Abstract
Chemical-intensive agriculture challenges environmental sustainability and biodiversity and must be changed. Minimizing the use of agrochemicals based on renewable resources can reduce or eliminate ecosystems and biodiversity threats. Nanochitosan as a sustainable alternative offers promising solutions for sustainable agricultural practices that work at multiple spatial and temporal scales throughout the plant growth cycle. This review focuses on the potential of nanochitosan in sustainable agricultural production and provides insights into the mechanisms of action and application options of nanochitosan throughout the plant growth cycle. We emphasize the role of nanochitosan in increasing crop yields, mitigating plant diseases, and reducing agrochemical accumulation. The paper discusses the sources of nanochitosan and its plant growth promotion, antimicrobial properties, and delivery capacity. Furthermore, we outline the challenges and prospects of research trends of nanochitosan in sustainable agricultural production practices and highlight the potential of nanochitosan as a sustainable alternative to traditional agrochemicals.
Keywords: nanochitosan, sustainable agriculture, renewable resources, antimicrobial, delivery system, SDGs
1. Introduction
Agriculture represents one of the cornerstones for human development. However, current agriculture often relies on the massive use of fertilizers and pesticides that adversely affect living organisms and ecosystems [1]. It is an important sustainable development goal (SDG) to achieve the environmentally sound management of chemicals and all wastes and significantly reduce their release into air, water, and soil in order to minimize their adverse impacts on human health and the environment [2]. Sustainable agricultural production patterns are a new trend of future agricultural development. Sustainable agriculture aims to provide sufficient nutritious food for all, while reducing environmental and health risks simultaneously [3]. The minimization of agrochemicals represents a fundamental tenet of sustainable agriculture, with the objective of reducing or eliminating adverse effects on living organisms and ecosystems [4]. Sustainable agriculture relies on the support of advanced technologies to provide more sustainable pesticides, fertilizers, and materials [5].
Nanomaterials and nanotechnologies offer new opportunities to address the challenges associated with agroecology [6]. Nanomaterials have a small size, tunable surface chemistry, and high-efficiency properties as a key driver in accelerating future sustainable agriculture [6]. Nanomaterials have been used to develop adsorbents, fertilizer agents, catalysts, antibacterial substances, and delivery systems. Among the available natural substances, nanochitosan is a biocompatible and biodegradable polymer with enormous structural modification potential that is expected to promote the development of sustainable agriculture [7].
Nanochitosan is derived from chitin, a critical biodegradable biomass polysaccharide, and is obtained by the chemical deacetylation of chitin under alkaline conditions. It can be obtained from renewable resources [8]. Chitin is the largest untapped renewable resource and one of the richest polymer polysaccharides in nature [5,9]. Nanochitosan exhibits multiscale architectures based on random copolymers of glucosamine and N-acetylglucosamine units and demonstrates a diverse range of morphologies. These include small oligomers, rod-shaped nanocrystals, elongated nanofibers, and hierarchical assemblies of nanofibers [10]. Nanochitosan is a non-toxic, bioadhesive, and biocompatible compound that is bioavailable and biodegradable [11,12]. The United States Food and Drug Administration has designated nanochitosan as a “generally recognized as safe” food additive [13]. A variety of distinct nanochitosan derivatives have been synthesized and applied in a diverse range of fields, including agriculture, food, textiles, and medicine [10,14]. Nanochitosan is a versatile and biocompatible material with the potential to be utilized throughout the entire crop growth cycle. It can be employed as a soil conditioner, plant growth regulator, vegetable and crop disease management agent, fruit antistaling agent, and seed coating agent. Nanochitosan is easily degraded in the environment, preventing the accumulation and enhancing the quantity of organic matter, thereby improving soil structure and health due to its high biodegradability [15,16]. In addition, nanochitosan can absorb and retain nutrients in the soil, including nitrogen and phosphorus, which are particularly beneficial when highly absorbent. Finally, nanochitosan is also anticipated to regulate plant pathogens and enhance crop protection through antimicrobial and biopesticide properties [17,18]. Nanochitosan can regulate the release and site-specific delivery of active ingredients by reducing the toxicity of pesticides to plants, enhancing their absorption, and improving solubility and stability [19]. The controlled release of its active ingredients can mitigate environmental threats to ecosystems and human health by enhancing the bioavailability of pesticides. These properties render nanochitosan an optimal material for the promotion of agroecology [7]. The mechanism of chitosan nanoparticles enhancing crop yield and mitigating plant diseases mainly involves the following aspects: firstly, as a soil conditioner, it regulates the soil microbial structure and improves soil quality; secondly, it directly regulates crops to promote growth; and finally, it directly kills pests and diseases or acts as a drug carrier.
