Abstract
Entamoeba histolytica virulence is related to a number of amebic components (lectins, cysteine proteinases, and amebapore) and host factors, such as intestinal bacterial flora. Trophozoites are selective in their interactions with bacteria, and the parasite recognition of glycoconjugates plays an important role in amebic virulence. Long-term monoxenic cultivation of pathogenic E. histolytica trophozoites, strains HK-9 or HM-1:IMSS, with Escherichia coli serotype O55, which binds strongly to the Gal/GalNAc amebic lectin, markedly reduced the trophozoites’ adherence and cytopathic activity on cell monolayers of baby hamster kidney (BHK) cells. Specific probes prepared from E. histolytica lectin genes as well as antibodies directed against the light (35-kDa) and heavy (170-kDa) subunits of the Gal/GalNAc lectin revealed a decrease in the transcription and expression of the light subunit in trophozoites grown monoxenically with E. coli O55. This effect was not observed when E. histolytica was grown with E. coli 346, a mannose-binding type I pilated bacteria. Our results suggest that the light subunit of the amebic lectin is involved in the modulation of parasite adherence and cytopathic activity.
The relative virulence of different strains of Entamoeba histolytica has been shown to vary as a consequence of changes in conditions of in vitro cultivation (15). The molecular mechanisms of such variations in virulence are not well understood. The virulence of E. histolytica has been proposed to be related to a number of amebic components: (i) a family of galactose (Gal)/N-acetyl-d-galactosamine (GalNAc)-specific lectins, which mediate the initial attachment of the parasite to the mucosal cells and enable resistance to lysis by serum complement (11, 27, 37, 41); (ii) small proteins known as amebapores, which form pores in membranes of target cells as well as in the cell walls of ingested bacteria (2, 18, 19, 23); and (iii) a family of six potent cysteine proteinases (CPs) which have considerable sequence homology (3, 12, 39, 43, 44). Although the specific role of each of these cysteine proteinases has not yet been established, their inhibition by antisense RNA (3, 4) has been shown to affect amebic virulence.
In addition to the amebic components, there are various host factors which contribute to and determine the virulence of E. histolytica trophozoites in the human host (31). One factor which has been suggested to play an important role in amebic virulence is the bacterial flora of the intestine (30). A number of studies have shown that the association of axenically grown E. histolytica trophozoites with certain types of bacteria enhanced their virulence (1, 10, 47). Cultivation of trophozoites with bacteria was also shown to alter some antigens of the ameba (5, 6). E. histolytica trophozoites attach and ingest bacteria either by using their membrane-associated lectin specific for Gal and GalNAc or by having their mannose-containing cell surface components serve as receptors for the mannose binding lectins of certain bacteria (7, 8, 30).
The ameba Gal/GalNAc lectin is a heterodimer consisting of heavy (170-kDa) and light (35- or 31-kDa) subunits (28, 36). The 170-kDa subunit has been suggested to play a crucial role in the carbohydrate recognition that mediates the interaction between the parasite and receptors on the mucosal cells (25, 34, 42). Recently a regulation of adherence and virulence by the cytoplasmic domain of the 170-kDa lectin subunit has been proposed (46). The involvement of the light lectin subunits in this process is not yet understood. Preliminary results recently obtained by subtractive hybridization have suggested that the avirulent E. histolytica strain Rahman has, among other defects, a deficiency in the expression of the 35-kDa light subunit (4a). In the present study we have investigated the effects of long-term monoxenic cultivation of E. histolytica HK-9 or HM-1:IMSS (with either Gal or Man binding bacteria) on amebic virulence. Surprisingly, a significant down regulation of E. histolytica adherence and cytopathic activity was observed when amebic trophozoites were monoxenically grown with the Gal-containing E. coli serotype O55 but not with the Man binding bacterium E. coli 346. This down regulation of virulence was found to be related to a decrease in the transcription and expression of the light (35-kDa) subunit of the ameba Gal/GalNAc lectin.
MATERIALS AND METHODS
Ameba cultures.
Trophozoites of E. histolytica HM-1:IMSS and HK-9 were cultured under axenic conditions in Diamond’s TYI-S-33 medium (16). The parasites were harvested at the exponential phase of growth, washed twice in a solution of phosphate-buffered saline (PBS), pH 7.0, and resuspended to the desired concentration for adherence and cytopathic assays. Monoxenic cultures of E. histolytica were established by growing amebic trophozoites with E. coli O55 or E. coli 346. The growth of bacteria in the monoxenic cultures was controlled by adding 5 μg of cefotaxime (Claforan; Hoechst, Frankfurt, Germany)/ml to each subculture. Several repetitions of monoxenic cultures of strains HM-1:IMSS and HK-9 were done. Seven separate cultures were initiated with E. coli serotype O55 (7), and four were initiated with E. coli 346, which has type I pili (24). The bacteria were grown separately in TYI-S-33 medium for 10 h at 37°C, and 1 ml of the bacterial culture was then added to the trophozoite cultures at each subculture. E. coli cells were radiolabeled as previously reported (8). The bacteria were grown at 37°C in Luria broth medium containing [14C]glucose (1 μCi/ml; specific activity, 346 mCi/mmol) for 4 h, harvested by centrifugation at 9,000 × g for 10 min, washed, and resuspended in saline solution to a concentration of 1010/ml (300 cpm/106 bacteria).
Adhesion and cytopathic activity on cell monolayers.
Monolayers of cultured BHK cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) with fetal calf serum (5%) to confluence in 24-well plates. For adhesion assays, the monolayer was fixed with 5% formaldehyde, washed twice with PBS, incubated with a solution of glycine (250 mM) for 30 min, and washed twice with PBS. Axenic and monoxenic E. histolytica trophozoites (2 × 105) from each strain were added to wells containing fixed monolayers in 1 ml of DMEM without serum and incubated at 37°C for 30 min. The number of parasites adherent to BHK cells was determined by counting the trophozoites that remained adhered to the cell monolayer after gentle decantation (two times) of the nonadhered trophozoites with warm DMEM. For cytopathic-activity assays, the rate of destruction of the BHK cell monolayer by axenic and monoxenic trophozoites was evaluated as previously described (30). In routine experiments, trophozoites of strain HM-1:IMSS (105) or strain HK-9 (2 × 105) were resuspended in DMEM without serum (1 ml), added to wells containing confluent monolayers, and incubated for 60 min at 37°C. The reaction was stopped by cooling the tissue culture plate at 4°C for 10 min, after which the plates were carefully washed twice with cold PBS. The amounts of mammalian cells that remained in the wells after the incubations were determined by staining with methylene blue and extraction of the dye as previously described (10). A ratio of 1,000 E. coli cells/trophozoite was used for the determination of the effect of short-term association of bacteria on the cytopathic effect. The standard deviation was calculated from triplicate wells in each experiment.
