Simple Summary
Saiva formosana Kato, 1929 is a species of lanternfly endemic to Taiwan. To date, there have been few reports on the morphology of adults and the fifth-instar nymph of this species. Saiva formosana can be confused with Pyrops watanabei (Matsumura, 1913) due to the morphological similarities exhibited by the nymphs of these two species. Elaeocarpus decipiens F. B. Forbes & Hemsl. has recently been reported to be the sole host plant for Saiva formosana. However, hatched egg masses of Saiva formosana have been detected on many other plant species. For the present study, we undertook a one-year investigation, lasting from May 2023 to April 2024, to record the different life stages of Saiva formosana and identify host plants. During the investigation, some egg masses were removed from tree trunks and reared in the laboratory in order to verify the species and explore any possible interaction between the hatching of this lanternfly species and the emergence of parasitic wasps. Finally, we compared the oviposition strategies and parasitic behaviors of wasps among the three lanternfly species, namely Saiva formosana, Pyrops watanabei, and Pyrops candelaria (Linnaeus, 1758).
Keywords: egg mass, Pyrops candelaria, Pyrops watanabei, Ficus fistulosa, Elaeocarpus decipiens, Heptapleurum heptaphyllum
Abstract
Since Saiva formosana Kato, 1929 was first reported as a new species in Taiwan; there have been few published reports on its ecology, and fundamental knowledge of this species is still lacking. The objectives of this study are to (1) determine the preferred plants of adults, egg-laying, and nymphs, (2) analyze the change in host plants with time and development, and (3) explore the relationship between the survival of eggs and parasitism by their wasps. We detected the adults of this species between May and September 2023, and again in April 2024, mainly on Elaeocarpus decipiens F. B. Forbes & Hemsl. During our investigation, we frequently observed parasitic wasps ovipositing on the egg masses. We established that most egg masses produced only Saiva nymphs or Anastatus adults. This lanternfly might better survive parasitic pressure by laying lower numbers of eggs per mass across a broader range of plant species. The first batch of hatching nymphs was found on Magnolia compresssa Maxim. on 20 June 2023; however, most nymphs in the second and third instars were detected on Ficus fistulosa Reinw. ex Blume, between August and October 2023. After the autumn, the occurrences of nymphs increased on Heptapleurum heptaphyllum (L.) Y. F. Deng which probably served as a shelter for overwintering.
1. Introduction
Invasive lanternflies, such as the notorious Lycorma delicatula (White, 1845), pose a severe threat to agriculture and the broader ecological environment [1]. In Taiwan, the longan lanternfly Pyrops candelaria (Linnaeus, 1758) has been reported since 2018 [2]. Although the impact on agriculture of this introduced lanternfly is not that serious compared to L. delicatula, its influence on the fauna and flora is still worth studying [3,4]. It may be the case that closely related native species are most vulnerable to invasion by P. candelaria due to their similar habitats. The more knowledge we obtain concerning these species, the clearer picture of their interaction we will gain. Ferrenberg and Denno [5] indicated that there has been a difference in opinion over the importance of competition, host plant resources, and natural enemies in community structures. Recent reviews have shown that interspecific competition can be an important factor in affecting the performance and population dynamics of herbivorous insects. In recent studies, the relationships between host plants and different stages of P. watanabei (Matsumura, 1913) have been established following the introduction of P. candelaria [2,6]. The introduced host plant, Triadica sebifera (L.) Small belonging to Euphorbiaceae, is relevant to the developmental stages of both lanternfly species. The coexistence of these two lanternfly species may complicate control strategies for P. candelaria [2,6]. In addition, during previous investigations, a third species of lanternfly, Saiva formosana Kato, 1929, was detected in some communal habitats.
A total of 13 species of Saiva are known to be distributed in Asian countries such as Cambodia, India, Indonesia, Malaysia, Myanmar, Sri Lanka, and Thailand [7]. In Taiwan, however, only S. formosana is considered an endemic species [7]. Saiva is one of the genera most closely related to Pyrops [8,9], and adults are easily differentiated, but eggs and nymphs are more difficult. After the discovery of S. formosana in 1929 [10], there were almost no further reports on this species until 2023, when Elaeocarpus decipiens F. B. Forbes & Hemsl. was reported to be the host plant for adults and only one fifth-instar nymph of this species [11]. The scarcity of references to S. formosana in the literature means that more information is required on the fundamental characteristics of this species. However, as we developed the ability to identify egg masses of S. formosana, we were able to determine that this species has a wide range of distribution in the mountainous areas of Taiwan. The findings of host plant shift obtained for the nymphal and adult stages of two Pyrops species suggested that S. formosana is probably hosted by plants other than E. decipiens at different developmental stages and times of the year [2,6].
In previous research, we also observed that egg parasitism by wasps upon S. formosana is prevalent. Therefore, in the present study, we sought to determine the range of hosts across different developmental stages, the preferred egg-laying sites, and the incidence of parasitism by wasps upon egg masses. In addition, we explored the relationship between the parasitism rate and the survival rate for the eggs of this lanternfly. In the United States and South Korea, an egg parasitoid, Anastatus orientalis Yang and Choi, 2015, has been selected and evaluated as a biological control agent for managing exotic Lycorma delicatula [12]. In the case of Pyrops candelaria, no implication of egg parasitoids has yet been taken as the control agent. However, an ancient record of the emergence holes of wasps on egg masses has been published [13]. Further research is required to determine whether the egg-parasitic wasp frequently found in the present study has the potential for expanding its host range from S. formosana to P. candelaria, either as a result of human activities or through gradual natural processes. In the Discussion Section of this article, we compare the oviposition strategies and parasitic behaviors of wasps among three species of lanternfly, namely S. formosana, P. watanabei, and P. candelaria.
2. Materials and Methods
Prior to this study, we detected many hatched egg masses that had no traces of wax and, therefore, were unlike the egg masses of Pyrops watanabei (Matsumura) or Pyrops candelaria (Linnaeus). We suspected that those egg masses belonged to Saiva formosana Kato. Accordingly, for the present study, we chose investigation sites (Table 1) with abundances of these hatched egg masses, located in Taipei City, New Taipei City, and Ilan County. We then investigated the different stages of S. formosana and their occurrences on various plants over the course of a single year from 1 May 2023 to 30 April 2024.
Table 1.