This review discusses the sources, applications, mechanisms, and prospects of nanochitosan in agroecology and clarify the necessity for the advancement of agroecology (Figure 1). We also summarize the mechanism of action and application scenarios of nanochitosan throughout the entire crop cycle, including its effects on crop yield, pesticide activity, and the reduction in agrochemical accumulation. Furthermore, the sources of nanochitosan and its effects on plant growth, antimicrobial properties, and delivery capacity are classified and discussed in detail. Finally, we discuss the challenges and provide an outlook for future nanochitosan research in agroecology, as well as the focus and trends.
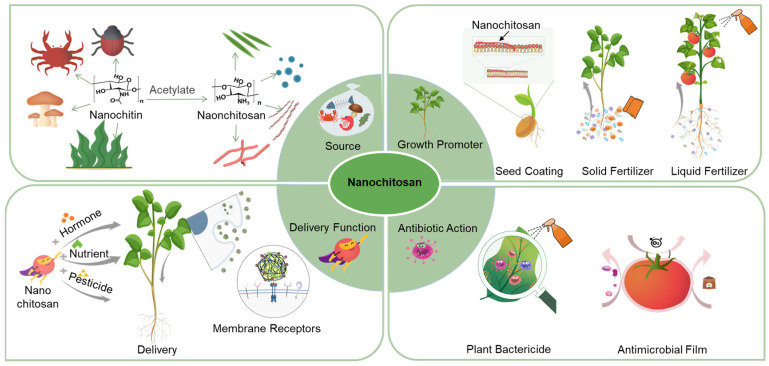
Figure 1.
Summary of the nanochitosan origin and application in multiple spatial and temporal scales throughout the plant growth cycle.
2. Source and Preparation of Nanochitosan
2.1. Source of Chitosan
Nanochitosan is derived from the chemical deacetylation of chitin under alkaline conditions. Chitin, a polymer found throughout the biosphere, is the second most abundant polymer after cellulose [20]. In more than 90% of animal species and insects, the primary component of the arthropod exoskeleton is a chitin-based complex. Currently, the majority of chitin production is derived from crab and shrimp shells from the canning industry, which avoids environmental pollution and nutrient waste [21]. Chitin is currently produced on a global scale, with an estimated annual production of between 1010 and 1012 tons [22].
2.2. Morphological Classification and Preparation Method of Nanochitosan
Nanochitosan encompasses chitosan nanofibers, nanoparticles, and nanocrystals that can be prepared by the deacetylation of chitin [23]. Chemical, enzymatic, microwave, and microbiological methods have been employed to prepare nanochitosan [24,25]. The chemical methods and processes involved are more straightforward and quicker, and the products have been shown to have more potent bioactivities. Furthermore, nanochitosan can be extracted through a combination of microbiological and biotechnological methods, including those involving Mucor rouxii, Absidia butleri, Abesidiacorrulea, pyrioceana blue, and waste citric acid bacteria [26,27]. The microbiological and biotechnological methods have considerable potential for development in the field of agroecology, due to its low energy and resource consumption, eco-friendliness, and high efficiency.
3. Nanochitosan Promotes Plant Growth
Nanochitosan is an environmentally friendly alternative to synthetic chemical agents for plant growth promotion due to its biocompatibility and biodegradability. This section aims to provide an overview of the progress of research on nanochitosan as a plant growth promoter (Table 1).
Table 1.
