Simple Summary
Insecticides, while boosting crop yields, can harm non-target insects like Vespa magnifica. This study explored the effects of four common insecticides: thiamethoxam, avermectin, chlorfenapyr, and β-cypermethrin, on Vespa magnifica. Although larval survival was unchanged, pupation and fledge rates dropped significantly. Enzyme assays showed stress responses with increased antioxidant activity and suppressed peroxidase levels. Transcriptomic analysis revealed heightened energy expenditure, impacting essential functions like flight and immunity. Key pathways related to metabolism and nervous system activity were also affected, potentially impairing learning, memory, and detoxification. These results highlight the broader ecological risks of insecticide exposure, emphasizing the need for better strategies to protect beneficial insect populations.
Keywords: Vespa magnifica, sublethal concentration, developmental calendar, transcriptome
Abstract
Insecticides are widely used to boost crop yields, but their effects on non-target insects like Vespa magnifica are still poorly understood. Despite its ecological and economic significance, Vespa magnifica has been largely neglected in risk assessments. This study employed physiological, biochemical, and transcriptomic analyses to investigate the impact of sublethal concentrations of thiamethoxam, avermectin, chlorfenapyr, and β-cypermethrin on Vespa magnifica. Although larval survival rates remained unchanged, both pupation and fledge rates were significantly reduced. Enzymatic assays indicated an upregulation of superoxide dismutase and catalase activity alongside a suppression of peroxidase under insecticide stress. Transcriptomic analysis revealed increased adenosine triphosphate-related processes and mitochondrial electron transport activity, suggesting elevated energy expenditure to counter insecticide exposure, potentially impairing essential functions like flight, hunting, and immune response. The enrichment of pathways such as glycolysis, hypoxia-inducible factor signaling, and cholinergic synaptic metabolism under insecticide stress highlights the complexity of the molecular response with notable effects on learning, memory, and detoxification processes. These findings underscore the broader ecological risks of insecticide exposure to non-target insects and highlight the need for further research into the long-term effects of newer insecticides along with the development of strategies to safeguard beneficial insect populations.
1. Introduction
Pesticides play a crucial role in modern agriculture and are widely used worldwide to control pests and protect crop yields. These chemicals include insecticides, acaricides, nematicides, herbicides, and plant growth regulators [1]. While effective against pests, insecticides also pose significant risks to non-target beneficial insects [2]. In the midst of the Sixth Great Species Extinction, the decline in arthropod populations underscores the urgency of understanding insecticides impacts on these species [3]. For instance, imidacloprid exposure lowers activity levels in Bombus impatiens, and phoxim and cypermethrin trigger detoxification enzymes in Meteorus pulchricornis [4,5]. However, the effects of insecticides on Vespa magnifica remain largely unexplored.
Vespa magnifica, a species of the order Hymenoptera, suborder Apocrita, family Vespidae and genus Vespa, is a holometabolous insect that undergoes four developmental stages: egg, larva, pupa, and adult. Both females and drones die before winter with only the queen surviving to overwinter and re-emerge in early March. The queen then constructs a nest and begins laying eggs. The full developmental cycle spans 36 to 43 d, consisting of 6–8 d for the egg stage, 10–15 d for the larval stage, and 16–20 d for the pupal stage [6]. The developmental timeline of Vespa magnifica is significantly influenced by factors such as the generation number, nutrient intake, and local climate [7]. As ground-nesting wasps, Vespa magnifica are exposed to insecticides throughout their life cycle, including during foraging, nesting, nursing, and hibernation. They can also be exposed to insecticides present in the air, plants, propolis, resins, and soil [8]. Exposure to insecticides disrupts the nervous system of Vespa magnifica, causing disorientation and lethargy, which in turn reduces hunting and nest-building efficiency. Additionally, it impairs development, particularly pupation and eclosion, leading to delays and abnormalities in larvae, thus decreasing their ability to successfully pupate and emerge as adults [9,10].
Vespa magnifica plays significant ecological and economic roles [11]. Ecologically, as a predatory insect, it helps regulate populations of other insects, including pests that can damage crops or disrupt ecosystems. For instance, in 1978, Shimen County in Hunan Province, China, successfully utilized wasps to control cotton bollworm infestations, dramatically reducing insecticide costs and usage [12]. Similarly, in the Shizuoka Prefecture, Japan, wasps were used for pest management in tea plantations, while in Feng County, Jiangsu Province, wasps were employed to control cotton bollworms, achieving a pest elimination rate of 98.5% [13]. Building on the homing behavior of Vespa velutina, Guo et al. designed portable wooden or wire mesh boxes for rearing hornets. These boxes, housing hornet colonies, were strategically placed in vegetable, fruit, and tea fields, enabling fast, thorough, and environmentally safe pest control [14]. Additionally, Vespa magnifica can prey on various pests such as seudaulacaspis pentagona, Cetonia aurata, Plutella xylostella, Locusta migratoria, Propylaea japonica, and Orius strigicollis [6]. Economically, Vespa magnifica indirectly benefits agriculture by reducing the need for chemical insecticides, leading to cost savings and more sustainable farming practices [15]. Additionally, its presence in ecosystems can enhance pollination indirectly by controlling herbivorous insects, contributing to healthier crop yields [16].
Thiamethoxam, a second-generation neonicotinoid insecticide, is considered the most widely used insecticide globally due to its high efficiency, broad-spectrum activity, and strong systemic properties. It acts on the nicotinic acetylcholine receptors in the insect nervous system. The 25% water-dispersible granule formulation of thiamethoxam is commonly used against pests such as Aonidiella aurantii (Hemiptera), Sitobion avenae (Aphididae), and Popillia japonica (Coleoptera) [17,18]. In recent years, the negative effects of thiamethoxam on non-target organisms, particularly bees, have garnered significant attention. For instance, prolonged or short-term exposure to thiamethoxam has been shown to impair bee flight ability [19]. Additionally, thiamethoxam causes sublethal effects in bees, damaging their central nervous system and leading to midgut injuries [20]. In social wasps, exposure to lethal and sublethal concentrations of thiamethoxam reduces mobility and disrupts the stability of worker wasps and the colony [21]. Brazilian wasps (Polybia paulista) exposed to LC50 and LC10 sublethal concentrations of thiamethoxam exhibit reduced activity, loss of the intestinal brush border, the presence of spherocrystals, and brain cell apoptosis characterized by loss of cell contact and pyknosis [22]. In response to these concerns, the European Union fully banned the registration of thiamethoxam for use outside permanent greenhouses in 2018 and proposed the revocation of all maximum residue limits for thiamethoxam on agricultural products in 2022 [23]. As a hymenopteran of economic significance, studying the toxicity of thiamethoxam on Vespa mandarinia could provide a solid research foundation and robust scientific data to support adjustments to China’s neonicotinoid insecticide registration policies and future technical trade negotiations with the EU and other members regarding neonicotinoid-related measures.
Avermectin, a macrolide antibiotic produced by streptomyces avermitilis, is known for its unique mechanism of action and strong insecticidal activity, exhibiting both stomach toxicity and contact effects. With the increasing use of 1.8% avermectin emulsifiable concentrate, concerns have arisen regarding its potential toxicity to non-target organisms in the environment [24]. This highlights the need to evaluate the sublethal effects of avermectin on wasps, particularly Vespa magnifica. Understanding the sublethal effects of avermectin is crucial, as these effects may not result in immediate mortality but can significantly impact the health and behavior of wasp populations. For instance, studies have shown that exposure to sublethal doses of avermectin can lead to alterations in metabolic functions in other species, such as reduced glycogen levels and lipid synthesis, along with the inhibition of key enzyme activities [25]. These metabolic disruptions can compromise energy balance, reproductive success, and overall fitness. In addition, avermectin has been found to impair cell proliferation and induce mitochondrial apoptosis in honeybee cells [26]. Such findings underscore the potential risk that avermectin poses to wasps, which may experience similar cellular and physiological disturbances. The induction of toxic effects and the disruption of normal physiological functions could lead to decreased foraging efficiency, impaired nest maintenance, and weakened colony dynamics. By elucidating the mechanisms through which avermectin affects wasps, we can better assess its risks to beneficial insect populations and contribute to the development of more sustainable pest management practices.
Chlorfenapyr is a novel aryl-substituted pyrrole compound with a broad spectrum of insecticidal activity, offering safety and long-lasting effects by targeting insect mitochondria [27]. The 12% emamectin benzoate–chlorfenapyr suspension is highly effective in controlling Orthopteran pests, such as locusts and katydids, as well as Lepidopteran pests, which are key prey for Vespa magnifica [28]. Field studies on sublethal toxicity in Osmia excavata exposed to chlorfenapyr showed significant delays in larval development and a marked reduction in emergence rates [29]. While the sublethal effects of chlorfenapyr on Apis mellifera have been documented [30], research on its effects on Vespa magnifica remains limited. Thus, evaluating the sublethal effects of chlorfenapyr on Vespa magnifica is crucial for assessing its ecological risks especially given the critical predatory and ecological role of Vespa magnifica.