Attachment of radiolabeled bacteria to trophozoites.
Attachment of radiolabeled bacteria to E. histolytica trophozoites was carried out as previously described (8). 14C-labeled E. coli cells (109) (300 cpm/106 bacteria) were incubated with E. histolytica trophozoites (106) in 1 ml of saline solution for 30 min at 4°C. Separation of bacteria which attached to the trophozoites and those that did not was performed by discontinuous density gradient centrifugation with Percoll (Pharmacia, Uppsala, Sweden). Bacteria layered between 100 and 60% Percoll, whereas amebae layered between 60 and 20% Percoll. The bacteria attached to trophozoites were counted in a scintillation fluid with a liquid scintillation counter.
Erythrophagocytosis assay.
Erythrophagocytosis was carried out as previously described (32). Human erythrocytes (HRBC) and E. histolytica trophozoites from axenic and monoxenic cultures were mixed in a ratio of 100:1 and incubated 15 min at 37°C. The noningested erythrocytes were lysed with distilled water, and the sedimented parasites were resuspended in formic acid. The average number of HRBC per trophozoite was determined with a calibration curve and by reading the absorbance at 397 nm.
Hemolytic activity.
Hemolysis of HRBC by intact trophozoites was performed as previously described (32). E. histolytica trophozoites from axenic and monoxenic cultures were mixed with HRBC in a ratio of 1:2,000 (trophozoites-HRBC) in hemolysis buffer {100 mM NaCl, 30 mM KCl, 100 mM sorbitol, 0.1% bovine serum albumin, and 10 mM PIPES [piperazine-N,N′-bis(2-ethanesulfonic acid)]–Tris (pH 6.8)} and incubated for 90 min at 37°C. The hemoglobin that was released in the supernatant was read at 570 nm.
Cysteine proteinase activity.
Proteinase activity was measured with the synthetic peptide benzyloxycarbonyl-l-arginyl-l-arginine-p-nitroanilide (Z-Arg-Arg-pNA; Bachem) as a substrate (20). CP activity was measured in total lysates of trophozoites in lysis buffer (1% Nonidet P-40 in PBS). One unit of activity is defined as the number of micromoles of substrate digested per minute per milligram of protein.
Alcohol dehydrogenase activity.
Enzyme activity (alcohol dehydrogenase) was assayed as previously described (20). The assay mixture contained 50 mM glycine-NaOH buffer, pH 9.5, 0.2 mM NADP+, and 20 mM 2-propanol. The rate of NADP+ reduction was monitored at 340 nm. One unit of enzyme activity was defined as the number of micromoles of substrate reduced per minute per milligram of protein.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blotting.
E. histolytica trophozoites were solubilized with 1% Nonidet P-40 in PBS in the presence of 50 μM protease inhibitor E-64. Proteins from whole E. histolytica lysates were resolved on 12% polyacrylamide gels (25 μg/lane) under reducing conditions (17), electrophoretically transferred to nitrocellulose membranes, and immunostained with Gal/GalNAc lectin antibodies. The membranes were incubated with monoclonal antibodies directed against the E. histolytica light (35-kDa) subunit (a gift of Barbara Mann, University of Virginia) and with polyclonal antibodies against the heavy (170-kDa) subunit (a gift of Samuel L. Stanley, St. Louis, Mo.). The blots were washed and incubated with horseradish peroxidase-conjugated antibodies and developed with an enhanced chemiluminescence kit (Boehringer Mannheim) according to the manufacturer’s conditions. Detection was done by autoradiography.
Dot blot and Northern blot hybridization.
For Northern blot hybridization, total RNA was prepared with an RNA isolation kit (TRI-Reagent; Molecular Research Center, Inc. Cincinnati, Ohio). Five micrograms of total RNA was size fractionated under denaturing conditions on 4% polyacrylamide gels containing 8 M urea. The RNA was transferred to a nylon membrane and hybridized under stringent conditions as described previously (9). For dot blot hybridization, 1 μg of total RNA from each strain was spotted onto nylon membranes. The different probes were prepared by PCR amplification of genomic DNA according to published gene sequences. The primers for the 35-kDa light-subunit gene were prepared according to EMBL database sequence accession no. M96024. The primers for the 170-kDa heavy-subunit gene were prepared according to the hgl3 sequence accession no. L14815. A set of primers for the amplification of the three amebapore genes (a, b, and c) (18, 21) (accession no. M83945, X76904, and X76903) was prepared. The probe used for actin was previously described (3). The labeled probes were prepared by random primer labeling (Rediprime; Amersham). Quantitation of labeled RNA was performed with an imaging densitometer (Bio-Rad).
RESULTS
Effect of bacteria on amebic virulence.
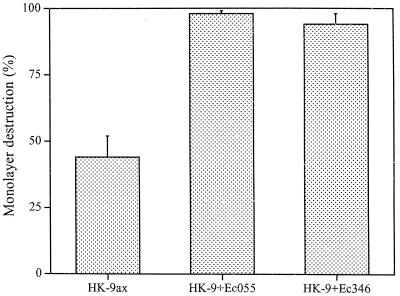
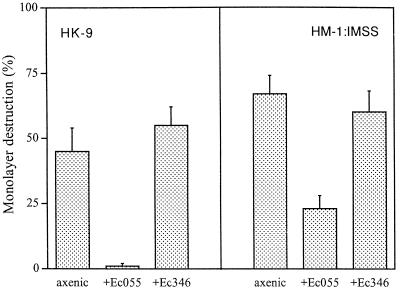
Previous studies have shown that E. histolytica trophozoites can attach and ingest bacteria either by using their membrane-associated lectins or by having their mannose-containing cell surface components serve as receptors for bacterial adhesins (8). As previously reported (10), short-term (30- to 60-min) coincubation of axenically grown E. histolytica trophozoites together with E. coli bacteria (serotype O55 or 346) and mammalian cell monolayers markedly increased their cytopathic activity (Fig. 1). In contrast, a marked decrease in cytopathic activity was observed when trophozoites of E. histolytica HK-9 or HM-1:IMSS were monoxenically cultivated with E. coli O55 for 1 month (Fig. 2). The decrease in cytopathic activity was reproducible and was observed in seven independently initiated monoxenic cultures. Coincubation of the monoxenically grown trophozoites with additional E. coli cells (O55 or 346) for short periods caused only a slight increase (up to 10%) in their cytopathic activity compared with the marked increase observed with axenic trophozoites (Fig. 1). Cultivation of trophozoites with a regular supplement of lethally irradiated (500 kilorads) E. coli O55 cells for 1 month had a similar, albeit less pronounced, effect in decreasing trophozoite adherence (data not shown). The decrease in cytopathic activity was observed only upon monoxenic cultivation with E. coli O55. Monoxenic cultivation of E. histolytica trophozoites with E. coli 346 did not cause a decrease in cytopathic activity (Fig. 2). Elimination of the E. coli cells from 1-month-old monoxenic cultures of strain HK-9 by addition of higher doses of antibiotic (20 μg/ml) resulted in a restoration of the cytopathic activity within 1 week (data not shown).