Investigation sites of the present study, with elevations and GPS coordinates. The stages of Saiva formosana Kato detected at the sites are also indicated.
| Investigation Sites | Elevations | GPS Coordinates | Stages Detected |
|---|---|---|---|
| Erbazi Botanical Garden, Xindian, New Taipei City |
285 m | 24.936, 121.498 | Adults, Eggs, and Nymphs |
| Miagaotai Peak Trail, Xinyi, Taipei City |
260 m | 25.015, 121.579 | Adults, Eggs, and Nymphs |
| Zhanghu Hiking Trail, Wenshan, Taipei City |
354 m | 21.966, 121.579 | Adults, Eggs, and Nymphs |
| Quantoumu Trail, Sanxing, Ilan County |
212 m | 24.658, 121.610 | Adults, Eggs, and Nymphs |
| Tukuyue Trail, Nangang, Taipei City |
272 m | 25.026, 121.635 | Adults and Eggs |
| Daijian Trail, Xizhi, New Taipei City |
350 m | 25.053, 121.665 | Eggs and Nymphs |
| Mt. Shitou Trail, Xindian, New Taipei City |
118 m | 24.959, 121.544 | Eggs |
| Dagemen Historical Trail, Shiding, New Taipei City |
330 m | 24.953, 121.667 | Eggs |
| Caonan Benyan Tree, Wenshan, Taipei City |
250 m | 24.968, 121.608 | Eggs |
| Paoma Historical Trail, Jiaoxi, Ilan County |
322 m | 24.849, 121.775 | Eggs |
| Maokong Gondola Station, Wenshan, Taipei City |
299 m | 24.968, 121.589 | Eggs |
| Houshanyue Trail, Wenshan, Taipei City |
350 m | 24.980, 121.599 | Eggs |
| Mt. Shiqiliao Trail, Sanxia, New Taipei City |
180 m | 24.957, 121.476 | Eggs |
The investigations were conducted at least twice every week in the daytime, and the first individual detected on 1 May 2023 was an adult on Elaeocarpus decipiens F. B. Forbes & Hemsl. The numbers of adults on each plant were noted until the last adult was found on 22 September of the same year. The plant species were also recorded when individuals were detected. In line with the recent studies on the genus Pyrops, the scientific names of the species and families of the plants used in this study referred to the same source [2,6]. The full scientific names with authors and date of publication are listed in Supplementary Materials Table S1. Moreover, to avoid confusion with egg masses laid by previous generations (i.e., prior to 2023), only unhatched masses were recorded in the present study. The egg masses of S. formosana are exposed without any cover, so it is easy to count them using photographs. The number of columns and of eggs per egg mass can then be noted. In the present study, we counted eggs from the first column on the left end of the mass and named the columns C1, C2, C3, and so on, moving rightwards, following Liu [14]. In addition, some egg masses were removed and reared in the laboratory. This was to confirm the species and to quickly distinguish between developing instars in the field. The nymphs subsequently reared from these eggs were taken to be the references of five instars, which differed in both size and color; they were used to differentiate developmental instars and then count the numbers of each instar during our investigation. As the side view of S. formosana nymph might be taken to be a nymph of P. watanabei [15], we took both dorsal and lateral profile photographs of each nymphal instar (Figure 1 and Figure 2). After 22 September 2023, no more adults were found until 20 April 2024, and our one-year study finally ended on 30 April 2024. Occurrences were recorded using visual means, typically the naked eye aided by an MT 14 flashlight (Ledlenser GmbH & Co. KG, Solingen, Germany) in high luminosity (up to 1000 lumens) as well as via a digital camera with a 60× optical zoom (COOLPIX B700, Nikon Co. Ltd., Tokyo, Japan), if we needed to check the higher parts of the canopy and on overcast days. A total of 40 egg masses were removed from the investigation sites and reared in the laboratory to explore the relationship between the survival of eggs and parasitism by their wasps (Anastatus sp.). The rearing period lasted from 7 June 2023 to 4 April 2024.
Figure 1.
Different stages of Saiva formosana. (A) A batch of newly hatched nymphs beside an egg mass on Acacia confusa. (B) A first instar on Ficus fistulosa. (C) A second instar on F. fistulosa. (D) A third instar on Heptapleurum heptaphyllum. (E) A fourth instar on H. heptaphyllum. (F) A fifth instar on H. heptaphyllum. (G) An adult on Elaeocarpus decipiens.
Figure 2.
Side views of nymphs of Saiva formosana. (A) A first instar on Acacia confusa. (B) A second instar on Ficus fistulosa. (C) A third instar on Machilus thunbergia. (D) A fourth instar on Heptapleurum heptaphyllum. (E) A fifth instar on H. heptaphyllum.
3. Results
3.1. Diagnosis of the Nymphs
In terms of the first-, second-, and third-instars of Saiva formosana Kato, the dorsal view of the abdomen is light color in the middle and darker on both edges. On the contrary, the abdomens of the nymphs of Pyrops candelaria and P. watanabei in the first to fourth instars are dark color in the middle and light color on both sides (see photographs in Supplementary Materials Figures S1–S4). The shape of the cephalic process is also a useful trait to distinguish among the three species. From lateral views, the cephalic processes of the fourth- and fifth-instar nymphs of S. formosana are flat like a sword; however, those of Pyrops spp. are curvy like the nip of a fountain pen (see photographs in Supplementary Materials Figures S1–S4).
3.2. Monthly Records of Different Stages
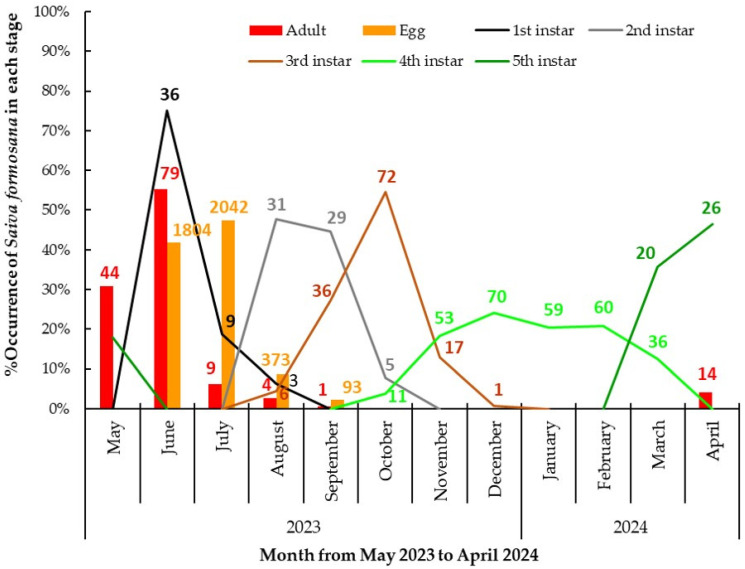
It can be seen from Figure 3 that in May 2023 and April 2024, fifth-instar nymphs and adults were both detected. In June 2023, 55.2% of the adults were recorded, and newly laid egg masses appeared. In addition, some newly hatched nymphs were found beside the egg masses. In July, the recorded proportion of egg masses reached a peak of 47.4%, while the number of adults declined distinctly. In August, we detected most stages, including adults, egg masses, and nymphs in the first to third instars. The nymphs in the third instar were recorded from August to December. Nymphs in the fourth instar were recorded for almost a full half-year, from October 2023 to March 2024. During the investigation in the autumn and winter, most of the nymphs were found to attach closely to twigs with their ventral side, especially at slightly concave points (Figure 4). Only nymphs in the fourth instar were detected in January and February. Nymphs of the fifth instar began to appear again from March 2024 onwards.