β-cypermethrin is a widely used pyrethroid insecticide that primarily exerts its insecticidal effect by disrupting the sodium ion channels in the nervous system, leading to impaired movement, spasms, paralysis, and eventually death [31]. However, sublethal doses of β-cypermethrin pose negative impacts on non-target insects, particularly important predatory species like Vespa magnifica. Studies have shown that sublethal doses of β-cypermethrin not only contaminate honeybee hives, causing severe harm to the colonies, but also inhibit the growth and development of other insects, such as the significant weight reduction observed in Spodoptera litura larvae [32,33]. Additionally, in natural ecosystems, pests like Solenopsis invicta are known to threaten bee colonies during breeding seasons, and the application of insecticides may interfere with Vespa magnifica’s predatory behavior and population stability [34]. Therefore, assessing the sublethal effects of β-cypermethrin on Vespa magnifica is crucial for understanding its ecological risks and providing a scientific foundation for insecticide management policies.
The aim of this study was to evaluate the effects of sublethal concentrations (LC10, LC20, and LC30) of thiamethoxam, avermectin, chlorfenapyr, and β-cypermethrin on the physiological development of Vespa magnifica using a feeding exposure method and to further investigate these impacts through transcriptome analysis. Utilizing a feeding exposure method, we assessed parameters such as larval survival, pupation rates, fledge success, body weight at various developmental stages, and enzyme activity. Advanced transcriptome analysis was conducted to pinpoint differentially expressed genes (DEGs), which was followed by in-depth gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analyses, with results further validated through reverse transcription quantitative polymerase chain reaction (RT-qPCR). This comprehensive approach provides a valuable foundation for further ecological risk assessments and the development of targeted pest management that mitigates unintended impacts on non-target species such as Vespa magnifica.
2. Materials and Methods
2.1. Ethical Guidelines
Vespa magnifica is neither a protected nor endangered species and is commonly found in rural and suburban areas of southern China. According to the legislation of the People’s Republic of China, research involving arthropods does not require ethical approval [35]. Our housing conditions and testing procedures comply with established animal welfare standards.
2.2. Animals
The Vespa magnifica specimens were acquired from the Wasp General Base at the Yunnan (Lufeng) Hatchery Park (102.016751° N, 25.043597° E) and reared at the School of Agriculture and Life Sciences, Kunming University. Rearing conditions were kept at a temperature of (25 ± 2) °C and a relative humidity of (60 ± 10)%. The diet included a nutritional solution (Wasp General Base at the Yunnan (Lufeng) Hatchery Park, Chuxiong, China), honey, and Locusta migratoria manilensis (Maoyuan Locust Breeding Base, Kunming, China). The experiment, conducted during the egg-laying period of the queen, used healthy one-day-old larvae. All specimens originated from the same colony and were starved for 2 h before the experiment.
2.3. Insecticides
In this study, commercial formulations were used instead of technical drug insecticides to reflect real-world exposure in Vespa magnifica habitats, providing more accurate data for ecological risk assessment and management [36]. According to our preliminary survey results, the insecticides used in this study are commonly applied in Vespa magnifica breeding areas across nine cities in Yunnan, China: Kunming, Dali, Chuxiong, Yuxi, Qujing, Mengzi, Pu’er, Xishuangbanna, and Mangshi [37]. These insecticides include thiamethoxam (25% water-dispersible granules, Chuan Dong Pesticide & Chemical Co., Ltd., Dazhou, China), avermectin (1.8% emulsifiable concentrate, Shandong Rongbang Chemical Co., Ltd., Binzhou, China), chlorfenapyr (12% suspension concentrate, Shandong Yilan Technology Co., Ltd., Weifang, China), and β-cypermethrin (4.5% microemulsion, Chengdu Bangnong Chemical Co., Ltd., Chengdu, China). Based on our previous acute toxicity studies, the LC10, LC20, and LC30 sublethal concentrations of the insecticides thiamethoxam, avermectin, chlorfenapyr, and β-cypermethrin were determined (Table 1).
Table 1.
Different sublethal concentrations of four insecticides to Vespa magnifica [37].
| Insecticides | Sublethal Concentrations (μg a.i./wasp) | ||
|---|---|---|---|
| LC10 | LC20 | LC30 | |
| Thiamethoxam | 2.42 × 10−6 | 5.07 × 10−6 | 6.34 × 10−6 |
| β-Cypermethrin | 8.24 × 10−5 | 1.74 × 10−4 | 3.09 × 10−4 |
| Avermectin | 2.28 × 10−6 | 4.42 × 10−6 | 6.29 × 10−6 |
| Chlorfenapyr | 6.43 × 10−6 | 1.06 × 10−5 | 1.80 × 10−5 |
2.4. Sublethal Effects of Thiamethoxam, Avermectin, Chlorfenapyr, and β-Cypermethrin on the Developmental Stages of Vespa magnifica
A basic larval diet was prepared consisting of 50% nutritional solution, 37% sterile water, 6% glucose, 6% locust juice, and 1% yeast extract, which can be stored at 4 °C for 3 days [38]. The locust juice was prepared by removing the wings and legs of locusts, which was followed by crushing them in a blender. The test insecticides formulations were dissolved in ultrapure water to create stock solutions to replace sterile water in the preparation of the larval diet (Table 1). Insecticide exposure was conducted within the combs of 1-day-old Vespa magnifica larvae. The larval combs were randomly divided into five groups with each group marked and consisting of 50 larvae: (1) control group (larval diet with sterile water); (2) thiamethoxam group (larval diet containing LC10, LC20, and LC30 concentrations of thiamethoxam); (3) avermectin group (larval diet containing LC10, LC20, and LC30 concentrations of avermectin); (4) chlorfenapyr group (larval diet containing LC10, LC20, and LC30 concentrations of chlorfenapyr); (5) β-cypermethrin group (larval diet containing LC10, LC20, and LC30 concentrations of β-cypermethrin). Every morning at 9:00 a.m., the combs were removed, and 20 µL of different insecticide solutions was fed to the larvae using a dropper. The combs were then returned to the Vespa magnifica breeding box with adult workers. Feeding continued for six days until the larvae were capped. During this period, adult Vespa magnifica were given a 50% honey solution and Locusta migratoria manilensis, and in turn, they fed the larvae. Observations were made at 12 h intervals (12 h, 24 h, 36 h, 48 h, 54 h, 60 h, 66 h, and 72 h). The number of dead larvae, pupae, and newly fledged Vespa magnifica was recorded with dead individuals promptly removed to prevent pathogen growth. Larval survival, pupation, and fledge rates were calculated, and body weights were measured. The artificial climate chamber was controlled by an air conditioner (KFR-35GW, Midea, China) and a humidifier (CS-3VWL, Midea, China) with the experiment conducted in an artificial climate chamber at 25 ± 2 °C and 50–60% relative humidity.
2.5. Evaluation of Antioxidant Enzyme and Peroxidase Activity in Vespa magnifica Exposed to Thiamethoxam, Avermectin, Chlorfenapyr, and β-Cypermethrin
To prepare 10 mL of LC10, LC20, and LC30 insecticide solutions for thiamethoxam, avermectin, chlorfenapyr, and β-cypermethrin, equal parts honey and water (1:1) were mixed. The control group received the same honey–water mixture. Each treatment was replicated three times with 20 wasps per group. Vespa magnifica were first fed the insecticide solution, which was followed by continuous access to the honey-water mixture. After 24 h, surviving wasps were stored at −80 °C for further analysis.
To prepare wasp abdominal worm specimens, 0.5 g of tissue was weighed, frozen in liquid nitrogen, and ground. After grinding, 5 mL of the corresponding enzyme kit extraction buffer was added. The mixture was then homogenized in an ice bath after thawing. It was centrifuged at 8000× g for 10 min at 4 °C using a high-speed refrigerated centrifuge (TGL-1650, Shuke Instrument Co., Ltd., Chengdu, China). The supernatant was collected, with one portion kept on ice for immediate analysis, and the remainder was frozen at −80 °C for future use. Superoxide dismutase (SOD) activity was measured at 560 nm, catalase (CAT) activity at 240 nm, and peroxidase (POD) activity at 470 nm using enzyme assay kits (Solarbio Science & Technology Co., Ltd., Beijing, China) on a spectrophotometer (CARY60, Shimadzu International Trading Co., Ltd., Shanghai, China).
2.6. RNA Sequencing
The impact on the Vespa magnifica became more pronounced as the sublethal concentrations increased [37]. To investigate the potential molecular mechanisms underlying Vespa magnifica exposure to thiamethoxam, avermectin, chlorfenapyr, and β-cypermethrin, we constructed a total of 15 sequencing libraries from adult Vespa magnifica treated with these insecticides. Specifically, Vespa magnifica were exposed to sublethal concentrations (LC10) of thiamethoxam, abamectin, chlorfenapyr, and β-cypermethrin for 24 h, while the control group received 50% honey water. After treatment, the surviving hornets were immediately dissected to extract the intestines, which were collected into 1.5 mL centrifuge tubes, treated with liquid nitrogen, and stored at −80 °C. Total RNA was extracted using the TaKaRa column kit (Baori Medical Biotechnology, Beijing, China), and RNA concentration and purity were measured with a NanodropTM (Nanodrop 2000, Thermo Fisher Scientific, Waltham, MA, USA). The RNA Integrity Number (RIN) was determined using the Agilent 2100 (G2938C, Agilent, Santa Clara, CA, USA). For single library construction, the total RNA must meet the following requirements: ≥1.0 µg in total amount, concentration ≥ 35.0 ng/µL, and OD 260/280 ≥ 1.8. A library was constructed using more than 1.0 µg of total RNA, which included mRNA purification, mRNA fragmentation, cDNA synthesis, end repair and A-tailing, adapter ligation, library selection, and quality control [39]. RNA sequencing and library construction were performed at Shanghai Meiji Biomedical Technology Co., Ltd., Shanghai, China.