FIG. 1.
Cytopathic activity of axenically (ax) grown E. histolytica HK-9 (2 × 105) in the absence or presence of added bacteria. Trophozoites were added to the monolayer together with E. coli (Ec) O55 or 346 at a ratio of 1,000:1 (bacteria-amebae) and incubated for 60 min at 37°C. The monolayer destruction was determined as described in Materials and Methods. The data represent the means and standard deviations of three independent experiments.
FIG. 2.
Cytopathic activity of E. histolytica HK-9 (2 × 105) and HM-1 (105) axenically and monoxenically cultivated with E. coli (Ec) O55 or 346 for 1 month. After 1 h of interaction, the monolayer destruction was determined as described in Materials and Methods. The data represent the means and standard deviations of three independent experiments.
Adhesion to cell monolayers and bacteria.
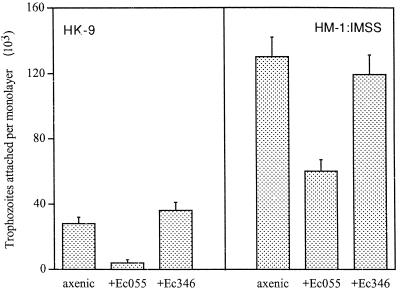
The adhesion levels of E. histolytica trophozoites grown in axenic and monoxenic conditions to fixed monolayers of BHK cells was determined. Axenically grown trophozoites of the less virulent strain, HK-9, had lower levels of adhesion than those of the highly virulent HM-1:IMSS strain (Fig. 3). This is in agreement with the differences observed in the cytopathic activities of both strains.
FIG. 3.
Adhesion of E. histolytica trophozoites (2 × 105) of strains HK-9 and HM-1 to BHK cell fixed monolayers after 30 min of interaction. The amebae were grown in axenic conditions and monoxenically cultivated with E. coli (Ec) O55 or 346 for 1 month. The trophozoites that remained attached to the monolayer after washing were counted. The data represent the means and standard deviations of three independent experiments.
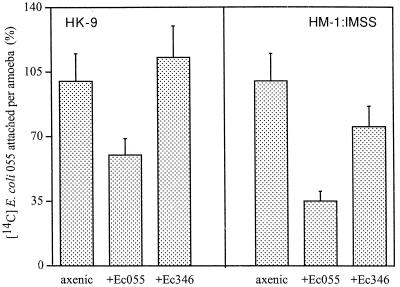
As previously reported (27), in both axenic strains the adherence to monolayers was inhibited (50 to 70%) by galactose (20 mg/ml). The level of adherence of both E. histolytica strains, HK-9 and HM1:IMSS, to fixed monolayers was affected when the trophozoites were grown with E. coli O55 for 1 month (Fig. 3). Adherence levels were not affected when trophozoites were grown with E. coli 346. Furthermore, the adherence of 14C-labeled E. coli O55 cells to E. histolytica trophozoites was reduced only in trophozoites which had grown monoxenically with E. coli O55 (Fig. 4). The adhesion of 14C-labeled E. coli 346 was not affected in trophozoites grown with either E. coli O55 or 346. The attachment of 14C-labeled E. coli O55 to axenic E. histolytica (HK-9 and HM-1) was inhibited (50 to 70%) with galactose (20 mg/ml) (data not shown).
FIG. 4.
Attachment of 14C-labeled E. coli O55 cells to trophozoites. E. coli (Ec) O55 (109) (300 cpm/106 bacteria) was added to freshly harvested and washed trophozoites of E. histolytica (HK-9 and HM-1:IMSS) (106) grown in axenic conditions and monoxenically cultivated with E. coli O55 or 346 for 1 month. The reaction was carried out in suspension for 30 min at 4°C. Nonadherent bacteria were separated by a Percoll gradient centrifugation as described in the text. Attachment of 14C-labeled E. coli O55 to axenic trophozoites was taken as 100%.
Erythrophagocytosis and hemolysis.
Rates of erythrophagocytosis are related to amebic adhesion and have been correlated to virulence (33, 42). E. histolytica HK-9 grown with E. coli O55 had lower hemolytic activity and lower levels of erythrophagocytosis than the axenic strain (Table 1). Monoxenic growth of trophozoites in the presence of E. coli 346 did not affect these parameters. Strain HM-1:IMSS grown with E. coli O55 was not significantly affected in its capability to lyse and ingest erythrocytes.
TABLE 1.
Erythrophagocytosis, hemolytic activity, and cysteine proteinase activity in axenic and monoxenic trophozoites of E. histolytica
| Straina | Erythrophagocytosis (HRBC/amebae)b | Hemolytic activity (OD570)c | Protease activity (U)d |
|---|---|---|---|
| HK-9 axenic | 7.9 ± 1.2 | 0.91 ± 0.05 | 79.0 ± 8.7 |
| HK-9 (EcO55) | 3.5 ± 1.1 | 0.22 ± 0.03 | 15.5 ± 3.8 |
| HK-9 (Ec346) | 7.5 ± 0.9 | 0.94 ± 0.05 | 19.1 ± 4.0 |
| HM-1 axenic | 11.9 ± 1.9 | 0.69 ± 0.03 | 20.5 ± 3.6 |
| HM-1 (EcO55) | 9.5 ± 1.1 | 0.72 ± 0.04 | 23.2 ± 4.5 |
| HM-1 (Ec346) | 12.2 ± 2.1 | 0.75 ± 0.06 | 18.8 ± 4.9 |
E. histolytica grown under axenic conditions or in monoxenic culture with E. coli (Ec) serotype O55 or 346 for 1 month.
Erythrophagocytosis rates (± standard deviation) of E. histolytica trophozoites after 15 min of incubation at 37°C in a ratio of 1:100 (amebae-HRBC).
