52(6):1101–1105. [PubMed] [Google Scholar]
- Gussoni E., Pavlath G. K., Lanctot A. M., Sharma K. R., Miller R. G., Steinman L., Blau H. M. Normal dystrophin transcripts detected in Duchenne muscular dystrophy patients after myoblast transplantation. Nature. 1992 Apr 2;356(6368):435–438. doi: 10.1038/356435a0. [DOI] [PubMed] [Google Scholar]
- Hirn M., Pierres M., Deagostini-Bazin H., Hirsch M., Goridis C. Monoclonal antibody against cell surface glycoprotein of neurons. Brain Res. 1981 Jun 15;214(2):433–439. doi: 10.1016/0006-8993(81)91208-7. [DOI] [PubMed] [Google Scholar]
- Huard J., Acsadi G., Jani A., Massie B., Karpati G. Gene transfer into skeletal muscles by isogenic myoblasts. Hum Gene Ther. 1994 Aug;5(8):949–958. doi: 10.1089/hum.1994.5.8-949. [DOI] [PubMed] [Google Scholar]
- Huard J., Labrecque C., Dansereau G., Robitaille L., Tremblay J. P. Dystrophin expression in myotubes formed by the fusion of normal and dystrophic myoblasts. Muscle Nerve. 1991 Feb;14(2):178–182. doi: 10.1002/mus.880140213. [DOI] [PubMed] [Google Scholar]
- Huard J., Roy R., Bouchard J. P., Malouin F., Richards C. L., Tremblay J. P. Human myoblast transplantation between immunohistocompatible donors and recipients produces immune reactions. Transplant Proc. 1992 Dec;24(6):3049–3051. [PubMed] [Google Scholar]
- Irintchev A., Draguhn A., Wernig A. Reinnervation and recovery of mouse soleus muscle after long-term denervation. Neuroscience. 1990;39(1):231–243. doi: 10.1016/0306-4522(90)90236-w. [DOI] [PubMed] [Google Scholar]
- Irintchev A., Salvini T. F., Faissner A., Wernig A. Differential expression of tenascin after denervation, damage or paralysis of mouse soleus muscle. J Neurocytol. 1993 Nov;22(11):955–965. doi: 10.1007/BF01218353. [DOI] [PubMed] [Google Scholar]
- Irintchev A., Wernig A. Muscle damage and repair in voluntarily running mice: strain and muscle differences. Cell Tissue Res. 1987 Sep;249(3):509–521. doi: 10.1007/BF00217322. [DOI] [PubMed] [Google Scholar]
- Irintchev A., Zeschnigk M., Starzinski-Powitz A., Wernig A. Expression pattern of M-cadherin in normal, denervated, and regenerating mouse muscles. Dev Dyn. 1994 Apr;199(4):326–337. doi: 10.1002/aja.1001990407. [DOI] [PubMed] [Google Scholar]
- Irintchev A., Zweyer M., Wernig A. Cellular and molecular reactions in mouse muscles after myoblast implantation. J Neurocytol. 1995 Apr;24(4):319–331. doi: 10.1007/BF01186543. [DOI] [PubMed] [Google Scholar]
- Karpati G., Ajdukovic D., Arnold D., Gledhill R. B., Guttmann R., Holland P., Koch P. A., Shoubridge E., Spence D., Vanasse M. Myoblast transfer in Duchenne muscular dystrophy. Ann Neurol. 1993 Jul;34(1):8–17. doi: 10.1002/ana.410340105. [DOI] [PubMed] [Google Scholar]
- Kinoshita I., Vilquin J. T., Tremblay J. P. Pretreatment of myoblast cultures with basic fibroblast growth factor increases the efficacy of their transplantation in mdx mice. Muscle Nerve. 1995 Aug;18(8):834–841. doi: 10.1002/mus.880180806. [DOI] [PubMed] [Google Scholar]
- Law P. K., Bertorini T. E., Goodwin T. G., Chen M., Fang Q. W., Li H. J., Kirby D. S., Florendo J. A., Herrod H. G., Golden G. S. Dystrophin production induced by myoblast transfer therapy in Duchenne muscular dystrophy. Lancet. 1990 Jul 14;336(8707):114–115. doi: 10.1016/0140-6736(90)91628-n. [DOI] [PubMed] [Google Scholar]