2.7. Transcriptome Sequencing, Gene Annotation and DEG Analysis
The raw data were obtained using the sequencing by synthesis (SBS) technology. Illumina HiSeq 2000 was used to analyze the data, removing adapter-containing reads, reads with undetermined base information, and low-quality reads. The effective reads (clean reads) for further analysis were obtained by checking the GC content and base error rate. Quality control of the raw data was performed using a Fastx_toolkit (http://hannonlab.cshl.edu/fastx_toolkit/, 0.0.14, accessed on 15 October 2023). Hisat 2 (http://ccb.jhu.edu/software/hisat2/index.shtml, 2.1.0, accessed on 20 October 2023) was used as the alignment tool for sequence alignment to the reference genome [40]. Gene annotation was performed using Swiss-Prot (ftp://ftp.uniprot.org/pub/databases/uniprot/current_release/knowledgebase/complete/uniprot_sprot.fasta.gz, 2022.10, accessed on 20 October 2023). Kallisto (https://pachterlab.github.io/kallisto/download, 0.46.0, accessed on 25 October 2023) was employed to quantify gene expression levels [41]. DESeq2 (http://bioconductor.org/packages/stats/bioc/DESeq2/, 1.24.0, accessed on 25 October 2023) was used to analyze the differential expression between the control group and the groups treated with thiamethoxam, avermectin, chlorfenapyr, and β-cypermethrin [42]. GO enrichment and KEGG pathway analyses for DEGs were conducted using Goatools (https://files.pythonhosted.org/packages/bb/7b/0c76e3511a79879606672e0741095a891dfb98cd63b1530ed8c51d406cda/goatools-0.8.9.tar.gz, 0.6.5, accessed on 30 October 2023).
2.8. Reverse Transcription Quantitative Polymerase Chain Reaction (RT-qPCR)
In insects, cytochrome P450 (CYP450) plays a crucial role in detoxification, breaking down harmful chemicals and reducing their toxicity. Selecting CYP450-related genes for RT-qPCR validation in Vespa magnifica provides insight into the activation of detoxification pathways in response to insecticide exposure, making these genes key markers for understanding the molecular mechanisms of insecticide resistance and tolerance [43]. GAPDH, a key enzyme in glycolysis, plays a vital role in cellular metabolism and is minimally influenced by environmental factors. Therefore, selecting GAPDH as the optimal internal reference gene for gene expression studies in the gut of Vespa magnifica ensures both the accuracy and consistency of experimental results [44]. To further verify the accuracy of the gene expression trends observed in RNA sequencing, 9 genes related to the CYP450 enzyme system were randomly selected from the DEGs for qRT-PCR validation (Table 2). The RT-qPCR reactions were performed using the SYBR® Premix Ex Taq™ II (Tli RNaseH Plus) kit (TaKaRa, Ōtsu City, Japan) and a real-time quantitative PCR instrument (StepOneTM 4376373, ThermoFisher, Waltham, MA, USA). The reaction system consisted of 10 μL of 2X ChamQ SYBR Color qPCR Master Mix, 0.8 μL of each forward and reverse primer, 10 μL of 50X ROX Reference Dye, 6 μL of ddH2O, and 2 μL of diluted cDNA. The RT-qPCR conditions were 95 °C for 5 min, followed by 40 cycles of 95 °C for 30 s, 55 °C for 30 s, and 72 °C for 40 s [45]. The relative expression of target genes was calculated using the comparative CT method (Relative expression = 2−ΔΔCT) [46].
Table 2.
Sequences of primers used for qRT-PCR analysis.
| Gene ID | KO Name | NCBI | Primer F | Primer R | Length (bp) |
|---|---|---|---|---|---|
| NODE_cov_102.801070_g8279_i0 | CYP4 | XP_035741425.1 | TTGTCCAGCCATATTTAC | TAGGTGCTATTAGTTTACGA | 127 |
| NODE_cov_98.805527_g8081_i0 | CYP4V | XP_035742762.1 | CGAAGCCATTCATAAACA | ATACTCCACGGTCTCCTC | 125 |
| NODE_cov_68.538621_g2989_i0 | CYP6 | XP_035741324.1 | TGAATGTATGGTTCCCAGTT | CAATCCGAAGGGCAAGTA | 137 |
| NODE_cov_74.509154_g2989_i0 | CYP61 | CAF1632358.1 | CTATACGAATTGGCTCTG | GAAATGTTACTGGTGGAT | 169 |
| NODE_cov_61.292950_g3449_i0 | CYP9 | XP_035722235.1 | CTTATTGCGTACTTGTCC | AATGATACCCTTCTCGTC | 127 |
| NODE_cov_53.046424_g4522_i0 | GST | XP_035723860.1 | GCTAACAACAGGACCATC | AGCCCATAATACAATAACCA | 149 |
| NODE_cov_80.862000_g15569_i0 | PPIB | KAH9408598.1 | TCCAAATTGGCGGTAAAG | TTGATAACCATCCAGCAC | 270 |
| NODE_cov_74.683208_g15569_i0 | UTG | XP_046836138.1 | ACATAGACCCATTATCACC | TACCCATAAGACCACCAT | 300 |
| NODE_cov_16.023701_g14314_i0 | GAPDH | KAI2807735.1 | CGATGTTCGTCGTTGGTG | TTTGGGTTGCCGTGATAG | 171 |
2.9. Statistical Analysis
SPSS v27.0 (SPSS Inc., Chicago, IL, USA) software was used to perform a Shapiro–Wilk test for normality and Levene test for chi-square. Significant analysis of variance (p < 0.05) was carried out using one-way ANOVA for variance and Tukey’s method after passing the test. The significance of differences in the larval survival rate, pupation rate, fledge rate, body weight and enzyme activity were calculated. Origin v21.0 drawing software was used for drawing.
3. Results
3.1. Effect of Sublethal Effects of Insecticides Exposure on Development, Body Weight, and Enzyme Activity in Vespa magnifica
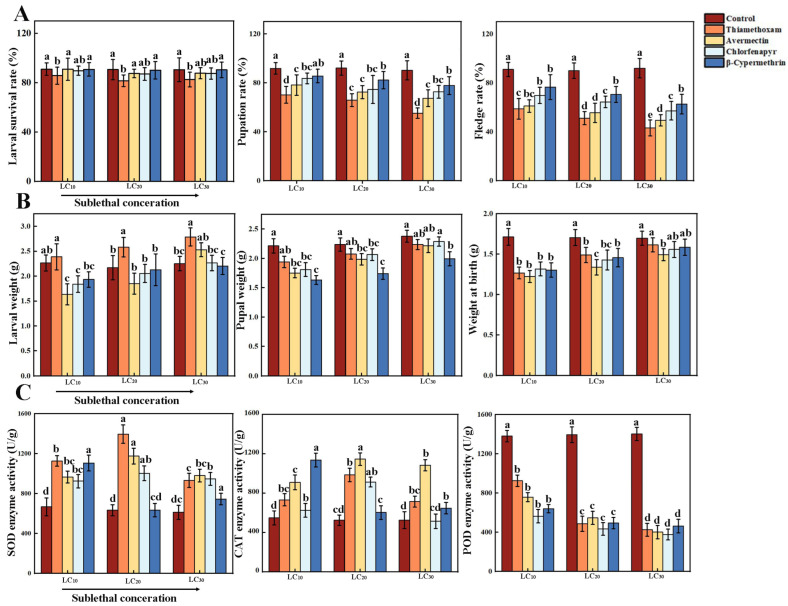
Under sublethal stress, thiamethoxam had a significant effect on the survival rate of Vespa magnifica larvae, while abamectin, chlorfenapyr, and β-cypermethrin did not show significant differences in larval survival rates. However, thiamethoxam, abamectin, chlorfenapyr, and β-cypermethrin all had significant effects on pupation and fledge rates with the differences becoming more pronounced as sublethal concentrations increased. Among them, the thiamethoxam LC30 treatment group showed the most notable reductions with pupation and eclosion rates significantly decreasing by 36.99% and 52.06%, respectively (p < 0.05, Figure 1A).
Figure 1.
Sublethal effects of insecticides exposure on development, body weight, and enzyme activity in Vespa magnifica. (A) Sublethal effects of insecticides exposure on larval survival, pupation and fledge rates of Vespa magnifica (n = 50, p < 0.05). (B) Body weight changes in Vespa magnifica larvae treated with sublethal concentrations of insecticides during the larval, pupal, and fledge stages (n = 50, p < 0.05). (C) SOD, CAT, and POD enzyme activity in Vespa magnifica following exposure to sublethal concentrations of insecticides (n = 20, p < 0.05). The different letters (a, b, c and d) above the bars indicate significant differences between the groups.
During the larval stage, larvae treated with thiamethoxam at LC30 showed a 25.9% increase in body weight compared to the control group (2.28 ± 0.018 g). In contrast, larvae treated with avermectin, chlorfenapyr, and β-cypermethrin exhibited lower body weights than the control, although their weights increased with higher concentrations. Across all treatments, body weights remained consistently lower than controls during both the pupal and fledging stages. No significant differences in body weight were observed between the chlorfenapyr and β-cypermethrin groups at emergence, which was possibly due to environmental adaptability in the insects (p < 0.05, Figure 1B).