Application of nanochitosan in promoting plant growth.
| Site of Action | Morphology | Applied Crop | Effect | Function | References |
|---|---|---|---|---|---|
| Soil | Nanochitosan solution | Pinus edulis | Improves plant water and nutrient uptake | 37% reduction in mortality | [21] |
| Soil | Nanochitosan particles |
Corn | Increased bacterial diversity; increase in the number of OTUs (operational taxonomic units); increased enzyme activity (FDA (fluorescein diacetate), dehydrogenase, and alkaline phosphatase) | The composition, diversity, and richness of rhizosphere microbial communities were improved; improved soil health | [22] |
| Soil | Nanochitosan –Cu |
Corn | Improved the activity of antioxidant enzymes in leaves, promoted the activity of maize source, and increased yield | Increased chlorophyll content (2-fold) and induced internode sucrose translocation (2.5–3.5-fold) to provide nutrients | [23] |
| Soil | Chitosan–urea nanoparticles | Potato | The fresh weight and dry weight of stems and leaves of potato were significantly increased; improved yield-related traits such as number of potatoes set and potato weight per plant | The contents of NH4+-N and NO3−-N in the soil were significantly reduced; the number of ammonia-oxidizing bacteria and nitrifying reducing bacteria in the soil decreased | [25] |
| Soil and foliar |
Nanochitosan particles |
Tomato | The density of root-knot nematodes decreased by 45.89–66.61% and the density of TMV (tobacco mosaic virus) decreased by 10.26–65.00% | Soil enzyme activity and increased plant defense enzyme activity | [9] |
| Soil and foliar |
Nanochitosan solution |
Tomatoes and potatoes | Control of bacterial wilt | Reduced morbidity and disease severity | [10] |
| Foliar | Nanochitosan–N fertilizer | Corn–soybean intercropping | The biological yield of maize increased, the occurrence of disease decreased by 78.93%, and the disease severity decreased by 71.85%; the biological yield of soybean increased, the occurrence of disease decreased by 81.64%, and the disease severity decreased by 77.63% | Promoted the absorption and utilization of nitrogen fertilizer and reduced nitrogen fertilizer loss | [5] |
| Foliar | Nanochitosan solution | Sesame | Improved resistance to Spodoptera litura, increased antioxidant enzyme activity, and improved nutritional quality | Activated the plant’s natural immunity to herbivorous insects and enhanced the production of defense metabolites | [6] |
| Foliar | Nanochitosan– gibberellin |
Sorghum | Gibberellin-modified nanochitosan acted as a biostimulant to enhance the growth of sorghum under salt stress, and plant height, fresh weight and dry weight increased | Increased chlorophyll levels and increased antioxidant enzyme (POD (peroxidase) and SOD (superoxide dismutase)) activity | [6] |
| Foliar | Nanochitosan–K | Strawberry | The total yield increased, the market yield increased, and the fruit firmness increased significantly | Increased the content of soluble and exchangeable potassium in soils; increased total soluble solids, vitamin C levels, acidity, total sugars, and anthocyanin levels in the fruit | [7] |
| Foliar | Nanochitosan solution |
Common beans | Plants treated with 62.5 mg/L nanochitosan showed higher chlorophyll content, plant height, fresh weight (stem and root), seed yield, and nutrient content | Increased organic matter, available nutrient content, and total bacterial count in soil and decreased Na% in fungal communities and plants | [8] |
| Foliar and seed | Nanochitosan–copper particles | Corn | Enhanced plant growth and yield, promoted nutrient uptake, enhanced plant defense response to disease, and improved plant growth and yield | Improved the activity of antioxidant enzymes and defense enzymes to control CLS (calcium lignosulfonate) disease | [20,28] |
| Seed | Nanochitosan–Bacillus spp. | Corn | Increased seed germination rate, plant height, root length, leaf area, fresh and dry weight, chlorophyll, carotenoids, and total sugar and protein content; promoted plant growth; enhanced defense response; and increased yield | Beneficial microbial communities in maize rhizosphere soil were improved | [11] |