- Lefaucheur J. P., Sébille A. Muscle regeneration following injury can be modified in vivo by immune neutralization of basic fibroblast growth factor, transforming growth factor beta 1 or insulin-like growth factor I. J Neuroimmunol. 1995 Mar;57(1-2):85–91. doi: 10.1016/0165-5728(94)00166-l. [DOI] [PubMed] [Google Scholar]
- Leiden J. M. Gene therapy--promise, pitfalls, and prognosis. N Engl J Med. 1995 Sep 28;333(13):871–873. doi: 10.1056/NEJM199509283331310. [DOI] [PubMed] [Google Scholar]
- Morgan J. E., Hoffman E. P., Partridge T. A. Normal myogenic cells from newborn mice restore normal histology to degenerating muscles of the mdx mouse. J Cell Biol. 1990 Dec;111(6 Pt 1):2437–2449. doi: 10.1083/jcb.111.6.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishioka Y. Application of Y chromosomal repetitive sequences to sexing mouse embryos. Teratology. 1988 Aug;38(2):181–185. doi: 10.1002/tera.1420380211. [DOI] [PubMed] [Google Scholar]
- Partridge T. A. Invited review: myoblast transfer: a possible therapy for inherited myopathies? Muscle Nerve. 1991 Mar;14(3):197–212. doi: 10.1002/mus.880140302. [DOI] [PubMed] [Google Scholar]
- Rando T. A., Blau H. M. Primary mouse myoblast purification, characterization, and transplantation for cell-mediated gene therapy. J Cell Biol. 1994 Jun;125(6):1275–1287. doi: 10.1083/jcb.125.6.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richler C., Yaffe D. The in vitro cultivation and differentiation capacities of myogenic cell lines. Dev Biol. 1970 Sep;23(1):1–22. doi: 10.1016/s0012-1606(70)80004-5. [DOI] [PubMed] [Google Scholar]
- Rose O., Rohwedel J., Reinhardt S., Bachmann M., Cramer M., Rotter M., Wobus A., Starzinski-Powitz A. Expression of M-cadherin protein in myogenic cells during prenatal mouse development and differentiation of embryonic stem cells in culture. Dev Dyn. 1994 Nov;201(3):245–259. doi: 10.1002/aja.1002010308. [DOI] [PubMed] [Google Scholar]
- Tremblay J. P., Malouin F., Roy R., Huard J., Bouchard J. P., Satoh A., Richards C. L. Results of a triple blind clinical study of myoblast transplantations without immunosuppressive treatment in young boys with Duchenne muscular dystrophy. Cell Transplant. 1993 Mar-Apr;2(2):99–112. doi: 10.1177/096368979300200203. [DOI] [PubMed] [Google Scholar]
- Watt D. J., Lambert K., Morgan J. E., Partridge T. A., Sloper J. C. Incorporation of donor muscle precursor cells into an area of muscle regeneration in the host mouse. J Neurol Sci. 1982 Dec;57(2-3):319–331. doi: 10.1016/0022-510x(82)90038-7. [DOI] [PubMed] [Google Scholar]
- Wernig A., Irintchev A. "Bystander" damage of host muscle caused by implantation of MHC-compatible myogenic cells. J Neurol Sci. 1995 Jun;130(2):190–196. doi: 10.1016/0022-510x(95)00034-y. [DOI] [PubMed] [Google Scholar]
- Wernig A., Irintchev A., Weisshaupt P. Muscle injury, cross-sectional area and fibre type distribution in mouse soleus after intermittent wheel-running. J Physiol. 1990 Sep;428:639–652. doi: 10.1113/jphysiol.1990.sp018232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yablonka-Reuveni Z., Seifert R. A. Proliferation of chicken myoblasts is regulated by specific isoforms of platelet-derived growth factor: evidence for differences between myoblasts from mid and late stages of embryogenesis. Dev Biol. 1993 Apr;156(2):307–318. doi: 10.1006/dbio.1993.1079. [DOI] [PubMed] [Google Scholar]