As the sublethal concentrations increased, the activity levels of SOD and CAT in Vespa magnifica treated with thiamethoxam, abamectin, and chlorfenapyr for 24 h initially rose and then declined compared to the control group. At the sublethal concentration of LC20, SOD activity peaked at 1396.21 U/g, and CAT activity reached a maximum of 986.26 U/g. In contrast, after 24 h of exposure to β-cypermethrin, SOD and CAT activity initially decreased and then increased with SOD activity peaking at 1106.20 U/g and CAT activity at 1137.81 U/g. Both enzymes exhibited activation effects. Additionally, as sublethal concentrations increased, the activity of POD in Vespa magnifica exposed to thiamethoxam, abamectin, chlorfenapyr, and β-cypermethrin for 24 h gradually decreased compared to the control group, showing an inhibitory effect on POD activity. At the sublethal concentration of LC30, thiamethoxam caused the highest inhibition of POD activity, reaching 425.60 U/g (p < 0.05, Figure 1C).
3.2. Transcriptome Sequencing, Gene Annotation and Analysis of DEGs
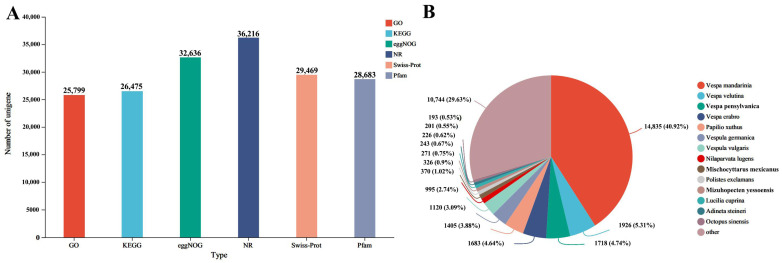
The total RNA yield for each sample was ≥1.0 µg with concentrations ≥35.0 ng/µL, an OD 260/280 ratio of ≥1.8, and an OD 260/230 ratio of ≥1.0. Raw reads from Vespa magnifica intestinal transcriptome sequencing ranged from 41.18 to 50.87 million. After quality control, 97.8% of the raw reads were retained (40.86 to 50.37 million) with Q20 and Q30 values exceeding 95% and 93%, respectively (Table 3). All genes and transcripts from the Vespa magnifica transcriptome were compared against six databases (NR, Swiss-Prot, Pfam, EggNOG, GO, KEGG) using BLAST. NR annotations showed the highest proportion (58.23%) under sublethal insecticide stress with 40.92% of sequences aligning with Vespa mandarinia (Figure 2A,B).
Table 3.
Transcriptome sequencing data.
| Sample | Raw Reads | Clean Reads | Average Base Error Rate (%) | Q20 (%) | Q30 (%) | GC (%) |
|---|---|---|---|---|---|---|
| Control 1 | 45,917,960 | 45,562,310 | 0.0273 | 96.27 | 94.11 | 35.97 |
| Control 2 | 44,923,142 | 44,359,422 | 0.0272 | 96.25 | 94.16 | 39.05 |
| Control 3 | 41,176,086 | 40,861,948 | 0.0276 | 95.92 | 93.66 | 37.23 |
| Thiamethoxam 1 | 46,030,496 | 45,668,930 | 0.0278 | 96.26 | 94.07 | 36.54 |
| Thiamethoxam 2 | 45,640,090 | 45,245,878 | 0.0269 | 96.32 | 94.18 | 34.08 |
| Thiamethoxam 3 | 46,618,708 | 46,124,634 | 0.0272 | 96.19 | 93.91 | 30.88 |
| Avermectin 1 | 50,873,080 | 50,371,152 | 0.0274 | 96.06 | 93.85 | 36.79 |
| Avermectin 2 | 42,207,426 | 41,867,502 | 0.0278 | 95.85 | 93.55 | 36.72 |
| Avermectin 3 | 48,891,090 | 48,240,970 | 0.0269 | 96.35 | 94.08 | 32.15 |
| Chlorfenapyr 1 | 41,813,518 | 41,388,414 | 0.0279 | 95.82 | 93.46 | 36.91 |
| Chlorfenapyr 2 | 46,337,574 | 45,928,132 | 0.0279 | 95.82 | 93.49 | 37.68 |
| Chlorfenapyr 3 | 46,115,384 | 45,661,982 | 0.0273 | 96.17 | 93.88 | 36.75 |
| β-Cypermethrin 1 | 44,129,178 | 43,744,382 | 0.0275 | 96.23 | 93.76 | 38.20 |
| β-Cypermethrin 2 | 44,483,326 | 44,100,214 | 0.0274 | 96.05 | 93.81 | 37.62 |
| β-Cypermethrin 3 | 46,039,540 | 45,601,858 | 0.0273 | 96.09 | 93.89 | 36.16 |
Figure 2.
Functional annotation and species distribution of unigenes in Vespa magnifica transcriptome. (A) Annotation result statistics of unigenes of Vespa magnifica transcriptome in different databases. (B) Species distribution of unigenes of Vespa magnifica NR database.
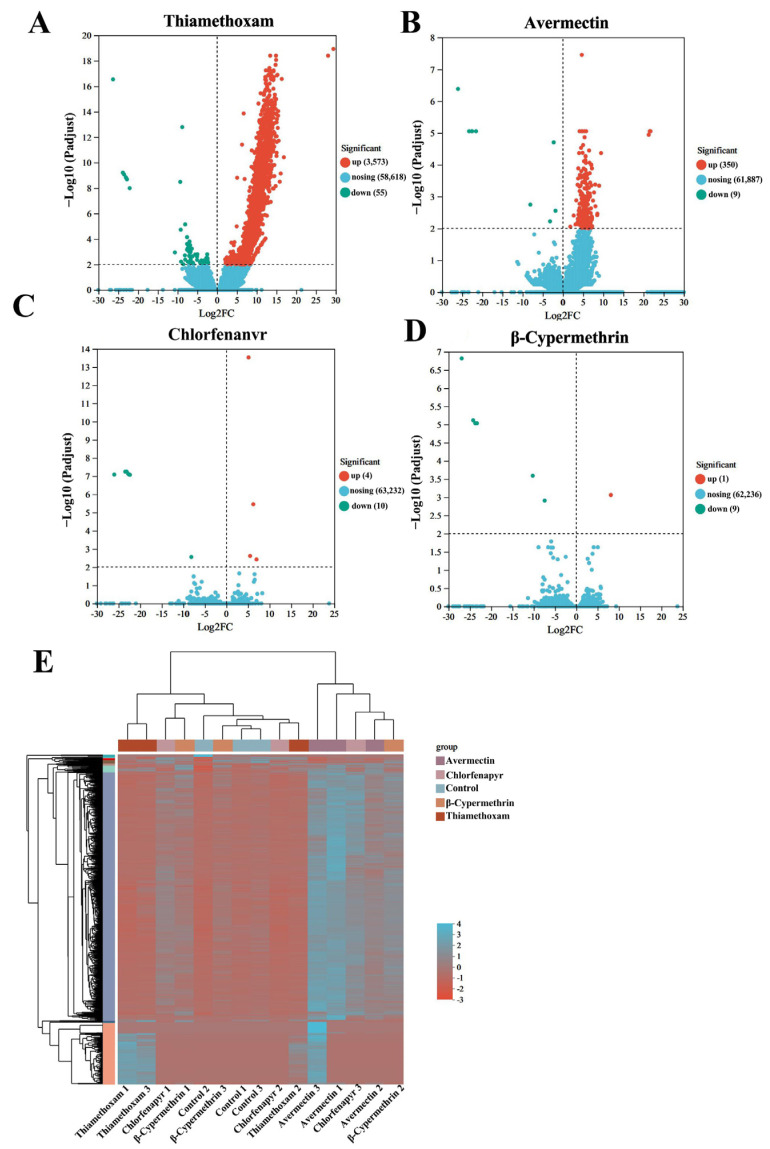
Volcano plot analysis revealed 3628 DEGs between the thiamethoxam and control groups, with 3572 upregulated and 55 downregulated (Figure 3A), indicating a strong upregulatory effect. Avermectin exposure resulted in 359 DEGs (350 upregulated, 9 downregulated) (Figure 3B), showing a milder impact compared to thiamethoxam. Chlorfenapyr led to 14 DEGs (4 up-regulated, 10 downregulated) (Figure 3C), primarily causing downregulation. β-cypermethrin had minimal influence, with 10 DEGs (one upregulated, nine downregulated) (Figure 3D). Cluster analysis grouped DEGs with similar expression patterns, which was likely associated with related metabolic and signaling pathways (Figure 3E).
Figure 3.
DEGs between the control and four insecticides exposure for 24 h. (A) DEGs induced by thiamethoxam at an LC10 dose. (B) DEGs induced by avermectin at an LC10 dose. (C) DEGs induced by chlorfenapyr at an LC10 dose. (D) DEGs induced by β-cypermethrin at an LC10 dose. (E) Cluster analysis of DEGs of Vespa magnifica.