Figure 3.
The percentage occurrence of Saiva formosana in each stage per month from 1 May 2023 to 30 April 2024. In terms of each stage, the sum of the 12 monthly percentage occurrences is 100% in one year. The numbers of individuals are labeled on the bars and curve lines.
Figure 4.
The whole body of a nymph in the fourth instar attached closely to the twigs of (A) Citrus maxima (19 December 2023), and (B) Machilus thunbergia (on 24 January 2024).
3.3. Records of Lanternfly Stages and Their Host Plants
3.3.1. Adults
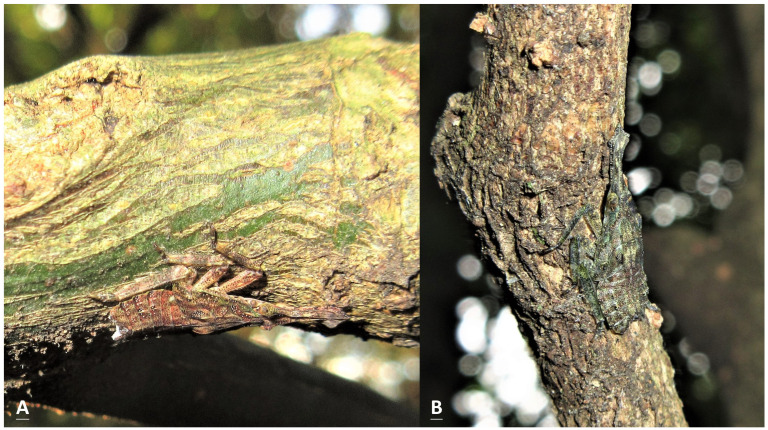
As we can see in Table 2, only six species of plants hosted the adults of S. formosana. Adults were detected from April to September, as shown in Figure 3, and 73.4% of these were found on Elaeocarpus decipiens F. B. Forbes & Hemsl. Approximately 14.7% of the adults were recorded on Magnolia compressa Maxim., and almost 7.0% were on Tetradium glabrifolium (Champ. ex Benth.) T. G. Hartley. On only one occasion were adults recorded on Triadica sebifera (L.) Small; however, on this occasion, five individuals appeared simultaneously, accompanied by 20 adults of Pyrops watanabei. Figure 5B shows a photograph of four lanternflies taken at that time. No adults were recorded on Heptapleurum heptaphyllum (L.) Y. F. Deng, Ficus fistulosa Reinw. ex Blume, Machilus zuihoensis Hayata, 1911, or M. thunbergii Siebold & Zucc. The average was 1.7 adults per plant. Adults feeding on the trunk or branch were sighted on E. decipiens, M. compressa, and T. glabrifolium (see Supplementary Materials Figure S5).
Table 2.
Numbers of adults of Saiva formosana on different plants, as recorded from 1 May to 22 September 2023 and from 20 to 25 April 2024.
| Plant Species | Family | n | No. Adults |
|---|---|---|---|
| Elaeocarpus decipiens | Elaeocarpaceae | 56 | 105 |
| Magnolia compressa | Magnoliaceae | 17 | 21 |
| Tetradium glabrifolium | Rutaceae | 8 | 10 |
| Triadica sebifera | Euphorbiaceae | 1 | 5 |
| Zanthoxylum ailanthoides | Rutaceae | 1 | 1 |
| Morus australis | Moraceae | 1 | 1 |
| Sum | - | 84 | 143 |
Figure 5.
Coexistence of two species of lanternflies, Saiva formosana (hallow arrow) and Pyrops watanabei (solid arrow) on the same tree. (A) A fourth instar of S. formosana and a fourth instar of P. watanabei on Heptapleurum heptaphyllum (25 December 2023). (B) Two adults of S. formosana and two adults of P. watanabei on Triadica sebifera (14 June 2023).
3.3.2. Egg Masses
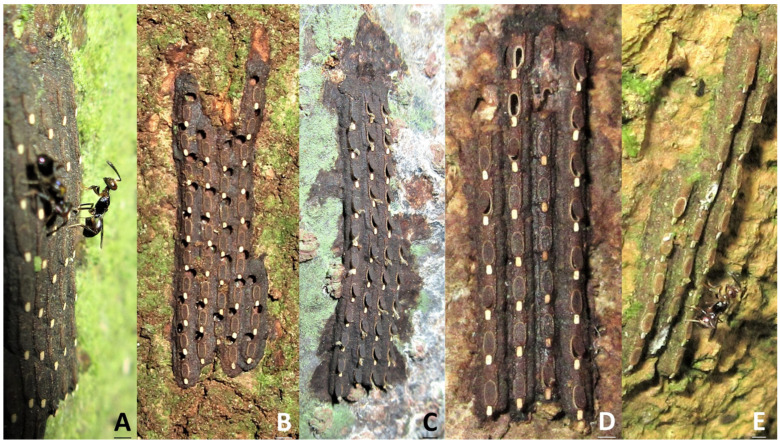
Although egg masses can be found on the higher parts of trunks or branches, most of the egg masses were detected on the bases of tree trunks, at heights below 50 cm. Only unhatched egg masses (Figure 6A) without any emergence holes (Figure 6B) were counted. As eggs can be re-covered by lids after hatching (Figure 6C,D) and because in the present study, hatched egg masses sometimes retained all their lids, our experience as researchers was required to avoid confusion between hatched and unhatched egg masses when recording our data. Most of the parasitic wasps were observed on newly laid egg masses (Figure 6A); however, we did observe a parasitic wasp attempting to oviposit on an old, hatched egg mass (Figure 6E). In total, 116 unhatched egg masses with 4291 eggs were found on 107 plants, including five dead tree trunks during the period from June to September 2023 (Table 3). The host plants belonged to 29 species of 21 families, and all of them were woody plants. Approximately 19.0%, 14.7%, and 7.8% of the egg masses were found on Heptapleurum heptaphyllum, Magnolia compressa, and Machilus zuihoensis, respectively. Only six egg masses were recorded on Elaeocarpus decipiens. A single egg mass was detected on Ficus fistulosa. No egg masses were recorded on any herbaceous plants. A total of 17 wasps (Anastatus sp.) were observed searching or ovipositing on 12 of the egg masses. The average number of egg masses per plant was nearly 1.1. Egg masses had between eight and forty-nine eggs, with an average figure (with standard error) of 37.0 ± 0.7. Furthermore, the eggs were arranged in one to seven columns (mean ± SE = 4.8 ± 0.1 columns/egg mass), with each column having between one and thirteen eggs. The average numbers of eggs per column, from the left to the right, were as follows: C1 = 6.6; C2 = 8.9; C3 = 8.8; C4 = 8.4; C5 = 6.8; and C6 = 4.1 eggs. Only one egg mass had a seventh column (C7); two eggs were recorded in this instance.