| Seed | Nanochitosan solution |
Cucumber | Increased germination rate (90%), enhanced seedling vigor (2665), and improved resistance to powdery mildew (66.6% disease protection) | Stimulated plant hormone content, induced the biosynthesis of defense-related enzymes, and enhanced plant growth | [12] |
| Seed | Nanochitosan–salicylic acid particles | Wheat | Increased the activity of seed reserve food remobilization enzyme; increased seedling vigor index (SVI) by 1.6 times, chlorophyll content by 1.46 times, and plant weight per pot | Promoted plant growth, improved antioxidant status, regulated reactive oxygen species (ROS) and malondialdehyde (MDA) content, and maintained cellular homeostasis | [13] |
| Seed | Nanochitosan | Corn | Improvement in growth parameters and soil quality, biomass increased and plant height (54%), leaf number (67.18%), photosynthetic pigment (65.62%), sugar (79.13%), protein (71.93%), phenol (136.57%), and flavonoids (167.61%) | The activity of antioxidant enzymes was increased: catalase (80.15%) and peroxidase (25.52%); the total number of soil bacteria increased (101%), phosphate (111%), the activity of soil enzymes was increased and dehydrogenase (94.88%), fluorescein diacetate (112%), and alkaline phosphatase (32.09%) | [29] |
| Seed | Nanochitosan– Ag |
Wheat | Promoted seed germination, improved seed quality, and reduced the incidence of disease | The fungal load was reduced by 100%, the albumin content increased by 4.25 times, and the glutenin content increased by 5.78 times | [15] |
| Seed | Nanochitosan– salicylic acid solution |
Lentil | Increased plant dry weight by 16% (after 35 days) and 40% (after 70 days) | Improved the mineral, soluble sugar, and pigment content, nitrogen content by about 40%, phosphorus content by about 52%; strengthened the defense mechanism (total phenols by about 2 times, peroxidase by about 7–69%, and polyphenol oxidase by about 16–50%) | [16] |
| Seed | Nanochitosan– polymer |
Wheat | The aerial part increased by 9.8–15.3 mm; the underground part increased by 11.3–17.5 mm | Promoted seed germination and seedling growth and increased the germination rate by 3.3–4.0% | [17] |
| Seed | Nanochitosan solution |
Corn | The germination rate increased to 96.97%, the plant height increased by 1.5 times, and the leaf area increased by 2 times | Promoted nutrient absorption, enhanced soil microbial activity, and improved the activity of soil health indicators such as dehydrogenase, fluorescein diacetate hydrolase, and alkaline phosphatase, and the activity was increased by 2 to 3 times | [18] |
| Seed | Nanochitosan– Pseudomonas particles |
Tomato | Increases the growth percentage of tomato (46.62%) and reduces the percentage of disease (115.85%) | Soil enzyme activity was improved, including urease (411%), phosphatase (488%), catalase (765%), β-glucosidase (194%), and antifungal enzymes (chitinase 1666% and glucanase 89%); improved the activity of defense enzymes in tomato plants under infection, including superoxide dismutase (64%), polyphenol oxidase (80%), cell-wall-bound peroxidase (180%), and phenylalanine ammonia-lyase (180%) |
[19] |
3.1. The Mechanisms of Nanochitosan Enhancing Plant Growth
The chitosan polymer exhibits a high density of positive charges and a profound affinity for cell membranes due to the presence of one amino group (-NH2) and two hydroxyl groups (-OH) within its monomeric structure [30]. The amino group confers a net positive charge to chitosan, enabling it to effectively bind with macromolecules, negatively charged lipids, and proteins. Chitosan can also form coordination bonds with metal ions to produce complexation and bind together. The hydroxyl functional group also serves as an electron acceptor, facilitating metabolic processes and signal transduction through binding to cellular receptors.