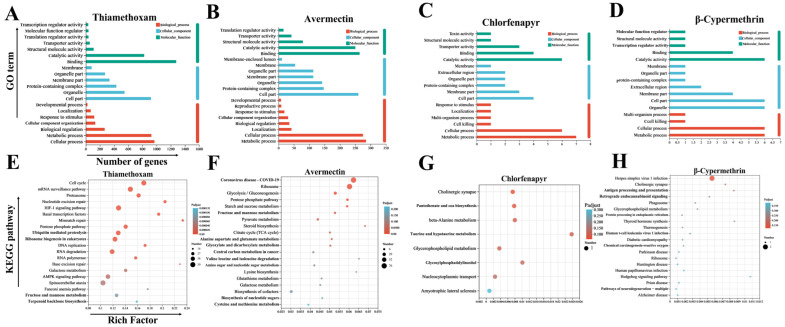
GO enrichment analysis of the DEGs revealed significant enrichment in biological process (BP), cellular component (CC), and molecular function (MF) categories, providing a comprehensive and detailed insight into the roles of genes within the organism [47]. In the BP category, the DEGs between thiamethoxam, abamectin, bromo-fipronil, β-cypermethrin, and the control group were primarily enriched in the subcategories of cellular process and metabolic process. In the CC category, the DEGs were mainly enriched in cell part, protein-containing complex, and organelles. In the MF category, DEGs were predominantly enriched in binding and catalytic activity (Figure 4A–D).
Figure 4.
GO and KEGG enrichment analysis of DEGs reveals key pathways and functions affected by insecticide treatments in Vespa magnifica. (A) GO aggregation map of DEGs in thiamethoxam and control. (B) GO aggregation map of DEGs in avermectin and control. (C) GO aggregation map of DEGs in chlorfenapyr and control. (D) GO aggregation map of DEGs in β-cypermethrin and control. (E) Enrichment analysis of KEGG pathway of thiamethoxam and control DEGs. (F) Enrichment analysis of KEGG pathway of avermectin and control DEGs. (G) Enrichment analysis of KEGG pathway of chlorfenapyr and control DEGs. (H) KEGG pathway enrichment analysis of cypermethrin and control DEGs.
In the transcriptome sequencing of the gut of Vespa magnifica, the DEGs were potentially involved in various functions such as detoxification, metabolism, and immunity. KEGG pathway enrichment analysis further revealed how these genes participate in specific metabolic pathways, signal transduction processes, or biological networks [48]. Compared to the control, thiamethoxam treatment resulted in the enrichment of 1371 KEGG metabolic pathways, involving 695 DEGs. The most significantly enriched pathways included the cell cycle, mRNA surveillance pathway, pentose phosphate pathway, nucleotide excision repair, basal transcription factors, HIF-1 signaling pathway, AMPK signaling pathway, and ribosome biogenesis in eukaryotes (Figure 4E). For avermectin, 462 KEGG pathways were enriched compared to the control group, involving 367 DEGs. The top ten enriched pathways possibly linked to detoxification properties included ribosome, glycolysis/gluconeogenesis, the pentose phosphate pathway, starch and sucrose metabolism, and pyruvate metabolism (Figure 4F). Chlorfenapyr treatment led to the enrichment of eight KEGG pathways, involving eight DEGs. The significantly enriched pathways included cholinergic synapse, pantothenate and CoA biosynthesis, beta-alanine metabolism, glycerophospholipid metabolism, and nucleocytoplasmic transport (Figure 4G). For β-cypermethrin, nine KEGG pathways were enriched, involving 21 DEGs. The significantly enriched pathways included herpes simplex virus 1 infection, cholinergic synapse, antigen processing and presentation, retrograde endocannabinoid signaling, and glycerophospholipid metabolism (Figure 4H).
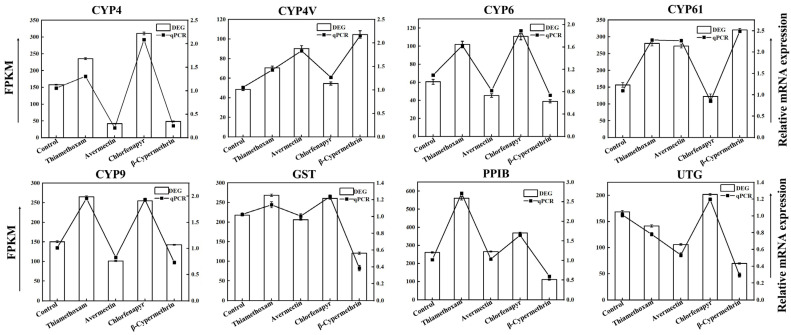
The RT-qPCR results were consistent with the RNA-seq data, confirming the reliability of the transcriptome analysis (Figure 5).
Figure 5.
qPCR validation of nine DEGs related to the CYP450 enzyme system confirms consistency with RNA-seq data.
4. Discussion
In this study, thiamethoxam, avermectin, chlorfenapyr, and β-cypermethrin had no significant effect on the survival of Vespa magnifica larvae. However, there were significant variations in pupation and fledge rates, which increased at sublethal concentrations. Notably, exposure to sublethal concentrations of insecticides, particularly thiamethoxam at LC30, led to a reduction in pupation rates to 36.99% and fledge rates to 52.06%, which can be attributed to various physiological disruptions. Thiamethoxam interacts with nicotinic acetylcholine receptors, causing neurotoxicity and developmental disorders [49]. In the LC30 treatment group, the reduction in pupation and fledge rates indicates a severe impairment in the larvae’s ability to undergo metamorphosis. Additionally, the energy required for detoxification diverts resources from critical developmental processes, further contributing to the decrease in both pupation and fledge rates [50]. Other insecticides, such as avermectin, chlorfenapyr, and β-cypermethrin, although less toxic to larval survival, still significantly affected pupation and fledge rates. As sublethal concentrations increased, the cumulative effects on pupation and fledge became more pronounced, reflecting the broader impact of sublethal stress on the development of Vespa magnifica. These findings align with research on Bombus terrestris larvae exposed to sublethal dinotefuran and reduced pupation rates in crabronid wasps exposed to thiamethoxam and imidacloprid [51,52]. Similar effects were observed in honey bees treated with chlorantraniliprole and propiconazole [53]. These findings emphasize the need to consider sublethal insecticide exposure when evaluating ecological risks, particularly for beneficial species like Vespa magnifica. In Vespa magnifica larvae exposed to LC30 concentrations of thiamethoxam, weight gain was observed. Thiamethoxam disrupts the endocrine system in larvae, affecting growth regulation and increasing the energy demands for detoxification, which leads to higher nutrient intake or storage [54]. After exposure, Vespa magnifica larvae alter their feeding behavior, redirecting energy toward detoxification and resulting in weight gain.
Sublethal insecticide exposure, particularly thiamethoxam, abamectin, chlorfenapyr, and β-cypermethrin, significantly impacted the enzyme activities of SOD, CAT, and POD in Vespa magnifica, which are critical for managing oxidative stress [55]. SOD and CAT, which neutralize harmful reactive oxygen species (ROS) [56], initially increased in response to thiamethoxam, abamectin, and chlorfenapyr, peaking at LC20 (SOD at 1396.21 U/g and CAT at 986.26 U/g). This suggests an early defense against oxidative stress. However, enzyme activity declined at higher concentrations, which was likely due to enzyme saturation or damage from prolonged stress [57]. β-cypermethrin showed a delayed response with SOD and CAT activity first decreasing and then peaking at lower concentrations (SOD at 1106.20 U/g and CAT at 1137.81 U/g), indicating a different oxidative stress pattern. POD, essential for breaking down peroxides, consistently decreased across all insecticide treatments, showing the strongest inhibition with thiamethoxam at LC30 (425.60 U/g). Since POD is responsible for breaking down peroxides, its inhibition implies that the Vespa magnifica are less capable of detoxifying these harmful by-products. As a result, the sustained high levels of ROS could lead to oxidative damage, impairing critical biological processes in adult Vespa magnifica, such as mobility, foraging, and overall survival [58]. Similar trends were observed in Microplitis mediator, Helicoverpa armigera, Diadegma semiclausum, and Spodoptera exigua when exposed to various insecticides [59,60]. Overall, these findings demonstrate that while SOD and CAT are initially upregulated to counteract insecticide-induced oxidative stress, prolonged exposure overwhelms these defenses. The inhibited POD activity further exacerbates oxidative damage, contributing to impaired functions in adult Vespa magnifica, such as reduced mobility, foraging ability, and overall survival.
The use of intestinal tissue for RNA sequencing in Vespa magnifica is essential because the gut plays a critical role in physiological processes like digestion, immunity, and detoxification. It is also the primary site for toxin exposure and response when studying the effects of insecticides [61]. By analyzing RNA expression in the gut, we can uncover molecular mechanisms involved in detoxification, immune responses, and other metabolic pathways affected by sublethal insecticide exposure [62]. This approach has been successfully applied in Apis mellifera studies to reveal how environmental stressors influence gene expression patterns in the gut [63]. This study employed high-throughput sequencing to analyze intestinal gene expression in Vespa magnifica under four insecticide stressors, revealing molecular responses and transcriptional regulation.
Thiamethoxam caused the strongest transcriptional response, with 3628 DEGs, mostly upregulated, affecting pathways like cell cycle, mRNA surveillance, and AMPK signaling, which are key to energy regulation [64]. This suggests increased energy demand and oxidative stress under thiamethoxam exposure. Research has shown similar transcriptional responses in other insects exposed to thiamethoxam, especially regarding gene expression changes linked to energy metabolism and oxidative stress. For example, a study on Apis mellifera exposed to thiamethoxam demonstrated significant changes in gene expression, particularly in genes associated with detoxification and stress responses [65]. Pathways such as energy production and oxidative stress management were notably affected, supporting findings that insecticide exposure can disrupt cellular processes essential for survival and adaptation.