Figure 6.
Egg masses of Saiva formosana. (A) An unhatched egg mass with two ovipositing parasitic wasps (Anastatus sp.). (B) An egg mass in which almost every egg has an emergence hole of a parasitic wasp. (C) A newly hatched egg mass with lids ajar and exuviae. (D) An egg mass in which only three eggs have lost their lids, while most eggs have retained theirs, and been re-covered. (E) An old, hatched egg mass with all the lids re-covered and a parasitic wasp. In contrast to the dark brown color of newly laid egg masses, this egg mass was light brown with apparent moss growth. It could be considered an egg mass laid prior to 2023.
Table 3.
Numbers of egg masses or eggs of Saiva formosana on different plants, as recorded from 5 June to 9 September 2023.
| Plant Species | Family | n a | No. Egg Masses |
No. Eggs |
No. Anastatus sp. b on Egg Masses |
|---|---|---|---|---|---|
| Heptapleurum heptaphyllum | Araliaceae | 20 | 22 | 791 | 0 |
| Magnolia compressa | Magnoliaceae | 16 | 17 | 647 | 3 |
| Machilus zuihoensis | Lauraceae | 9 | 9 | 327 | 0 |
| Mallotus paniculatus | Euphorbiaceae | 8 | 8 | 306 | 2 |
| Acacia confusa | Fabaceae | 4 | 7 | 281 | 1 |
| Acer serrulatum | Sapindaceae | 6 | 7 | 255 | 1 |
| Elaeocarpus decipiens | Elaeocarpaceae | 6 | 6 | 239 | 4 |
| Dead tree c | - | 5 | 5 | 199 | 0 |
| Machilus thunbergii | Lauraceae | 5 | 5 | 175 | 1 |
| Turpinia formosana | Staphyleaceae | 4 | 4 | 117 | 0 |
| Wendlandia formosana | Rubiaceae | 2 | 2 | 86 | 1 |
| Quercus glauca | Fagaceae | 2 | 2 | 79 | 0 |
| Ficus ampelos | Moraceae | 2 | 2 | 62 | 2 |
| Ilex micrococca | Aquifoliaceae | 1 | 2 | 78 | 0 |
| Cleyera japonica | Pentaphylacaceae | 2 | 2 | 71 | 0 |
| Trema orientalis | Cannabaceae | 1 | 2 | 70 | 1 |
| Randia cochinchinensis | Rubiaceae | 1 | 1 | 49 | 0 |
| Ficus fistulosa | Moraceae | 1 | 1 | 34 | 0 |
| Zanthoxylum ailanthoides | Rutaceae | 1 | 1 | 47 | 0 |
| Ilex asprella | Aquifoliaceae | 1 | 1 | 43 | 0 |
| Hydrangea chinensis | Hydrangeaceae | 1 | 1 | 42 | 0 |
| Macaranga tanarius | Euphorbiaceae | 1 | 1 | 41 | 0 |
| Ilex ficoidea | Aquifoliaceae | 1 | 1 | 41 | 0 |
| Prunus persica | Rosaceae | 1 | 1 | 38 | 0 |
| Glochidion rubrum | Phyllanthaceae | 1 | 1 | 37 | 1 |
| Triadica cochinchinensis | Euphorbiaceae | 1 | 1 | 35 | 0 |
| Ardisia sieboldii | Primulaceae | 1 | 1 | 31 | 0 |
| Citrus maxima | Rutaceae | 1 | 1 | 27 | 0 |
| Diospyros eriantha | Ebenaceae | 1 | 1 | 23 | 0 |
| Daphniphyllum glaucescens | Daphniphyllaceae | 1 | 1 | 20 | 0 |
| Sum | - | 107 | 116 | 4291 | 17 |
a only plants with unhatched egg masses were noted. b,c species were not identified.
3.3.3. Nymphs
Nymphs usually remained under branches or twigs. In total, 617 nymphs were found on 477 plants, more or less throughout the year from May 2023 to April 2024 (Table 4). The host plants belonged to 13 species of 12 families. Nearly 32.4% and 30.6% of the nymphs were recorded on Ficus fistulosa and Heptapleurum heptaphyllum, respectively. Moreover, 20.3% were recorded on two Machilus species; namely M. zuihoensis and M. thunbergii. All the nymphal stages were recorded on F. fistulosa, although only one egg mass was detected on this tree, as can be seen in Table 3. Only one nymph was recorded on Elaeocarpus decipiens. On Magnolia compressa, a batch of 36 newly hatched nymphs were observed beside an egg mass. Excluding newly hatched nymphs close to egg masses, nearly 87.3% of the first- and second-instar nymphs were recorded on F. fistulosa. Approximately one-half of the third-instar nymphs were found on F. fistulosa, with the other half being found on H. heptaphyllum and M. thunbergia. More than 41.2% of the fourth-instar nymphs were found on H. heptaphyllum. During winter, we made three sightings of the fourth-instar nymphs of this lanternfly, together with the nymphs of P. watanabei in the third or fourth instar, on the same tree of H. heptaphyllum (Figure 5A). Over 62% of the nymphs in the last instar were found on H. heptaphyllum, though we did not detect any adults on this plant. The average was 1.3 nymphs per plant. Feeding by nymphs was difficult to be observed in the field because the proboscis is short and hidden between legs. However, more than 50 nymphs and two instars were detected on F. fistulosa, H. heptaphyllum, M. zuihoensis, and M. thunbergii. Therefore, these four species of plants might be food plants and winter shelters for nymphs. Other plants may be served as the egg-laying or temporary resting sites in which the newly hatched or fourth-instar nymphs can be detected.
Table 4.