Hemolytic activity (± standard deviation) of E. histolytica after 90 min of incubation with HRBC in a ratio of 1:2,000 (amebae-HRBC). OD570, optical density at 570 nm.
Cysteine proteinase activity (± standard deviation) measured as micromoles of substrate (Z-Arg-Arg-pNA) digested per minute per milligram of protein (U).
Proteolytic activity and alcohol dehydrogenase activity.
Proteolytic activity was measured in axenically and monoxenically grown trophozoites of E. histolytica. The levels of cysteine proteinase activity were 80% lower in trophozoites of strain HK-9 grown with either E. coli O55 or 346. In contrast, no significant difference was found between axenic HM-1:IMSS and the monoxenically grown trophozoites (Table 1). The alcohol dehydrogenase activity of E. histolytica was not affected in trophozoites grown with either of the E. coli strains (78 and 65 U for HK-9 and HM-1:IMSS, respectively).
Immunodetection of light and heavy subunits of Gal/GalNAc lectin.
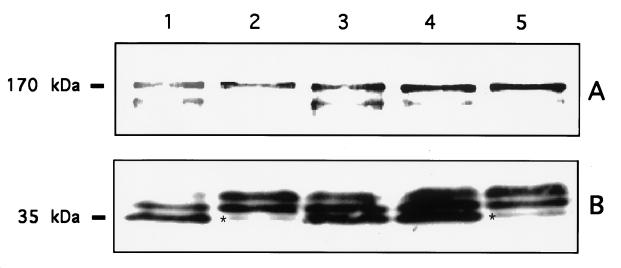
Polyclonal antibodies against the 170-kDa subunit and monoclonal antibodies against the 35-kDa subunit of the Gal/GalNAc lectin were used in Western blots to analyze and to compare the levels of the two lectin components in the axenic and the monoxenically grown trophozoites. The polyclonal antibody recognized a main band of 170 kDa that was not significantly affected when the amebae were grown for 1 month with either of the E. coli strains (Fig. 5A). A minor band, with a slightly lower molecular mass (>170 kDa), appeared to be weaker only in trophozoites grown with E. coli O55. As previously demonstrated (29), the monoclonal antibody against the light subunit recognized three bands with similar patterns in axenic trophozoites (HK-9 and HM-1). The main lower band corresponds to a 35-kDa protein, in accordance with molecular markers (Fig. 5B). The band pattern observed in strains HK-9 and HM-1 grown with E. coli O55 was different in that the lower band was almost missing. This effect was not observed in HK-9 grown with E. coli 346. The lower band in the 35-kDa region was clearly less intense only in trophozoites grown with E. coli O55 (Fig. 5B, lanes 2 and 5), a condition that, as shown above, affected trophozoite cytopathic activity.
FIG. 5.
Western blot analysis of the light and heavy subunits of Gal/GalNAc lectin from E. histolytica trophozoites axenically and monoxenically grown with E. coli for 1 month. Western immunoblots were interacted with polyclonal (A) and monoclonal (B) antibodies directed against the heavy and light Gal/GalNAc subunits, respectively. Horseradish peroxidase-conjugated antibodies were used as a secondary antibody and developed by enhanced chemiluminescence. Lanes: 1, axenic HK-9; 2 and 3, HK-9 grown with E. coli O55 and 346, respectively; 4, axenic HM-1; 5, HM-1 grown with E. coli O55. The asterisks indicate the 35-kDa bands that are almost missing in trophozoites grown with E. coli O55.
RNA levels of Gal/GalNAc lectin subunits and amebapore.
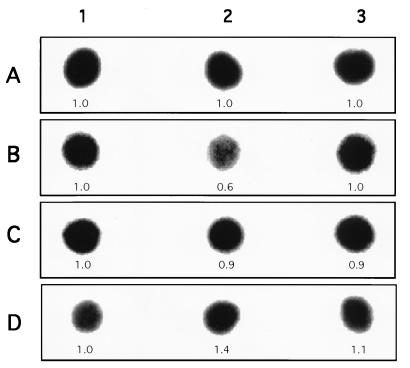
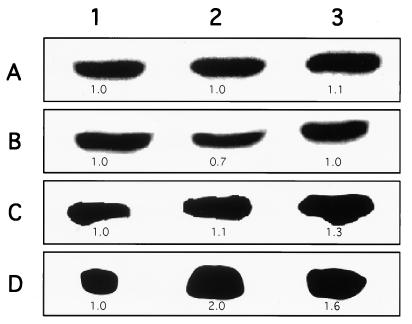
Dot and Northern hybridization analysis of total RNA extracted from E. histolytica trophozoites (axenically and monoxenically grown) was done with labeled DNA probes from the genes coding for the light and heavy subunits of Gal/GalNAc lectin as well as with the gene coding for the amebapore. Comparison by imaging densitometry of the RNA levels of the 35-kDa light subunit lectin showed a decrease of about 50% in trophozoites of strain HK-9 (Fig. 6 and 7) and 30% in trophozoites of strain HM-1 (Fig. 8) grown monoxenically with E. coli O55. The RNA levels of trophozoites grown with E. coli 346 were not affected (Fig. 7 and 8). As a standard control, the blot was also probed with an actin probe. Interestingly, the expression of amebapore appears to double in trophozoites monoxenically grown with E. coli O55, whereas there is only a slight increase (20 to 30%) in amoebae grown with E. coli 346 (Fig. 7 and 8).
FIG. 6.
RNA levels of HK-9 axenically and monoxenically grown with E. coli. Dot blot hybridization of amebic RNA with labeled DNA probes from actin (A), light (B) and heavy (C) Gal/GalNAc lectin subunits, and amebapore (D) is shown. Lanes: 1, axenic HK-9; 2 and 3, HK-9 grown with E. coli O55 and 346, respectively. The values below each dot indicates the optical density, taking the HK-9 axenic value (1.0) as the reference for each probe. Identical amounts of RNA were placed in each blot.