Avermectin had a milder effect on Vespa magnifica, causing significant changes with 359 DEGs, but the numbers of upregulated genes and affected pathways were fewer than with thiamethoxam. The enriched pathways, including the ribosome, glycolysis, pentose phosphate pathway, and pyruvate metabolism, are crucial for energy generation and carbohydrate metabolism [66]. This suggests that under avermectin exposure, Vespa magnifica may adjust its metabolism to enhance energy utilization and carbohydrate breakdown in response to increased energy demands. Similar metabolic adjustments have been reported in other insects. For example, studies on Helicoverpa armigera revealed an altered expression in energy production pathways, including glycolysis and pyruvate metabolism, highlighting a metabolic shift to meet insecticide-induced energy demands [67]. Research on Spodoptera frugiperda also demonstrated significant changes in gene expression related to glycolysis and ATP synthesis following avermectin exposure, reinforcing the findings in Vespa magnifica [68].
The chlorfenapyr treatment altered the expression of 14 DEGs in Vespa magnifica, with 10 genes downregulated, indicating an inhibitory effect. KEGG analysis revealed few affected pathways, including cholinergic synapse and β-alanine metabolism. The cholinergic synapse enrichment suggests potential disruptions in neural signaling that could indirectly impact energy metabolism, while β-alanine metabolism indicates a possible inhibition of energy acquisition processes [69]. Similar studies have documented significant transcriptional changes in other insects due to chlorfenapyr. For example, research on Bombyx mori found a downregulation of genes related to metabolic processes and energy regulation [70]. Another study on Frankliniella occidentalis reported alterations in gene expression affecting amino acid metabolism [71]. This indicates that the insecticide can inhibit processes crucial for energy acquisition, affecting the insect’s overall energy balance.
β-cypermethrin had a minimal impact on Vespa magnifica, affecting only 10 DEGs, which were primarily downregulated. KEGG enrichment analysis revealed pathways such as herpes simplex virus 1 infection and antigen presentation, indicating a focus on immune responses rather than direct energy metabolism. Similar effects have been observed in other insects. For instance, a study on Spodoptera litura showed that exposure to β-cypermethrin mainly influenced immune-related genes with little impact on metabolic pathways [72]. Additionally, research on Solenopsis invicta also indicated alterations in gene expression related to immune response mechanisms and stress pathways [73], further suggesting a minimal effect on energy metabolism compared to other insecticides.
Across all insecticides, DEGs were enriched in functions related to detoxification, metabolism, and immunity. ATP-related processes, essential for maintaining energy balance, were consistently upregulated in response to all treatments, indicating an increased energy demand for detoxification. This included the upregulation of purine metabolism, nucleoside triphosphate catabolism, and mitochondrial electron transport. Overall, Vespa magnifica exhibits varying degrees of resistance and adaptation to these insecticides through targeted gene regulation. Notably, the mitochondrial electron transport in Vespa mandarinia is significantly enhanced when exposed to insecticides, which is crucial for ATP synthesis and physiological functions [74]. Insufficient energy can impair flight, hunting, and immunity, reducing the organism’s ability to detoxify insecticides and increasing mortality risk.
Detoxification metabolism enables insects such as Vespa magnifica to handle external toxins by converting lipophilic substances into water-soluble forms for easier excretion [75]. The identification of nine DEGs related to the CYP450 enzyme system in Vespa magnifica under various LC10 insecticide treatments highlights the critical role of detoxification pathways in the organism’s response to chemical stressors. These genes, including members of the CYP4, CYP4V, CYP6, CYP61, and CYP9 families, as well as GST, PPIB, and UTG, are known for their involvement in detoxification and xenobiotic metabolism [76]. The observed upregulation of these genes suggests that Vespa magnifica relies heavily on the CYP450 system and associated detoxification mechanisms to mitigate the effects of insecticide exposure.
5. Conclusions
This study highlights the sublethal effects of various insecticides, including thiamethoxam, abamectin, chlorfenapyr, and β-cypermethrin, on the development, body weight, enzyme activity, and gene expression in Vespa magnifica. While larval survival rates showed no significant changes, both pupation and fledge rates significantly declined as insecticide concentrations increased. Additionally, the body weight of Vespa magnifica increased during the larval, pupal, and fledge stages. The activation of SOD and CAT enzymes, along with the inhibition of POD in adult Vespa magnifica, indicates the triggering of oxidative stress.
At the molecular level, thiamethoxam induced the strongest transcriptional response with 3628 DEGs significantly upregulated. Pathways related to ATP processes and mitochondrial electron transport were notably affected, reflecting the increased energy demands for detoxification. Avermectin and chlorfenapyr triggered milder transcriptional responses with key metabolic pathways such as glycolysis, pentose phosphate, and pyruvate metabolism being upregulated to meet the energy needs of the Vespa magnifica. In contrast, β-cypermethrin had the least impact with key pathways like herpes simplex virus 1 infection and antigen presentation being downregulated, affecting immune responses. The regulation of detoxification pathways, particularly the CYP450 enzyme system, highlights Vespa magnifica’s response to insecticide stress.
In conclusion, this study uncovers the sublethal effects of various insecticides on development, body weight, enzyme activity, and gene expression in Vespa magnifica with significant differences observed in detoxification processes and energy metabolism regulation. These findings emphasize the broader ecological risks of insecticide exposure to non-target insects and underscore the need for further research on the long-term effects of newer insecticides and strategies to protect beneficial insect populations.
Author Contributions
Experimental design, software, data analysis, writing, Q.H. and S.F.; Experimental operation and validation, K.L. and F.S.; Experimental design, data analysis, X.C., Y.L. and R.M.; Writing and editing, funding acquisition, supervision, Z.L. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
All the sample raw reads obtained in this study have been deposited at the National Center for Biotechnology Information (accession number: PRJNA1141747).
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research was funded by the National Natural Science Foundation of China (32260132), the Yunnan Provincial Ten Thousand People Plan (YNWR-QNBJ-2018-161), the Joint Special Project for Basic Research of Local Universities in Yunnan Province (202301BA070001-105), and the Frontier Research Team of Kunming University 2023.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Tudi M., Daniel Ruan H., Wang L., Lyu J., Sadler R., Connell D., Chu C., Phung D.T. Agriculture development, pesticide application and its impact on the environment. Int. J. Environ. Res. Public Health. 2021;18:1112. doi: 10.3390/ijerph18031112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kenko D.B.N., Ngameni N.T., Awo M.E., Njikam N.A., Dzemo W.D. Does pesticide use in agriculture present a risk to the terrestrial biota? Sci. Total Environ. 2023;861:160715. doi: 10.1016/j.scitotenv.2022.160715. [DOI] [PubMed] [Google Scholar]
- 3.Cowie R.H., Bouchet P., Fontaine B. The sixth mass extinction: Fact, fiction or speculation? Biol. Rev. Camb. Philos. Soc. 2022;97:640–663. doi: 10.1111/brv.12816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sheng S., Wang J., Zhang X.R., Liu Z.X., Yan M.W., Shao Y., Zhou J.C., Wu F.A., Wang J. Evaluation of sensitivity to phoxim and cypermethrin in an endoparasitoid, Meteorus pulchricornis (Wesmael) (Hymenoptera: Braconidae), and its parasitization efficiency under insecticide stress. J. Insect Sci. 2021;21:10. doi: 10.1093/jisesa/ieab002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raine N.E., Rundlöf M. Pesticide exposure and effects on non-Apis bees. Annu. Rev. Entomol. 2024;69:551–576. doi: 10.1146/annurev-ento-040323-020625. [DOI] [PubMed] [Google Scholar]
- 6.Dong D.Z., Wang Y.Z. The Wasps of Yunnan: Hymenoptera: Vespoidea. Henan Science and Technology Press; Zhengzhou, China: 2017. [Google Scholar]