Numbers of nymphs in different instars of Saiva formosana recorded on different plant species from 2 May 2023 to 25 April 2024.
| Plant Species | Family | n | No. Nymphs | Total a | ||||
|---|---|---|---|---|---|---|---|---|
| 1st Instar |
2nd Instar |
3rd Instar |
4th Instar |
5th Instar |
||||
| Ficus fistulosa | Moraceae | 165 | 6 | 56 | 62 | 64 | 12 | 200 |
| Heptapleurum heptaphyllum | Araliaceae | 176 | 0 | 1 | 37 | 129 | 22 | 189 |
| Machilus zuihoensis | Lauraceae | 47 | 0 | 0 | 0 | 50 | 17 | 67 |
| Machilus thunbergii | Lauraceae | 41 | 0 | 7 | 30 | 18 | 3 | 58 |
| Magnolia compressa | Magnoliaceae | 1 | 36 b | 0 | 0 | 0 | 0 | 36 |
| Callicarpa formosana | Lamiaceae | 22 | 0 | 0 | 0 | 30 | 1 | 31 |
| Ilex uraiensis | Aquifoliaceae | 8 | 0 | 0 | 3 | 7 | 0 | 10 |
| Citrus maxima | Rutaceae | 7 | 0 | 0 | 0 | 8 | 0 | 8 |
| Acacia confusa | Fabaceae | 1 | 6 b | 0 | 0 | 0 | 0 | 6 |
| Premna serratifolia | Lamiaceae | 5 | 0 | 0 | 0 | 5 | 1 | 6 |
| Turpinia formosana | Staphyleaceae | 2 | 3 b | 1 | 0 | 0 | 0 | 4 |
| Elaeocarpus decipiens | Elaeocarpaceae | 1 | 0 | 0 | 0 | 1 | 0 | 1 |
| Glochidion zeylanicum | Phyllanthaceae | 1 | 0 | 0 | 0 | 1 | 0 | 1 |
| Sum | - | 477 | 51 | 65 | 132 | 313 | 56 | 617 |
a total number of nymphs of all instars on a plant species. b the newly hatched nymphs were found beside an egg mass on 20 June and 12 July 2023.
3.4. Changes in Host Plant Preference
3.4.1. According to Time
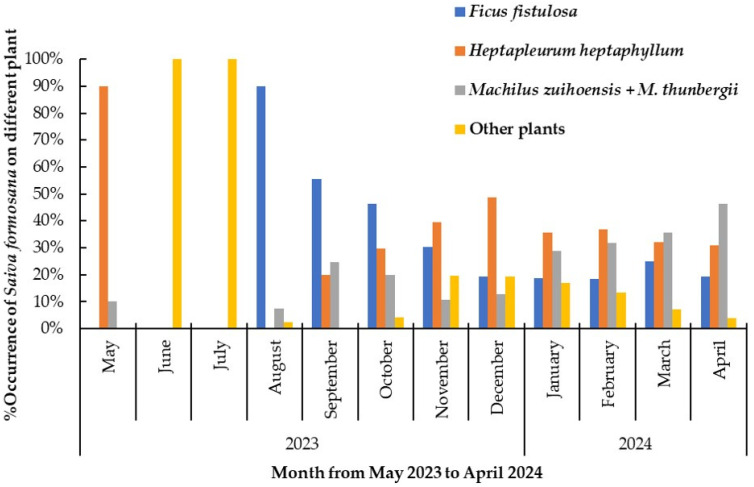
As can be seen in Figure 7, a trend in the host preference of nymphs toward Ficus fistulosa suddenly emerged, at a peak rate of 90.0%, in August 2023, before declining to approximately 55.4% and 46.2% in September and October, respectively. The preference of nymphs for F. fistulosa then continued to decline slowly, from 30.3% to 19.2%, between November 2023 and April 2024. In contrast, another trend was recorded for occurrence on Heptapleurum heptaphyllum; this emerged after the end of summer and increased with time from 20.0% in September 2023 to 48.7% in December. The level of occurrence then remained above 30% from January to April 2024. Another trend was recorded for the occurrence of nymphs on Machilus zuihoensis and M. thunbergii. This ranged from 10% to 25% between September and December of 2023, but then rose to a higher level of between 29% and 46% from January to April 2024. Finally, the trend for the occurrence of nymphs on plants other than the four species mentioned above was remarkably 100% in both June and July 2023, but less than 20% in all the other months.
Figure 7.
The percentage occurrence of nymphs of Saiva formosana on different host plants per month from 1 May 2023 to 30 April 2024. The percentage occurrence of this lanternfly is 100% in each month.
3.4.2. According to the Developmental Stage
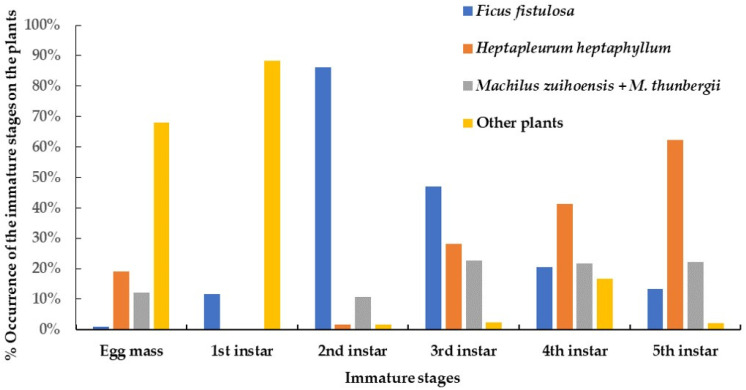
Two opposite trends in host plant preference according to different developmental stages can be seen in Figure 8. First, the preference of nymphs toward Heptapleurum heptaphyllum steadily increased from 1.5% in the second instar to 62.2% in the fifth instar. Over the same developmental period, the preference for Ficus fistulosa decreased from 86.2% to 13.3%. In addition, the possibility of detecting nymphs on Machilus zuihoensis and M. thunbergia was approximately 10–20%. Finally, 68.1% of the egg masses and 88.2% of the nymphs in the first instar were recorded on plants other than the four species mentioned above.
Figure 8.
The shift of host range with different developmental immature stages from the egg mass to the nymph in the fifth instar of Saiva formosana recorded on different host plants from 1 May 2023 to 30 April 2024.
3.5. Egg Masses and Parasitic Wasps
In total, 1529 eggs were counted from 40 egg masses. In terms of the eggs, the hatching rate was 45.5% (695 first-instar nymphs), and the emergence rate was 20.9% (320 adult wasps). In addition, no more than one adult wasp emerged from any individual egg, and no more than one emergence hole was left above any lid (Figure 6B). The remaining eggs were deemed abortive (514 eggs, 33.6%), as no insects were harvested. In terms of the egg masses (Table 5), more than 40% were with parasitic wasps. Overall, 90% of the egg masses either emerged only adult wasps (lose–win situation) or hatched only nymphs of the lanternfly (win–lose situation).
Table 5.
Rearing experiment for egg masses. Forty egg masses were reared in the laboratory to explore the impact of egg parasitism on the survival of eggs. Four situations are presented, with the numbers and chances of egg masses harvesting lanternfly nymphs only, parasitic wasps (Anastatus sp.) only, both, or none. “Win” denotes there is any nymph or wasp harvested from an egg mass. “Lose” denotes there is no nymph or wasp harvested from an egg mass.
| No. Egg Masses |
Win–Lose a | Win–Win b | Lose–Win c | Lose–Lose d | ||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | |
| 40 | 22 | 55.0 | 2 | 5.0 | 14 | 35.0 | 2 | 5.0 |
a only the first-instar nymphs of Saiva formosana hatched from the egg mass. b both lanternfly nymphs and adult parasitic wasps were harvested. c only adult parasitic wasps emerged from the egg mass. d no insects were harvested.