Nanochitosan has been demonstrated to enhance plant resistance to abiotic stress by inducing the production of key defense enzymes, including catalase, polyphenol oxidase, phenylalanine ammonia-lyase, and peroxidase. These enzymes are crucial in protecting plant cells from oxidative damage caused by reactive oxygen species [31]. Phenylalanine deaminase can induce plant metabolism. Polyphenol oxidase can catalyze phenolic substances to synthesize lignin, the raw material for building cell wall structure. In addition, nanochitosan can reduce chloroplast membrane disruption and increase chlorophyll content and the total photosynthesis of plants. This is achieved by reducing ethylene concentration and inhibiting chlorophyll enzyme activity [32]. Following the application of chitosan to leaves, α-amylase activity, chlorophyll content, and endogenous hormone content can be adjusted to further alter stress resistance and transpiration, thereby effectively increasing plant biomass. The expression of nitric oxide, an important signaling molecule in plant defense mechanisms, was significantly increased following exposure to nanochitosan [33].
Nanochitosan naturally serves as a source of carbon, oxygen, nitrogen, and phosphorus nutrients for plants. Moreover, nanochitosan as a nanocarrier can also support plant macronutrients (calcium, potassium, nitrogen, sulfur, phosphorus, and magnesium) and micronutrients (manganese, copper, nickel, zinc, boron, iron, and chloride) through its functional groups [34]. The cationic properties of chitosan can be employed to enhance the anion exchange capacity of the soil or growing medium, which is usually much lower than the cation exchange capacity. The leaching of anionic nutrient fertilizers (e.g., nitrates and phosphates) in soils treated with chitosan can be reduced [35].
3.2. The Application of Nanochitosan on the Plant Growth Cycle
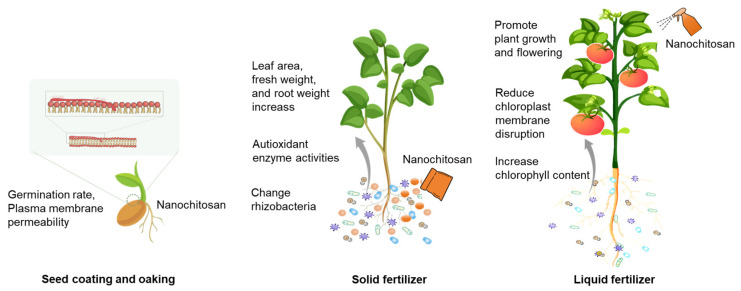
Nanochitosan as a sustainable alternative offers promising solutions for sustainable agricultural practice that work at multiple spatial and temporal scales throughout the plant growth cycle (Figure 2).
Figure 2.
The application of nanochitosan during the plant growth cycle.
Nanochitosan is an appropriate seed coating material due to its favorable film formation, adhesion, and hygroscopicity properties. Nanochitosan can form a seed coating on the seed surface, alter the permeability of the seed plasma membrane, and accelerate the germination and germination rate. The germination rate, mean germination time, germination value, picking value, and mean daily germination rate of seeds were affected by the nanochitosan environment. For example, chickpea seeds treated with chitosan from thiamine nanoparticles exhibited 90% germination compared to 75% germination with water. The nanochitosan treatment resulted in a reduction in the average germination time of pepper seeds by 4.9 to 5.3 days. The treatment promoted seedling emergence and growth as well as improved pepper growth parameters, including total leaf area, fresh weight, and root weight [36].
The application of a nanochitosan coating resulted in enhanced seed tolerance to abiotic stress, as evidenced by increased peroxidase, catalase, phenylalanine ammonia-lyase, and malondialdehyde (MDA) levels, which serve as indicators of lipid peroxidation. The MDA was also reduced by the increased antioxidant activity. Seedlings coated with nanochitosan resulted in elevated levels of defense enzymes and a tenfold increase in auxin content [37]. Chitosan-soaked groundnut seeds showed significant increases in germination, lipase activity, gibberellin, and indoleacetic acid levels [38]. Nanochitosan–glycinebetaine pretreatment reduced the relative permeability of the maize plasma membrane under heat and drought stresses conditions, reduced the damage of heat stress on maize, and also to improved drought hardiness [39]. It is worth noting that the optimal concentration of nanochitosan was found to be 30 ppm, while nanochitosan at a concentration of 90 ppm can lead to adverse effects on the majority of the traits studied [40].