- 7.Wilson E.E., Mullen L.M., Holway D.A. Life history plasticity magnifies the ecological effects of a social wasp invasion. Proc. Natl. Acad. Sci. USA. 2009;106:12809–12813. doi: 10.1073/pnas.0902979106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elisei T., Junior C., Junior A.J.F. Management of social wasp colonies in eucalyptus plantations (Hymenoptera:Vespidae) Sociobiology. 2012;59:1167–1174. [Google Scholar]
- 9.Desneux N., Decourtye A., Delpuech J.M. The sublethal effects of pesticides on beneficial arthropods. Annu. Rev. Entomol. 2007;52:81–106. doi: 10.1146/annurev.ento.52.110405.091440. [DOI] [PubMed] [Google Scholar]
- 10.Siahaya V.G. Effect of sublethal dose/concentration on various insect behaviors. Agrologia. 2021;10:123–135. doi: 10.30598/ajibt.v10i1.1296. [DOI] [Google Scholar]
- 11.Tong L., Liu Z.C., Guo C.W., Tang W.J., Deng W.H., Dong D.Z. Effects of body weight, temperature and relative humidity on the overwintering of Vespa magnifica queen. Biotic. Resour. 2021;43:633–637. [Google Scholar]
- 12.Dong D.Z., Wang Y.Z., Le Y.G. Evaluation of the benefits and harms relationship of social wasps. J. South. Agric. Univ. 2000;22:441–442. [Google Scholar]
- 13.Chi C.L., Li Q., Ma L. Biological characteristics and controlling pest effects of Vespinae insect: A review. J. South. Agri. 2018;49:1139–1146. [Google Scholar]
- 14.Guo Y.J., Wang J.A., Tao S.B. Mobile wasp removal technology. Plant Doc. 2018;32:23–26. [Google Scholar]
- 15.Dang L.J., Guo Y.J., Dong B.G., Zhang X.M., Wang J.A., Tao S.B. The win-win validation of economic wasp breeding and green pest control. Chin. Livest. Poult. Breed. 2022;18:60–462. [Google Scholar]
- 16.Tan X., Huang Y., Yu Y.J. The transformation of small wasps into a major industry in Longling. Ethn. Today. 2016;1:20–423. [Google Scholar]
- 17.Calvo-Agudo M., Dregni J., González-Cabrera J., Dicke M., Heimpel G.E., Tena A. Neonicotinoids from coated seeds toxic for honeydew-feeding biological control agents. Environ. Pollut. 2021;289:117813. doi: 10.1016/j.envpol.2021.117813. [DOI] [PubMed] [Google Scholar]
- 18.Yang G., Lv L., Di S., Li X., Weng H., Wang X., Wang Y. Combined toxic impacts of thiamethoxam and four pesticides on the rare minnow (Gobiocypris rarus) Environ. Sci. Pollut. Res. Int. 2021;28:5407–5416. doi: 10.1007/s11356-020-10883-0. [DOI] [PubMed] [Google Scholar]
- 19.Thompson H., Overmyer J., Feken M., Ruddle N., Vaughan S., Scorgie E., Bocksch S., Hill M. Thiamethoxam: Long-term effects following honey bee colony-level exposure and implications for risk assessment. Sci. Total Environ. 2019;654:60–71. doi: 10.1016/j.scitotenv.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 20.Miotelo L., Mendes Dos Reis A.L., Rosa-Fontana A., Karina da Silva Pachú J., Malaquias J.B., Malaspina O., Roat T.C. A food-ingested sublethal concentration of thiamethoxam has harmful effects on the stingless bee Melipona scutellaris. Chemosphere. 2022;288:132461. doi: 10.1016/j.chemosphere.2021.132461. [DOI] [PubMed] [Google Scholar]
- 21.Crispim P.D., de Oliveira V.E.S., Batista N.R., Nocelli R.C.F., Antonialli-Junior W.F. Lethal and sublethal dose of thiamethoxam and its effects on the behavior of a non-target social wasp. Neotrop. Entomol. 2023;52:422–430. doi: 10.1007/s13744-023-01028-2. [DOI] [PubMed] [Google Scholar]
- 22.Batista N.R., Farder-Gomes C.F., Nocelli R.C.F., Antonialli-Junior W.F. Effects of chronic exposure to sublethal doses of neonicotinoids in the social wasp Polybia paulista: Survival, mobility, and histopathology. Sci. Total Environ. 2023;904:166823. doi: 10.1016/j.scitotenv.2023.166823. [DOI] [PubMed] [Google Scholar]
- 23.Guo L.Y., Yuan L.F., Cui S.J., Zhou C., Ye G.B. Adjustment of management policy of neonicotinoid pesticide thiamethoxam in EU and suggestions for countermeasures. Qual. Saf. Agro-Prod. 2022;12:87–93. [Google Scholar]
- 24.Kai C., Yuan S., Wang D., Liu Y., Chen F., Qi D. Basic amino acid-modified lignin-based biomass adjuvants: Synthesis, emulsifying activity, ultraviolet protection, and controlled release of avermectin. Langmuir. 2021;37:12179–12187. doi: 10.1021/acs.langmuir.1c02113. [DOI] [PubMed] [Google Scholar]
- 25.Radwan M.A., Gad A.F. Insights into the ecotoxicological perturbations induced by the biocide Abamectin in the white snail, Theba pisana. J. Environ. Sci. Health Part B. 2022;57:201–210. doi: 10.1080/03601234.2022.2044708. [DOI] [PubMed] [Google Scholar]
- 26.Obregon D., Guerrero O., Sossa D., Stashenko E., Prada F., Ramirez B., Duplais C., Poveda K. Route of exposure to veterinary products in bees: Unraveling pasture’s impact on avermectin exposure and tolerance in stingless bees. PNAS Nexus. 2024;3:68. doi: 10.1093/pnasnexus/pgae068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang P., Yan X., Yu B., He X., Lu L., Ren Y. A comprehensive review of the current knowledge of chlorfenapyr: Synthesis, mode of action, resistance, and environmental toxicology. Molecules. 2023;28:7673. doi: 10.3390/molecules28227673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jia B., Zhang J., Hong S., Chang X., Li X. Sublethal effects of chlorfenapyr on Plutella xylostella (Lepidoptera: Plutellidae) Pest Manag. Sci. 2023;79:88–96. doi: 10.1002/ps.7175. [DOI] [PubMed] [Google Scholar]
- 29.Song Y., Li L., Li C., Lu Z., Ouyang F., Liu L., Yu Y., Men X. Comparative ecotoxicity of insecticides with different modes of action to Osmia excavata (Hymenoptera: Megachilidae) Ecotoxicol. Environ. Saf. 2021;212:112015. doi: 10.1016/j.ecoenv.2021.112015. [DOI] [PubMed] [Google Scholar]
- 30.Nai Y.S., Chen T.Y., Chen Y.C., Chen C.T., Chen B.Y., Chen Y.W. Revealing pesticide residues under high pesticide stress in Taiwan’s agricultural environment probed by fresh Honey Bee (Hymenoptera: Apidae) Pollen. J. Econ. Entomol. 2017;110:1947–1958. doi: 10.1093/jee/tox195. [DOI] [PubMed] [Google Scholar]
- 31.Ullah S., Zuberi A., Alagawany M., Farag M.R., Dadar M., Karthik K., Tiwari R., Dhama K. Cypermethrin induced toxicities in fish and adverse health outcomes: Its prevention and control measure adaptation. J. Environ. Manag. 2018;206:863–871. doi: 10.1016/j.jenvman.2017.11.076. [DOI] [PubMed] [Google Scholar]
- 32.Albero B., Miguel E., García-Valcárcel A.I. Acaricide residues in beeswax. Implications in honey, brood and honeybee. Environ. Monit. Assess. 2023;195:454. doi: 10.1007/s10661-023-11047-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang X., Hafeez M., Chen H.Y., Li W.T., Ren R.J., Luo Y.S., Abdellah Y.A.Y., Wang R.L. DIMBOA-induced gene expression, activity profiles of detoxification enzymes, multi-resistance mechanisms, and increased resistance to indoxacarb in tobacco cutworm, Spodoptera litura (Fabricius) Ecotoxicol. Environ. Saf. 2023;267:115669. doi: 10.1016/j.ecoenv.2023.115669. [DOI] [PubMed] [Google Scholar]
- 34.Vandermeer J., Flores J., Longmeyer J., Perfecto I. Spatiotemporal foraging dynamics of Solenopsis invicta (Hymenoptera: Formicidae) and its potential effects on the spatial structure of interspecific competition. Environ. Entomol. 2022;51:1040–1047. doi: 10.1093/ee/nvac058. [DOI] [PubMed] [Google Scholar]
- 35.Zhao Y.J., Chen G.L. Study on the ethical review system for laboratory animal welfare. J. Jinzhou Med. Univ. (Soc. Sci. Ed.) 2024;22:41–45. [Google Scholar]
- 36.Sparks T.C., Nauen R. IRAC: Mode of action classification and insecticide resistance management. Pestic. Biochem. Physiol. 2015;121:122–128. doi: 10.1016/j.pestbp.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 37.Hu Q.M., Meng R.Y., Liu S.S., Gao J.W., Wang H., Cao Y.R., Liu Z.C. Acute toxicity and risk assessment of four insecticides to Vespa magnifica (Hymenoptera: Vespidae) Acta Entomol. Sin. 2024;67:1086–1095. [Google Scholar]
- 38.Organization for Economic Co-operation and Development (OECD) Test No. 237: Honey Bee (Apis melifera) Larval Toxicity Test, Single Exposure. OECD; Paris, France: 2013. [Google Scholar]
- 39.de Lima L.A., Reinmets K., Nielsen L.K., Marcellin E., Harris A., Köpke M., Valgepea K. RNA-seq sample preparation kits strongly affect transcriptome profiles of a gas-fermenting bacterium. Microbiol. Spectr. 2022;10:e0230322. doi: 10.1128/spectrum.02303-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Borodina T., Adjaye J., Sultan M. A strand-specific library preparation protocol for RNA sequencing. Methods Enzymol. 2011;500:79–98. doi: 10.1016/B978-0-12-385118-5.00005-0. [DOI] [PubMed] [Google Scholar]
- 41.Pertea M., Pertea G.M., Antonescu C.M., Chang T.C., Mendell J.T., Salzberg S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015;33:290–295. doi: 10.1038/nbt.3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Love M.I., Anders S., Huber W. Beginner’s guide to using the DESeq 2 package. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nauen R., Bass C., Feyereisen R., Vontas J. The role of cytochrome P450s in insect toxicology and resistance. Annu. Rev. Entomol. 2022;67:105–124. doi: 10.1146/annurev-ento-070621-061328. [DOI] [PubMed] [Google Scholar]