4. Discussion
This is the first article to reveal the morphology of all the developmental stages in a species of the genus Saiva, from egg masses, to nymphs in five different instars, to adults. The adults and fifth-instar nymphs of S. formosana Kato [11] and S. gemmata (Westwood) [16] have previously been described in the literature. These two species can be easily identified by their respective morphologies, and from color patterns visible in the ecological photographs taken in the field [16]. However, to avoid any risk of mistaken identity, photographs of dorsal and lateral profile views should be taken for reference. In previous studies, we obtained photographs of S. formosana, Pyrops watanabei (Matsumura), and P. candelaria (Linnaeus) for the purposes of comparison. In the back and side views, it can be seen that in the first- and second-instar nymphs of these three lanternfly species, the head and thorax are both blacker and darker, compared with the third-, fourth-, and fifth-instar nymphs [6,15]. In light of the findings of the present study, we suggest that the lateral view of the nymph reported in Constant and Pham [15] is a third instar of S. formosana, instead of P. watanabei. Furthermore, the color of the older three instars, including the third, fourth, and fifth instars, of P. watanabei is just brown with light patterns [6,15], whereas those of S. formosana are brown with a shade of green (Figure 1D,F, Figure 2C–E and Figure 4; the photograph in Constant and Pham [15]) or red (Figure 1E and Figure 4A). These two lanternfly species could exist in the same habitat, even on the same tree. Were they to coexist, it is most likely that their nymphs in the third or fourth instar would be found on Heptapleurum heptaphyllum (L.) Y. F. Deng in the proximity of Triadica sebifera (L.) Small during overwintering [6]. In the present study, we detected five adults of S. formosana on T. sebifera. In the previous studies, it was found that nymphs and adults of P. candelaria and P. watanabei had preferences towards T. sebifera [2,6]. In light of these findings, it is reasonable to assume that the coexistence of the above-mentioned three lanternfly species will become widespread in Taiwan in the future. In addition, the results of the present study indicate that most of the main host plants for S. formosana and P. candelaria are different. Hence, we may speculate that the greatest survival stress facing S. formosana is from egg parasitism rather than invasion by P. candelaria. By contrast, it may be that the stress facing P. watanabei from invasion by P. candelaria is worthy of greater concern, owing to their communal key hosts, T. sebifera and T. cochinchinensis Lour., for nymphs as well as adults.
The egg masses of S. formosana are similar to those of P. candelaria and P. watanabei, except for three biological traits. First, there are no wax covers on the egg masses of S. formosana; in contrast, the egg masses of P. candelaria [2] and P. watanabei [6,17] have thin and thick layers, respectively, of white wax. The record for the existence of Lycorma delicatula (White) in Taiwan is problematic according to Lin et al. [11], but the egg masses of endemic L. meliae Kato do have a covering layer of gray wax [Hsu, unpublished data]. Second, the size of the egg mass matters. In this study, we established that S. formosana has the lowest number of eggs in masses, with average figures of 37.0 eggs and 4.8 columns per mass. These may be compared with corresponding figures (unpublished data, Hsu) for P. candelaria (n = 30, mean ± SE = 82.5 ± 3.8 eggs, 8.4 ± 0.3 columns) and P. watanabei (n = 30, 134.8 ± 2.6 eggs, 11.4 ± 0.4 columns). The egg mass of Penthiocodes atomaria (Weber, 1801) is also without a wax cover; however, the size of this mass is bigger than that of S. formosana [18]. Finally, the levels of egg parasitism are different in the three species. The prevalent egg parasitism in S. formosana leads to the frequent appearance of emergence holes on hatched egg masses. In comparison, in the cases of P. candelaria and P. watanabei, egg parasitism by wasps was found to be rare in the former, and absent in the latter. No parasitic wasps were observed during our previous investigation of 186 egg masses of P. watanabei, and only 2 out of 203 egg masses of P. candelaria were found with ovipositing parasitic wasps (unpublished data, Hsu). According to Fatouros et al. [19], egg coating may be considered one of the factors leading to escape from egg parasitism. Regarding S. formosana, the exposed egg mass may play an important role in the prevalence of egg parasitism. However, the smaller sizes of egg clutch may be the reason why this species still exhibited a 45.5% egg survival rate in the laboratory in the present study. The result of the egg-mass rearing experiment conducted in this study revealed that after being parasitized by wasps, most of the masses had no eggs that survived. We think a much better hatch rate may be obtained in the field based on the report of L. delicatula [20]. Many species in Fulgoridae are characterized by the production of copious amounts of wax [21]. Insect wax may have other functions than escaping from predation and parasitism, such as camouflage [22], protection against desiccation [23], etc. Therefore, the issues of full functions performed by wax coverings on lanternflies need more research to address. Moreover, the types and compositions of waxes in adults, nymphs, and eggs are different [24]. In terms of S. formosana, no wax was observed on adults or egg masses, but wax attachments were found on the rear ends of nymphs (Figure 1B,C and Figure 4A). In addition, wax was often seen on the nymphs of S. formosana, P. watanabei, and P. candelaria during rearing in our laboratory. Bosuang et al. [25] indicated that wax is protective against predators and mildew.
Knowledge of changes in host plants can be crucial when evaluating the ecological impacts of the invasive species and determining control strategies [2]; however, the strategy of changes in host plants may be utilized by female insects to oviposit on a broader range of hosts; such insects may even oviposit on unsuitable hosts to make it harder for predators or parasitoids to find their eggs and early-stage instars [26,27]. In this study, S. formosana had a broad range of oviposition sites, including 29 species of plants. Additionally, nymphs preferred host plants that differed from those preferred by adults. The host preference for egg-laying by female S. formosana indicated that general site criteria may be more important than species-specific criteria. Like the cases in P. candelaria and P. watanabei, some egg masses were laid on dead trees [2,6].
For the convenience of investigation, we chose close-at-hand locations with low elevations (118–354 m) in northern Taiwan. However, in June 2024, we found that the distribution of this species could include areas at an elevation as high as 1300 m, in Jinfeng Township, Taitung County, in the mountain of the southern tip of Taiwan (unpublished data, Hsu). The question of whether the preference of this species towards its main host plants is different on higher mountains needs further study to answer. Lin et al. [11] pointed out that Elaeocarpus decipiens was the sole host for the adults and a nymph of S. formosana. In this study, we recorded the adults of S. formosana mostly in May and June, when E. decipiens bloomed [28]. Nevertheless, we also recorded more than ten adults on both Magnolia compressa Maxim. and Tetradium glabrifolium (Champ. ex Benth.) T. G. Hartley. In addition, in the present study, we established that nymphs had no preference towards Elaeocarpus decipiens. Instead, we found that nymphs had preferences for Ficus fistulosa Reinw. ex Blume, 1825, H. heptaphyllum, Machilus zuihoensis Hayata, and M. thunbergii Siebold & Zucc.