FIG. 7.
Total-RNA levels of Gal/GalNAc lectin subunits and amebapore from axenic and E. coli-cocultured E. histolytica trophozoites of strain HK-9. Northern hybridization of amebic RNA with labeled DNA probes from actin (A), light (B) and heavy (C) Gal/GalNAc lectin subunits, and amebapore (D) is shown. Lanes: 1, axenic HK-9; 2 and 3, HK-9 grown with E. coli O55 and 346, respectively, for 1 month; 4, HK-9 grown with E. coli O55 for 7 months. The values indicate the optical density of each band, taking the value of axenic HK-9 (1.0) as the reference for each probe.
FIG. 8.
Total-RNA levels of Gal/GalNAc lectin subunits and amebapore from axenic and E. coli-cocultured E. histolytica trophozoites of strain HM-1:IMSS. Northern hybridization of amebic RNA with labeled DNA probes from actin (A), light (B) and heavy (C) Gal/GalNAc lectin subunits, and amebapore (D) is shown. Lanes: 1, axenic HM-1; 2 and 3, HM-1 grown with E. coli O55 and 346, respectively. The values indicate the optical density of each band, taking the value of axenic HK-9 (1.0) as the reference for each probe.
Variations in the cytopathic activity of E. histolytica trophozoites monoxenically cultivated with E. coli O55 or 346 for longer periods.
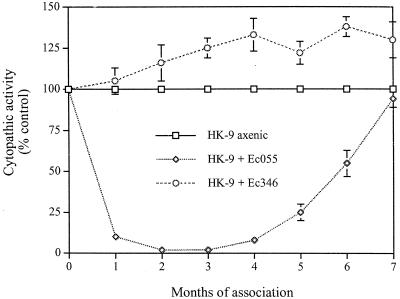
As described above, a dramatic decrease in adherence and cytopathic activity was observed when trophozoites of E. histolytica were monoxenically grown with E. coli O55 for 1 month. We examined the adherence and cytopathic activities of E. histolytica trophozoites of strain HK-9 grown in monoxenic conditions with E. coli O55 or 346 for 7 months. After 4 months of monoxenic culture, the trophozoites began to recover their cytopathic activity, and it reached the original level after 7 months (Fig. 9). Adherence, erythrophagocytosis, and hemolytic activity also recovered, while proteolytic activity remained lower (data not shown). The cytopathic activity of amebae grown with E. coli 346 for several months was 25% higher (Fig. 9). RNA levels of the light-subunit Gal/GalNAc lectin recovered to the original level after 7 months of monoxenic culture with E. coli O55 (Fig. 7, column 4). The amebapore RNA levels remained higher.
FIG. 9.
Cytopathic activity of E. histolytica HK-9 axenically and monoxenically cultivated with E. coli (Ec) O55 or 346 for several months. The destruction of the BHK cell monolayer by E. histolytica trophozoites was evaluated as described in Materials and Methods. The data are expressed as percentages of the cytopathic activity control values (axenic HK-9). Each point is an average of three determinations. The bars indicate standard errors.
DISCUSSION
As previously reported, short-term (30- to 60-min) coincubation of axenically grown E. histolytica trophozoites with E. coli cells significantly increases their cytopathic activity. This increase in activity is assumed to be caused by the stimulation of the amebic electron transfer activities due to the destruction of the toxic H2O2 molecules which accumulate in the trophozoites by the catalase from the ingested bacteria (10). In the present work, we have examined the effects of long-term monoxenic cultivation of E. histolytica trophozoites with bacterial strains that are usually present in the human intestine on adherence and in vitro virulence parameters. Monoxenic cultures of E. histolytica (strains HM-1:IMSS and HK-9) were established with two E. coli strains that use different mechanisms to interact with E. histolytica trophozoites. E. coli serotype O55 has galactose and N-acetyl galactosamine residues on its surface lipopolysaccharide that are recognized by the Gal/GalNAc lectin of E. histolytica trophozoites, whereas E. coli serotype 346 uses its type I pilus lectin to interact with the mannose residues on the ameba surface (7, 8, 24). Monoxenic cultivation of strains HK-9 and HM-1:IMSS with E. coli O55 for 1 month surprisingly decreased their cytopathic activities as well as their capabilities to adhere to mammalian cells or to additional E. coli O55 cells. This decrease was consistently observed in all the monoxenic cultures that were repeatedly initiated. Interestingly, the decrease in adherence and cytopathic activity caused by E. coli O55 appears to be temporary, and after 4 months of monoxenic cultivation, both adherence and cytopathic activity start to increase again. On the other hand, monoxenic cultivation of the same amebic strains with E. coli 346 did not affect their cytopathic activities or adherence capabilities. These findings indicate that the bacterial effect on amebic virulence depends on whether the association is short or long term as well as on the type of adherence that the trophozoites use to attach the bacteria (1, 6, 8). The inhibition of adherence observed in trophozoites grown with E. coli serotype O55, where the trophozoites use the amebic lectin as the mode of attachment, and not in those grown with the mannose binding E. coli 346 suggested that this down regulating effect was due to some impairment in the functionality of the amebic Gal/GalNAc lectin.