Nanochitosan can also be sprayed on the leaf surface to promote plant growth and flowering due to its better dispersibility and cell affinity. Nanochitosan is often classified as an elicitor for activating plant genes underlying secondary metabolite biosynthesis pathways. Nanochitosan was found to increase plant resistance to S. litura by activating endogenous signaling cascades (Ca2+ influx and phytohormone accumulation) and enhancing the production of defense metabolites (such as sesamolin and shanzhiside methylester) via upregulating defense metabolite biosynthetic genes. The highest resistance of sesame plants to S. litura and the alleviation of plant oxidative stress by increasing the activities of antioxidant enzymes were achieved by the foliar application of 100 mg L−1 nanochitosan [41].
Nanochitosan has also been demonstrated to be an effective agent for combating the common plant pathogen Xanthomonas. It can be sprayed on plants to induce the accumulation of bioactive secondary metabolites. Foliar applications of chitosan during flowering can increase the number of flowers, the weight per plant, and the yield per forage of chamomile. Low concentrations of chitosan (10 mg L−1) were found to effectively induce vegetative development in Brassica napus. The foliar application of chitosan has been demonstrated to promote tomato growth and affects the expression of endogenous chitinase-encoding genes and mycorrhiza formation [42]. The application of lactic acid chitosan to the foliage of plants has been demonstrated to promote the accumulation of bioactive substances [43]. Nanochitosan at a concentration of 2–4 g L−1 promotes the growth of maize plants by increasing the chlorophyll content, α-amylase activity, and endogenous hormone content of maize seedling leaves [44]. The rise in organic acids indicated an enhanced stress resistance mechanism in maize plants following an enhanced nanochitosan treatment. The foliar spray application of nanochitosan is a viable method for cultivating medicinal and aromatic plants, which are known to possess superior health benefits and medicinal properties. This practice effectively stimulates the accumulation of selected phenolic compounds in basil and lemon balm [43].
In addition to seed coating and foliar spraying, nanochitosan can also be added into the soil. The application of nanochitosan at a rate of 6 mg kg−1 in soil has been demonstrated to enhance nitrogen accumulation, dry matter, grain yield, crude protein concentration, and the translocation of nutrients from vegetative organs to grains following anthesis. The positive effect of nanochitosan is attributed to the activation of PEPC (phosphoenolpyruvate carboxylase), SPS (sucrose phosphate synthase), and crucial metabolic enzymes in flag leaves and spikes [45]. It can be used in a sustainable biofertilization strategy that enhances plant growth and improves health-promoting compounds in wheat. The combination of nanochitosan with Bacillus and Rhizobium has been demonstrated to have a growth-promoting effect on maize. After treatment with nanochitosan, seed germination significantly increases from 60% to 96.97%. Additionally, the plant height, leaf area, and the alcohols and acids in plant metabolites exhibited notable enhancements [29]. Notably, the content of esters and aldehydes exhibited a considerable increase. The application of nanochitosan (40 mg L−1) in conjunction with Pseudomonas spp. resulted in a notable enhancement of plant vigor, including an increase in plant height, chlorophyll, and carotenoid content in maize plants. Soil enzyme activities, including dehydrogenase and alkaline phosphatase, were significantly increased. The combination of nanochitosan with Bacillus spp. in maize has been demonstrated to enhance plant height, leaf area, seed germination, and organic acid production in response to stress tolerance [29]. The application of nanochitosan has been demonstrated to contribute to the bioremediation of heavy-metal-contaminated soils. Nanochitosan can improve the Cd phytoremediation capacity of Datura stramonium L. The link of nanochitosan with κ-carrageenan has been shown to effectively remove and immobilize Cd2+ from both water (750.2 μmol/g) and soil (992.7 μmol/g) [46,47].
4. The Antimicrobial Properties of Nanochitosan
Nanochitosan exhibits excellent antimicrobial properties and significantly inhibits various bacteria, fungi, and viruses [5]. Since Allen [48] first proposed the broad-spectrum antibacterial activity of chitosan, the antibacterial properties of chitosan and its derivatives have been a subject of great interest to researchers. This section aims to provide an overview of the research progress of nanochitosan as an antimicrobial agent and its potential applications in agriculture.