Lanternfly species have diversified life histories. The nymphs of S. formosana, P. watanabei, and P. candelaria require five instars to become adults, whereas those of L. delicatula require only four [29]. Although Saiva is the genus most closely related to Pyrops, the life history of S. formosana is more similar to that of P. watanabei than P. candelaria due to a longer nymphal stage and the tendency of overwintering on blooming H. heptaphyllum [30]. Mainly, nymphs in the fourth instars of S. formosana and the third or fourth instar of P. watanabei take H. heptaphyllum as winter shelters [6], whereas P. candelaria and L. meliae survive the winter as adults mainly in the higher canopy of longan and eggs on Melia azedarach L., respectively (unpublished data, Hsu). However, the earlier nymphal instars of S. formosana prefer Ficus fistulosa, which is more likely found in humid valleys [31,32]. This might explain why in some drier areas rich in E. decipiens and M. compressa, we detected no egg masses of S. formosana. As for the food plants, the feeding behavior performed by adults was sighted on E. decipiens, M. compressa, and T. glabrifolium. In terms of nymphs, only the feeding on H. heptaphyllum has been observed because of a photograph taken from the front of a fifth-instar nymph. The mating and adult emergence have never been found probably due to the timing being in the night or early morning. It is not like the easy sighting of those behaviors in P. candelaria and P. watanabei on T. sebifera [2,6].
5. Conclusions
In the present study, we established that while the adults of Saiva formosana prefer Elaeocarpus decipiens, they may also be detected on Magnolia compressa and Tetradium glabrifolium. They tend to lay a few eggs per mass on a broad range of trees. This may be a good strategy for this lanternfly species to survive under the stress of its parasitic wasps, even though its eggs lack the protection of a wax cover. We suggest that egg masses, both hatched and unhatched, can be used for monitoring lanternflies as they are immobile, have a year-round presence, and are much easier to locate than nymphs or adults. In this study, we usually detected egg masses in the humid valley, probably due to the preference for Ficus fistulosa exhibited by early-instar nymphs. In addition, the older nymphal instars of S. formosana have a high rate of occurrence on Heptapleurum heptaphyllum after November. This overwintering strategy of this lanternfly species is similar to that of Pyrops watanabei. We may say, then, that the coexistence of S. formosana and P. watanabei in the same habitat is not unusual in Taiwan. Our results also indicated that the main host plants of S. formosana and P. candelaria are different. Therefore, the survival stress facing this lanternfly from invasion by P. candelaria is probably insignificant. Furthermore, we suggest that the prevalent egg parasitism of S. formosana may cast new light on a possible way to imply biological control agents for the invasive lanternfly, P. candelaria, in Taiwan. Finally, the identity of the parasitic wasp is an issue that must be addressed in future studies by specialists in the field of taxonomy.
Acknowledgments
We especially appreciate Yueh-Ling Yang’s assistance, as she was passionate about working with us and gave many good ideas for this study.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/insects15110841/s1, Table S1. Checklist of Saiva formosana on different plants species. Figure S1. Dorsal views of Pyrops candelaria nymphs in various instars. Figure S2. Lateral views of Pyrops candelaria nymphs in various instars. Figure S3. Dorsal views of Pyrops watanabei nymphs in different instars. Figure S4. Lateral views of Pyrops watanabei nymphs indifferent instars. Figure S5. Adult Saiva formosana feeding on various trees.
Author Contributions
Conceptualization, M.-H.H., M.-L.W. and L.-J.W.; methodology, M.-H.H. and L.-J.W.; Validation, M.-H.H. and L.-J.W.; formal analysis, M.-H.H.; investigation, M.-H.H. and L.-J.W.; resources, M.-H.H. and M.-L.W.; data curation, M.-H.H.; writing—original draft preparation, M.-H.H.; writing—review and editing, M.-H.H. and L.-J.W.; visualization, M.-H.H. and and L.-J.W.; Supervision, M.-L.W.; Funding acquisition, M.-L.W. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
The original contributions presented in the study are included in the article and Supplementary Materials, further inquiries could be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This study was funded by the Taiwan Forestry Research Institute, grant numbers No. 112AS-7.1.3.-FI-G1 and No. 113AS-7.1.3.-FI-G1.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Kim S., Kuhn A., Raupp M.J., Martison H. Host preferences of spotted lanternfly and risk assessment of potential tree hosts in managed and semi-natural landscapes. Florida Entomol. 2024;106:74–82. doi: 10.1653/024.106.0202. [DOI] [Google Scholar]
- 2.Hsu M.-H., Yang Y.-L., Wu M.-L., Wang L.-J. Host plants of the immature stages of the invasive longan lanternfly, Pyrops candelaria (L.) (Hemiptera, Fulgoridae) in Taiwan. Insects. 2021;12:1022. doi: 10.3390/insects12111022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin Y.-S., Liao J.-R., Shiao S.-F., Ko C.-C. Origin and potential expansion of the invasive longan lanternfly, Pyrops candelaria (Hemiptera: Fulgoridae) in Taiwan. Biology. 2021;10:678. doi: 10.3390/biology10070678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin H., Song W., Ma D., Yang C., Yao Y., Liu R., Hao L., Wu D., Wang S., Jiang J., et al. Screening and characterization of a new Iflavirus virus in the fruit tree pest Pyrops candelaria. Insects. 2024;15:625. doi: 10.3390/insects15080625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferrenberg S.M., Denno R.F. Competition as a factor underlying the abundance of an uncommon phytophagous insect, the salt-marsh planthopper Delphacodes penedetecta. Ecol. Entomol. 2003;28:58–66. doi: 10.1046/j.1365-2311.2003.00479.x. [DOI] [Google Scholar]
- 6.Hsu M.-H., Yang Y.-L., Wu M.-L., Wang L.-J. Shift of host range for the immature stages of the lanternfly, Pyrops watanabei (Matsumura) (Hemiptera, Fulgoridae) native to Taiwan. Insects. 2022;13:826. doi: 10.3390/insects13090826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bourgoin T. FLOW (Fulgoromopha Lists on the Web): A Knowledge and a Taxonomy Database Dedicated to Planthoppers (Insecta, Hemiptera, Fulgoromorpha, Fulgoroidea). Version 8, Updated. [(accessed on 14 August 2024)]. Available online: https://flow.hemiptera-databases.org/flow/