The amebic Gal/GalNAc lectin is a 260-kDa heterodimeric glycoprotein consisting of heavy (170-kDa) and light (35- and 31-kDa) subunits linked by disulfide bonds (28, 36). The 170-kDa subunit has a multidomain that includes a single transmembrane span near the carboxy terminus, with a short putative cytoplasmic tail (25). The 170-kDa subunit has been suggested to contain a carbohydrate-binding domain (25, 44) which mediates adhesion to target cells. The large subunit is encoded by a family of five hgl genes (40), and the deduced amino acid sequences of the putative cytoplasmic domains of the sequenced genes revealed several potential phosphorylation sites (45), suggesting that the lectin could be involved in cell signaling and that the signal transduction may occur by way of phosphorylation. Some sequence homology with the autophosphorylation site of the epidermal growth factor receptor (35) has been identified. Recently, a decrease of lectin activity was observed in transfectants that overexpress the cytoplasmic domain, suggesting that it competes for a transduction signal (46). The light subunits (31 and 35 kDa) of the lectin are present in two isoforms: the 31-kDa isoform is glycosyl phosphatidyl inositol anchored, and the 35-kDa isoform is more highly glycosylated (28). The light subunit is encoded by a family of lgl genes located at six loci in the genome (38, 40) and consists of several polypeptide chains with considerable antigenic homology. A monoclonal antibody against the 35-kDa light subunit recognizes three bands on Western blots (29) but did not inhibit adherence of the trophozoites to CHO cells. The role of the light subunits in the functionality and Gal/GalNAc binding affinity of the lectin are not yet fully understood. We have recently found by subtractive hybridizations of cDNA representative libraries that the avirulent E. histolytica strain Rahman has, in addition to other deficiencies, a significantly lower level of the light-subunit gene transcript than the virulent strain HM-1:IMSS, whereas the transcript levels of the 170-kDa gene and several other genes, such as the actin gene, were comparable (4a). In the present study we show that in the adherence- and cytopathic-activity-deficient trophozoites of strains HK-9 and HM-1:IMSS, which were cultivated with E. coli serotype O55, the levels of RNA of the heavy (170-kDa) subunit were normal. Western blots reacted with a polyclonal antibody against the 170-kDa subunit show that the major band was also not affected; however, a minor band of smaller molecular mass was weaker in trophozoites grown with E. coli O55. Our findings for the light (35-kDa) subunit are clearer. Both Western and Northern blotting show that trophozoites monoxenically grown for 1 month with E. coli O55 had significantly reduced levels of the 35-kDa RNA and protein. This was not seen in the control trophozoites monoxenically grown with the mannose binding E. coli 346. The pattern of the light-subunit bands that reacted on Western blots with the monoclonal antibody in trophozoites of strains HK-9 and HM1:IMSS grown with E. coli O55 differed from those seen in the trophozoites axenically or monoxenically grown with E. coli 346 in that the main lower band (35 kDa) was clearly less intense.
Our findings suggest that the inhibition of expression of one of the light-subunit components could prevent the correct assembly of the S-S linked hololectin molecules, causing a defective function in the interaction of the amebae with mammalian cells and Gal-containing bacteria. The signal to reduce the expression of the light subunit is apparently generated as a consequence of the very extensive binding by the ameba lectin molecules to the Gal/GalNAc residues on the surfaces of the excess E. coli O55 cells present. The occupation of the Gal/GalNAc lectin binding sites most likely causes a clustering of the surface lectin molecules, which may trigger a transduction signal. The evidence for such a proposed signal mechanism is currently being sought. In general, we have found that the effects of monoxenic growth with E. coli O55 were more pronounced on the less virulent trophozoites of E. histolytica HK-9 than in those of the more virulent strain, HM-1:IMSS. This implies that the regulatory mechanisms and the effects of external factors may differ between strains.
Gene expression in trophozoites grown in the absence or presence of bacteria appears to vary in a number of genes. Our results show that the genes coding for amebapore are expressed significantly more in trophozoites grown with E. coli O55. The role of amebapore in virulence is not yet fully understood. The higher expression of amebapore could be related to the reported bacteriolytic activity of this small protein (18, 19). On the other hand, the RNA levels of amebapore in E. histolytica trophozoites grown with E. coli 346 did not increase, so this hypothesis needs to be studied in more detail.
CPs are considered an important virulence factor in the pathogenesis of amebiasis (39, 43). Nevertheless CPs are apparently not a main factor in the cytopathic activity of intact trophozoites. In a recent report we have shown that inhibition of expression of cysteine proteinases (90%) by antisense RNA did not affect the cytopathic effect of E. histolytica HM-1:IMSS but inhibited its ability to cause liver lesions in hamsters (3, 4). The correlation between virulence and levels of CPs in a strain are not yet well understood. A comparison between the levels of CPs of axenically grown trophozoites of strain HK-9 and those of the same strain grown with either of the E. coli strains (346 or O55) showed dramatically lower levels for both of the bacterium-associated amebae. However, in spite of the low levels of CPs, only the trophozoites grown with E. coli O55 displayed low cytopathic activity. The levels of CPs in strain HM1:IMSS grown with bacteria were not significantly different from those in the axenically grown trophozoites.
It has been repeatedly demonstrated that the relative levels of virulence of axenically cultured trophozoites vary. A gradual decrease in the ability of trophozoites to induce liver abscesses in hamsters is known to occur following prolonged growth in culture (26). Repeated passage through hamster liver or growth in the presence of high cholesterol helps restore virulence (13, 14, 22). The molecular mechanisms that regulate these down and up variations in virulence are not known. In the present work we have observed that following the association of trophozoites with E. coli serotype O55 there is a gradual decrease in cytopathic activity which is accompanied by a significant decrease in the expression of a lectin component. This decrease in adherence and cytopathic activity, however, is only temporary (1 to 3 months), and after three more months of monoxenic growth, there is a gradual recovery of cytopathic activity. This is accompanied by an increase in the RNA levels of the light subunit of the Gal/GalNAc lectin. The mechanism of this down and up regulation of gene expression is under investigation. Its elucidation will help us understand the genome plasticity that enables the effective adaptation of the ameba to changes in growth culture and nutrients as well as host factors and conditions.
In conclusion, the modulation of amebic virulence, at least in some cases, appears to be due to down regulation of the expression of a 35-kDa lectin subunit gene. We show that this regulation can be induced by long-term cultivation with a bacterium that tightly attaches to the Gal/GalNAc lectin. The pathway which leads to transcription regulation is under investigation.
ACKNOWLEDGMENTS
F. Padilla-Vaca was supported by a postdoctoral fellowship from CONACyT (Consejo Nacional de Ciencia y Tecnologia, Mexico). This work was supported in part by a grant from the Center for Emerging Diseases as well as from the Avicenne Program of the E.U.
We thank Barbara Mann (University of Virginia) for the monoclonal antibody against the 35-kDa subunit, S. Stanley (University of Washington) for antibodies against the 170-kDa subunit, and I. Ofek (University of Tel Aviv) for E. coli 346.