- 44.Kozera B., Rapacz M. Reference genes in real-time PCR. J. Appl. Genet. 2013;54:391–406. doi: 10.1007/s13353-013-0173-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bustin S.A., Benes V., Garson J.A., Hellemans J., Huggett J., Kubista M., Mueller R., Nolan T., Pfaffl M.W., Shipley G.L., et al. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009;55:611–622. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
- 46.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 47.Ashburner M., Ball C.A., Blake J.A., Botstein D., Butler H., Cherry J.M., Davis A.P., Dolinski K., Dwight S.S., Eppig J.T., et al. Gene ontology: Tool for the unification of biology. The Gene Ontology Consortium. Nat. Genet. 2000;25:25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ogata H., Goto S., Sato K., Fujibuchi W., Bono H., Kanehisa M. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 1999;27:29–34. doi: 10.1093/nar/27.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Simon-Delso N., Amaral-Rogers V., Belzunces L.P., Bonmatin J.M., Chagnon M., Downs C., Furlan L., Gibbons D.W., Giorio C., Girolami V., et al. Systemic insecticides (neonicotinoids and fipronil): Trends, uses, mode of action and metabolites. Environ. Sci. Pollut. Res. Int. 2015;22:5–34. doi: 10.1007/s11356-014-3470-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tomizawa M., Casida J.E. Neonicotinoid insecticide toxicology: Mechanisms of selective action. Annu. Rev. Pharmacol. Toxicol. 2005;45:247–268. doi: 10.1146/annurev.pharmtox.45.120403.095930. [DOI] [PubMed] [Google Scholar]
- 51.Heneberg P., Bogusch P., Astapenková A., Řezáč M. Neonicotinoid insecticides hinder the pupation and metamorphosis into adults in a crabronid wasp. Sci. Rep. 2020;10:7077. doi: 10.1038/s41598-020-63958-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kato Y., Kikuta S., Barribeau S.M., Inoue M.N. In vitro larval rearing method of eusocial bumblebee Bombus terrestris for toxicity test. Sci. Rep. 2022;12:15783. doi: 10.1038/s41598-022-19965-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ricke D.F., Lin C.H., Johnson R.M. Pollen treated with a combination of agrochemicals commonly applied during almond bloom reduces the emergence rate and longevity of Honey Bee (Hymenoptera: Apidae) Queens. J. Insect Sci. 2021;21:5. doi: 10.1093/jisesa/ieab074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Casida J.E., Durkin K.A. Neuroactive insecticides: Targets, selectivity, resistance, and secondary effects. Annu. Rev. Entomol. 2013;58:99–117. doi: 10.1146/annurev-ento-120811-153645. [DOI] [PubMed] [Google Scholar]
- 55.Gul H., Gadratagi B.G., Güncan A., Tyagi S., Ullah F., Desneux N., Liu X. Fitness costs of resistance to insecticides in insects. Front. Physiol. 2023;14:1238111. doi: 10.3389/fphys.2023.1238111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Felton G.W., Summers C.B. Antioxidant systems in insects. Arch. Insect Biochem. Physiol. 1995;29:187–197. doi: 10.1002/arch.940290208. [DOI] [PubMed] [Google Scholar]
- 57.Banerjee B.D., Seth V., Ahmed R.S. Pesticide-induced oxidative stress: Perspectives and trends. Rev. Environ. Health. 2001;16:1–40. doi: 10.1515/REVEH.2001.16.1.1. [DOI] [PubMed] [Google Scholar]
- 58.Birben E., Sahiner U.M., Sackesen C., Erzurum S., Kalayci O. Oxidative stress and antioxidant defense. World Allergy Organ J. 2012;5:9–19. doi: 10.1097/WOX.0b013e3182439613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jia B.T., Hong S.S., Zhang Y.C., Cao Y.W. Effect of sublethal concentrations of abamectin on protective and detoxifying enzymes in Diagegma semclausum. J. Envir. Entomol. 2016;38:990–995. [Google Scholar]
- 60.Bai L.S., Xu J.J., Zhao C.X., Chang Y.L., Dong Y.L., Zhang K.G., Li Y.Q., Li Y.P., Ma Z.Q., Liu X.L. Enhanced hydrolysis of β-cypermethrin caused by deletions in the glycin-rich region of carboxylesterase 001G from Helicoverpa armigera. Pest. Manag. Sci. 2021;77:2129–2141. doi: 10.1002/ps.6242. [DOI] [PubMed] [Google Scholar]
- 61.Dong Z.X., Li L.W., Li X.J., Li L.J., Zhao J., Zhao C.H., Huang Q., Gao Z., Chen Y., Zhao H.M., et al. Glyphosate exposure affected longevity-related pathways and reduced survival in asian honey bees (Apis cerana) Chemosphere. 2024;351:141199. doi: 10.1016/j.chemosphere.2024.141199. [DOI] [PubMed] [Google Scholar]
- 62.Marín M.J., Pérez C.A.E., Domínguez S.R., Rebeca D.S., Latorre A., Moya A. Adaptability of the gut microbiota of the German cockroach Blattella germanica to a periodic antibiotic treatment. Microbiol. Res. 2024;287:127863. doi: 10.1016/j.micres.2024.127863. [DOI] [PubMed] [Google Scholar]
- 63.Aufauvre J., Misme-Aucouturier B., Viguès B., Texier C., Delbac F., Blot N. Transcriptome analyses of the honeybee response to Nosema ceranae and insecticides. PLoS ONE. 2014;9:e91686. doi: 10.1371/journal.pone.0091686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Manjon C., Troczka B.J., Zaworra M., Beadle K., Randall E., Hertlein G., Singh K.S., Zimmer C.T., Homem R.A., Lueke B., et al. Unravelling the molecular determinants of bee sensitivity to neonicotinoid insecticides. Curr. Biol. 2018;28:1137–1143. doi: 10.1016/j.cub.2018.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shi T.F., Wang Y.F., Liu F., Qi L., Yu L.S. Sublethal effects of the neonicotinoid insecticide thiamethoxam on the transcriptome of the honey Bees (Hymenoptera: Apidae) J. Econ. Entomol. 2017;110:2283–2289. doi: 10.1093/jee/tox262. [DOI] [PubMed] [Google Scholar]
- 66.Rigoulet M., Bouchez C.L., Paumard P., Ransac S., Cuvellier S., Duvezin-Caubet S., Mazat J.P., Devin A. Cell energy metabolism: An update. Biochim. Biophys. Acta Bioenerg. 2020;1861:148276. doi: 10.1016/j.bbabio.2020.148276. [DOI] [PubMed] [Google Scholar]
- 67.Tian L., Gao X., Zhang S., Zhang Y., Ma D., Cui J. Dynamic changes of transcriptome of fifth-instar Spodoptera litura larvae in response to insecticide. 3 Biotech. 2021;11:98. doi: 10.1007/s13205-021-02651-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shu H., Lin Y., Zhang Z., Qiu L., Ding W., Gao Q., Xue J., Li Y., He H. The transcriptomic profile of Spodoptera frugiperda differs in response to a novel insecticide, cyproflanilide, compared to chlorantraniliprole and avermectin. BMC Genom. 2023;24:3. doi: 10.1186/s12864-022-09095-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Picciotto M.R., Higley M.J., Mineur Y.S. Acetylcholine as a neuromodulator: Cholinergic signaling shapes nervous system function and behavior. Neuron. 2012;76:116–129. doi: 10.1016/j.neuron.2012.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shao Y., Xin X.D., Liu Z.X., Wang J., Zhang R., Gui Z.Z. Transcriptional response of detoxifying enzyme genes in Bombyx mori under chlorfenapyr exposure. Pestic. Biochem. Physiol. 2021;177:104899. doi: 10.1016/j.pestbp.2021.104899. [DOI] [PubMed] [Google Scholar]
- 71.Gao Y., Kim M.J., Kim J.H., Jeong I.H., Clark J.M., Lee S.H. Transcriptomic identification and characterization of genes responding to sublethal doses of three different insecticides in the western flower thrips, Frankliniella occidentalis. Pestic. Biochem. Physiol. 2020;167:104596. doi: 10.1016/j.pestbp.2020.104596. [DOI] [PubMed] [Google Scholar]
- 72.Xu L., Mei Y., Liu R., Chen X., Li D., Wang C. Transcriptome analysis of Spodoptera litura reveals the molecular mechanism to pyrethroids resistance. Pestic. Biochem. Physiol. 2020;169:104649. doi: 10.1016/j.pestbp.2020.104649. [DOI] [PubMed] [Google Scholar]
- 73.Siddiqui J.A., Luo Y., Sheikh U.A.A., Bamisile B.S., Khan M.M., Imran M., Hafeez M., Ghani M.I., Lei N., Xu Y. Transcriptome analysis reveals differential effects of beta-cypermethrin and fipronil insecticides on detoxification mechanisms in Solenopsis invicta. Front. Physiol. 2022;13:1018731. doi: 10.3389/fphys.2022.1018731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sinenko S.A., Tomilin A.N. Metabolic control of induced pluripotency. Front. Cell. Dev. Biol. 2024;11:1328522. doi: 10.3389/fcell.2023.1328522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ahmed Laskar A., Younus H. Aldehyde toxicity and metabolism: The role of aldehyde dehydrogenases in detoxification, drug resistance and carcinogenesis. Drug. Metab. Rev. 2019;51:42–64. doi: 10.1080/03602532.2018.1555587. [DOI] [PubMed] [Google Scholar]
- 76.Feyereisen R. Insect CYP genes and P450 enzymes. Insect Mol. Biol. 2011;8:236–316. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the sample raw reads obtained in this study have been deposited at the National Center for Biotechnology Information (accession number: PRJNA1141747).