- 8.Bucher M., Condamine F.L., Luo Y., Wang M., Bourgoin T. Phylogeny and diversification of planthoppers (Hemiptera: Fulgoromorpha) based on a comprehensive molecular dataset and large taxon sampling. Mol. Phylogen. Evol. 2023;186:107862. doi: 10.1016/j.ympev.2023.107862. [DOI] [PubMed] [Google Scholar]
- 9.Urban J.M., Cryan J.R. Entomologically famous, evolutionarily unexplored: The first phylogeny of the lanternfly family Fulgoridae (Insecta: Hemiptera: Fulgoroidea) Mol. Phylogen. Evol. 2009;50:471–484. doi: 10.1016/j.ympev.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 10.Kato M. Descriptions of some new Formosan Homoptera. Trans. Nat. Hist. Soc. Formosa. 1929;19:540–551. (In Japanese) [Google Scholar]
- 11.Lin Y.-S., Liao J.-R., Shiao S.-F., Ko C.-C. Lanternflies (Hemiptera: Fulgoridae) of Taiwan. Zool. Studies. 2023;62:7. doi: 10.6620/ZS.2023.62-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Broadley H.J., Sipolski S.J., Pitt D.B., Hoelmer K.A., Wang X.-Y., Cao L.-M., Tewksbury L.A., Hagerty T.J., Barlett C.R., Russel A.D., et al. Assessing the host range of Anastatus orientalis, an egg parasitoid of spotted lanternfly (Lycorma delicatula) using eastern US non-target species. Front. Insect Sci. 2023;3:1154697. doi: 10.3389/finsc.2023.1154697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kerchaw J.C.W., Kirkaldy G.W. A memoir on the anatomy and life-history of the homopterous insect Pyrops candelaria (or “candle-fly”) Zool. Jahrb. Abt. Syst. 1910;29:105–124. [Google Scholar]
- 14.Liu H. Oviposition selection in spotted lanternfly: Impact of habitat and substrate on egg mass size and hatchability. Front. Insect Sci. 2022;2:932433. doi: 10.3389/finsc.2022.932433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Constant J., Pham H.T. Review of the clavatus group of the lanternfly genus Pyrops (Hemiptera: Fulgoromorpha: Fulgoridae) Eu. J. Taxon. 2017;305:1–26. doi: 10.5852/ejt.2017.305. [DOI] [Google Scholar]
- 16.Constant J., Phauk S., Bourgoin T. Updating lanternflies biodiversity knowledge in Cambodia (Hemiptera: Fulgoromorpha: Fulgoridae) by optimizing field work surveys with citizen science involvement through Facebook networking and data access in FLOW website. Belgian J. Entomol. 2016;37:1–16. [Google Scholar]
- 17.Wang C.-H., Wu I.-H., Lu C.-N. Population Monitoring of Pyrops watanabei after Its Removal from the Protected Species List. Forestry Bureau; Taipei, Taiwan: 2011. 83p. Project Report No. 100-30. (In Chinese) [Google Scholar]
- 18.Constant J., Barlett C. New records and species in five planthopper families from Keo Seima Wildlife Sanctuary, Cambodia with checklist of Cambodia planthoppers (Hemiptera: Fulgoromorpha) Belgian J. Entomol. 2019;83:1–27. [Google Scholar]
- 19.Fatouros N.E., Cusumano A., Bin F., Polaszek A., van Lenteren J.C. How to escape from insect parasitoids: A review of potential factors explaining parasitoid absence across the Insecta. Proc. R. Soc. B. 2020;287:20200344. doi: 10.1098/rspb.2020.0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu H. Oviposition substrate selection, egg mass characteristics, host preference, and life history of the spotted lanternfly (Hemiptera: Fulgoridae) in North America. Environ. Entomol. 2019;48:1452–1468. doi: 10.1093/ee/nvz123. [DOI] [PubMed] [Google Scholar]
- 21.Mason R.T., Fales H.M., Jones T.H., O’Brien L.B., Taylor T.W., Hogue C.L., Blum M.S. Characterization of fulgorid waxes (Homoptera: Fulgoridae: Insecta) Insect Biochem. 1989;19:737–740. doi: 10.1016/0020-1790(89)90054-1. [DOI] [Google Scholar]
- 22.Hischen F., Reiswich V., Kupsch D., De Mecquenem N., Riedel M., Himmelsbach M., Weth A., Heiss E., Armbruster O., Heitz J., et al. Adaptive camouflage: What can be learned from the wetting behavior of the tropical flat bugs Dysodius lunatus and Dysodius magnus. Biol. Open. 2017;6:1209–1218. doi: 10.1242/bio.026070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Locke M. Secretion of wax through the cuticle of insects. Nature. 1959;184:1967. doi: 10.1038/1841967a0. [DOI] [Google Scholar]
- 24.Bourgoin T. Les Glandes Tégumentaires ches les Tèttigometridae (Hemiptera, Fulgoromorpha) Annls. Soc. Ent. Fr. N.S. 1986;22:139–144. doi: 10.1080/21686351.1986.12278415. [DOI] [Google Scholar]
- 25.Bosuang S., Audibert C., Porion T., Chan C.L. A Guide to Lanternflies of Borneo. Natural History Publications (Borneo); Kota Kinabalu, Malaysia: 2017. 119p [Google Scholar]
- 26.Uyi O., Keller J.A., Swackhamer E., Hoover K. Performance and host association of spotted lanternfly (Lycorma delicatula) among common woody ornamentals. Sci. Report. 2021;11:15774. doi: 10.1038/s41598-021-95376-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Faraji F., Janssen A., Sabelis M.W. Oviposition patterns in a predatory mite reduce the risk of egg predation caused by prey. Ecol. Entomol. 2002;27:660–664. doi: 10.1046/j.1365-2311.2002.00456.x. [DOI] [Google Scholar]
- 28.Hsieh W.Y. The plant species belonged to Elaeocarpus, Elaeocarpaceae native to Taiwan and their close related alien species, Ceylon-olive Elaeocarpus. Taiwan For. J. 2019;45:62–66. (In Chinese) [Google Scholar]
- 29.Kim J.G., Lee E.-H., Seo Y.-M., Kim N.-Y. Cyclic behavior of Lycorma delicatula (Insecta: Hemiptera: Fulgoridae) on host plants. J. Insect Behav. 2011;24:423–435. doi: 10.1007/s10905-011-9266-8. [DOI] [Google Scholar]
- 30.Hsu C.-K., Chen F.-H., Wu C.-C., Wang L.-J. Forestry Research Newsletter, Vol. 25, No. 1. Taiwan Forestry Research Institute; Taipei, Taiwan: 2018. Business Opportunities in Blooming Trees: A Preliminary Study on Honey Plants in Lienhuachih Research Center; pp. 28–35. (In Chinese) [Google Scholar]
- 31.Chou F.-S., Lin W.-C., Hsui Y.-R., Huang C.-C. Forest types and succession in the Lienhuachih experimental forest of central Taiwan. Taiwan J. For. Sci. 2020;35:257–275. (In Chinese) [Google Scholar]
- 32.Liao C.-K., Chou F.-S., Lin C.-C. Succession of a degraded low-elevation mountain forest. Taiwan J. For. Sci. 2014;29:S13–S26. (In Chinese) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article and Supplementary Materials, further inquiries could be directed to the corresponding author.