REFERENCES
- 1.Anaya-Velazquez F, Padilla-Vaca F. Effect of intestinal bacteria on the virulence of Entamoeba histolytica. Arch Med Res. 1992;23:183–185. [PubMed] [Google Scholar]
- 2.Andra J, Berninghausen O, Leipe M. Potency of amoebapores compared to that of other membrane-permeating peptides. Arch Med Res. 1997;28:156–157. [PubMed] [Google Scholar]
- 3.Ankri S, Stolarsky T, Mirelman D. Antisense inhibition of expression of cysteine proteinases does not affect Entamoeba histolytica cytopathic or haemolytic activity but inhibits phagocytosis. Mol Microbiol. 1998;28:777–785. doi: 10.1046/j.1365-2958.1998.00837.x. [DOI] [PubMed] [Google Scholar]
- 4.Ankri S, Stolarsky T, Bracha R, Padilla-Vaca F, Mirelman D. Antisense inhibition of expression of cysteine proteinases affects Entamoeba histolytica-induced formation of liver abscess in hamsters. Infect Immun. 1999;67:421–422. doi: 10.1128/iai.67.1.421-422.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4a.Ankri, S., et al. Submitted for publication.
- 5.Bhattacharya A, Ghildyal R, Prasad J, Bhattacharya S, Diamond L S. Modulation of a surface antigen of Entamoeba histolytica in response to bacteria. Infect Immun. 1992;60:1711–1713. doi: 10.1128/iai.60.4.1711-1713.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhattacharya A, Anand M T, Jayshree P, Yadav N, Bhattacharya S. Molecular changes in Entamoeba histolytica in response to bacteria. J Eukaryot Microbiol. 1998;45:28S–33S. doi: 10.1111/j.1550-7408.1998.tb04521.x. [DOI] [PubMed] [Google Scholar]
- 7.Bracha R, Mirelman D. Adherence and ingestion of Escherichia coli serotype O55 by trophozoites of Entamoeba histolytica. Infect Immun. 1983;40:882–887. doi: 10.1128/iai.40.3.882-887.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bracha R, Kobiler D, Mirelman D. Attachment and ingestion of bacteria by trophozoites of Entamoeba histolytica. Infect Immun. 1982;36:396–406. doi: 10.1128/iai.36.1.396-406.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bracha R, Nuchamowitz Y, Mirelman D. Molecular cloning of a 30-kilodalton lysine-rich surface antigen from a nonpathogenic strain. Infect Immun. 1995;63:917–925. doi: 10.1128/iai.63.3.917-925.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bracha R, Mirelman D. Virulence of Entamoeba histolytica trophozoites. Effects of bacteria, microaerobic conditions, and metronidazole. J Exp Med. 1984;160:353–368. doi: 10.1084/jem.160.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Braga L L, Ninomiya H, McCoy J J, Eacker S, Wiedmer T, Pham C, Wood S, Sims P J, Petri W A., Jr Inhibition of the complement membrane attack complex by the galactose-specific adhesin of Entamoeba histolytica. J Clin Investig. 1992;90:1131–1137. doi: 10.1172/JCI115931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bruchhaus I, Jacobs T, Leippe M, Tannich E. Entamoeba histolytica and Entamoeba dispar: differences in numbers and expression of cysteine proteinase genes. Mol Microbiol. 1996;22:255–263. doi: 10.1046/j.1365-2958.1996.00111.x. [DOI] [PubMed] [Google Scholar]
- 13.Das S R, Ghoshal S. Restoration of virulence to rat of axenically grown Entamoeba histolytica by cholesterol and hamster liver passage. Ann Trop Med Parasitol. 1976;70:439–443. doi: 10.1080/00034983.1976.11687144. [DOI] [PubMed] [Google Scholar]
- 14.Diamond L S, Phillips B P, Bartgis I L. A comparison of the virulence of nine strains of axenically cultivated Entamoeba histolytica in hamster liver. Arch Investig Med. 1974;5:423–426. [PubMed] [Google Scholar]
- 15.Diamond L S. Lumen dwelling protozoa: Entamoeba, Trichomonads and Giardia. In: Jensen J B, editor. In vitro cultivation of protozoan parasites. Boca Raton, Fla: CRC Press; 1983. pp. 76–110. [Google Scholar]
- 16.Diamond L S, Harlow D R, Cunnick C C. A new medium for the axenic cultivation of Entamoeba histolytica and other Entamoeba. Trans R Soc Trop Med Hyg. 1978;72:431–432. doi: 10.1016/0035-9203(78)90144-x. [DOI] [PubMed] [Google Scholar]
- 17.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 18.Leippe M, Andra J, Nickel R, Tannich E, Muller-Eberhard H J. Amoebapores, a family of membranolytic peptides from cytoplasmic granules of Entamoeba histolytica: isolation, primary structure, and pore formation in bacterial cytoplasmic membranes. Mol Microbiol. 1994;14:895–904. doi: 10.1111/j.1365-2958.1994.tb01325.x. [DOI] [PubMed] [Google Scholar]
- 19.Leippe M. Amoebapores. Parasitol Today. 1997;13:178–183. doi: 10.1016/s0169-4758(97)01038-7. [DOI] [PubMed] [Google Scholar]
- 20.Leippe M, Sievertsen H J, Tannich E, Horstmann R D. Spontaneous release of cysteine proteinase but not of pore-forming peptides by viable Entamoeba histolytica. Parasitology. 1995;111:569–574. doi: 10.1017/s0031182000077040. [DOI] [PubMed] [Google Scholar]
- 21.Leippe M, Tannich E, Nickel R, van der Goot G, Pattus F, Horstmann R D, Muller-Eberhard H J. Primary and secondary structure of the pore-forming peptide of pathogenic Entamoeba histolytica. EMBO J. 1992;11:3501–3506. doi: 10.1002/j.1460-2075.1992.tb05432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lujan H D, Diamond L S. Cholesterol requirement and metabolism in Entamoeba histolytica. Arch Med Res. 1997;28:96–97. [PubMed] [Google Scholar]
- 23.Lynch E C, Rosenberg I M, Gitler C. An ion-channel forming protein produced by Entamoeba histolytica. EMBO J. 1982;1:801–804. doi: 10.1002/j.1460-2075.1982.tb01250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Madison B, Mann B, Clegg S, Abraham N. Type 1 fimbrial shafts of Escherichia coli and Klebsiella pneumoniae influence sugar-binding specificities of their FimH adhesins. Infect Immun. 1994;62:843–848. doi: 10.1128/iai.62.3.843-848.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mann B J, Chung C Y, Dodson J M, Ashley L S, Braga L L, Snodgrass T L. Neutralizing monoclonal antibody epitopes of the Entamoeba histolytica galactose adhesin map to the cysteine-rich extracellular domain of the 170-kilodalton subunit. Infect Immun. 1993;61:1772–1778. doi: 10.1128/iai.61.5.1772-1778.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mattern C F T, Keister D B, Natovitz P C. Virulence of Entamoeba histolytica upon continuous axenic cultivation. Arch Investig Med. 1982;13:185–190. [PubMed] [Google Scholar]
- 27.McCoy J J, Mann B J, Petri W A., Jr Adherence and cytotoxicity of Entamoeba histolytica or how lectins let parasites stick around. Infect Immun. 1994;62:3045–3050. doi: 10.1128/iai.62.8.3045-3050.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCoy J J, Mann B J, Vedvick T S, Petri W A., Jr Structural analysis of the light subunit of the Entamoeba histolytica galactose-specific adherence lectin. J Biol Chem. 1993;268:24223–24231. [PubMed] [Google Scholar]
- 29.McCoy J J, Weaver A M, Petri W A., Jr Use of monoclonal anti-light subunit antibodies to study the structure and function of the Entamoeba histolytica Gal/GalNAc adherence lectin. Glycoconjugate J. 1994;11:432–436. doi: 10.1007/BF00731279. [DOI] [PubMed] [Google Scholar]
- 30.Mirelman D. Ameba-bacterial relationship in amebiasis. In: Ravdin J I, editor. Amebiasis. Human infection by Entamoeba histolytica. New York, N.Y: John Wiley and Sons; 1988. pp. 351–369. [Google Scholar]
- 31.Mirelman D. Ameba-bacterium relationship in amebiasis. Microbiol Rev. 1987;51:272–284. doi: 10.1128/mr.51.2.272-284.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mora-Galindo J, Gutierrez-Lozano M, Anaya-Velazquez F. Entamoeba histolytica: kinetics of hemolytic activity, erythrophagocytosis and digestion of erythrocytes. Arch Med Res. 1997;28:S200–S201. [PubMed] [Google Scholar]
- 33.Orozco E, Guarneros G, Martinez-Palomo A, Sanchez T. Entamoeba histolytica: phagocytosis as a virulence factor. J Exp Med. 1983;158:1511–1521. doi: 10.1084/jem.158.5.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Petri W A, Smith R D, Jr, Achlesinger P H, Murphy C F, Ravdin J I. Isolation of the galactose-binding lectin that mediates the in vitro adherence of Entamoeba histolytica. J Clin Investig. 1987;80:1238–1244. doi: 10.1172/JCI113198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Petri W A, Jr, Snodgrass T L, Jackson T F H G, Gathiram V, Simjee A E, Chadee K, Chapman M D. Monoclonal antibodies directed against the galactose-binding lectin of Entamoeba histolytica enhance adherence. J Immunol. 1990;144:4803–4809. [PubMed] [Google Scholar]
- 36.Petri W A, Jr, Chapman M D, Snodgrass T L, Mann B J, Broman J, Ravdin J I. Subunit structure of the galactose and N-acetyl-D-galactosamine-inhibitable adherence lectin of Entamoeba histolytica. J Biol Chem. 1989;264:3007–3012. [PubMed] [Google Scholar]
- 37.Petri W A, Jr, Schnaar R L. Purification and characterization of galactose- and N-acetylgalactosamine-specific adhesin lectin of Entamoeba histolytica. Methods Enzymol. 1995;253:98–104. doi: 10.1016/s0076-6879(95)53011-8. [DOI] [PubMed] [Google Scholar]
- 38.Purdy J E, Mann B J, Shugart E C, Petri W A., Jr Analysis of the gene family encoding the Entamoeba histolytica galactose-specific adhesin 170 kDa subunit. Mol Biochem Parasitol. 1993;62:53–60. doi: 10.1016/0166-6851(93)90177-y. [DOI] [PubMed] [Google Scholar]
- 39.Que X, Reed S L. The role of extracellular cysteine proteinases in pathogenesis of Entamoeba histolytica invasion. Parasitol Today. 1997;13:190–194. doi: 10.1016/s0169-4758(97)01043-0. [DOI] [PubMed] [Google Scholar]
- 40.Ramakrishnan G, Ragland B D, Purdy J E, Mann B J. Physical mapping and expression of gene families encoding the N-acetyl D-galactosamine adherence lectin of Entamoeba histolytica. Mol Microbiol. 1996;19:91–100. doi: 10.1046/j.1365-2958.1996.356885.x. [DOI] [PubMed] [Google Scholar]
- 41.Ravdin J I. Human infection by Entamoeba histolytica. In: Ravdin J I, editor. Amebiasis: human infection by Entamoeba histolytica. New York, N.Y: John Wiley and Sons; 1988. pp. 166–176. [Google Scholar]
- 42.Ravdin J I, Guerrant R L. Role of adherence in cytopathic mechanisms of Entamoeba histolytica. Study with mammalian tissue culture cells and human erythrocytes. J Clin Investig. 1981;68:1305–1313. doi: 10.1172/JCI110377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reed S L, Bouvier J, Pollack A S, Engel J C, Brown M, Hirata K, Que X, Eakin A, Hagblom P, Gillin F, McKerrow J H. Cloning of a virulence factor of Entamoeba histolytica. J Clin Investig. 1993;91:1532–1540. doi: 10.1172/JCI116359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tannich E, Scholze H, Nickel R, Horstmann R D. Homologous cysteine proteinase of pathogenic and nonpathogenic Entamoeba histolytica: differences in structure and expression. J Biol Chem. 1991;266:4798–4803. [PubMed] [Google Scholar]
- 45.Tannich E, Ebert F, Horstmann R D. Primary structure of the 170 kDa surface lectin of pathogenic Entamoeba histolytica. Proc Natl Acad Sci USA. 1991;88:1849–1853. doi: 10.1073/pnas.88.5.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vines R R, Ramakrishnan G, Rogers J B, Lockhart L A, Mann B J, Petri W A., Jr Regulation of adherence and virulence by the Entamoeba histolytica lectin cytoplasmic domain, which contains a B2 integrin motif. Mol Biol Cell. 1998;9:2069–2079. doi: 10.1091/mbc.9.8.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wittner M, Rosenbaum R M. Role of bacteria in modifying virulence of Entamoeba histolytica. Studies of amebae from axenic cultures. Am J Trop Med Hyg. 1970;19:755–761. doi: 10.4269/ajtmh.1970.19.755. [DOI] [PubMed] [Google Scholar]