4.1. The Antibacterial Mechanisms of Nanochitosan
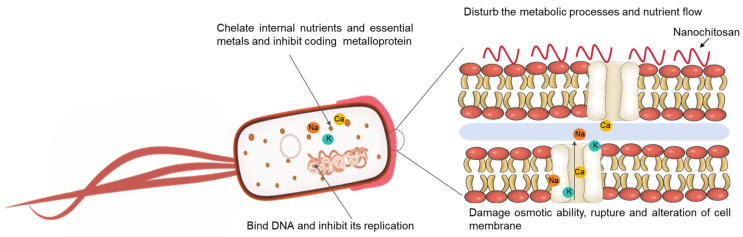
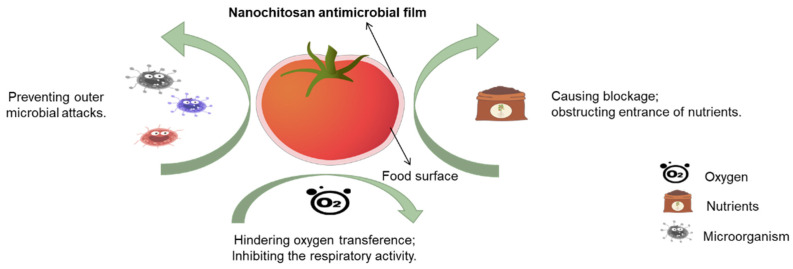
Nanochitosan exhibits a variety of inhibitory effects against bacteria, fungi, and viruses. A nanochitosan polymer has one amino group (-NH2) at the C-2 location and two hydroxyl groups (-OH) at the C-3 and C-6 locations. The -NH2 group imparts a net-positive charge to nanochitosan, allowing it to interact with anionic molecules, including structural and functional anionic protein and cellular membrane phospholipids. It is notable that the C6-OH group has hyperactive -OH groups that can rotate freely with minimal steric hindrance. The -OH functional group acts as an electron acceptor and hastens signal transduction by engaging with cell receptors and accelerating metabolic events. Consequently, nanochitosan has four primary modes of action: (1) The metabolic processes and nutrient flow of the microorganism are disrupted by the formation of a dense polymeric membrane on the pathogen’s surface, resulting from the deposition of chitosan [49]. (2) It interacts electrostatically with the cell membrane, causing increased permeability of the pathogenic organism’s cell envelope, rupture and alteration of the cell membrane, osmotic damage, and eventual death [50]. (3) It forms complexes with certain metal ions, inhibiting the metalloproteins’ activity and microbial growth [51]. (4) It binds to negatively charged phosphate groups in the nucleic acid backbone, thereby inhibiting mRNA transcription and protein translation [52]. This results in the release of LPS from the outer membrane, thereby increasing the permeability of the outer membrane (Figure 3).
Figure 3.
The antibacterial mechanisms of nanochitosan.
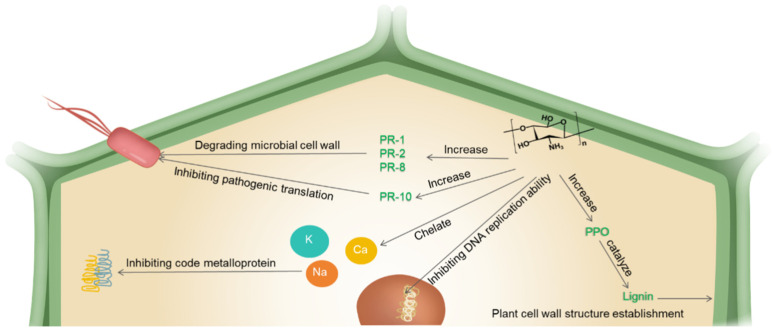
In addition to nanochitosan’s direct effect on pathogenic bacteria, nanochitosan also has an indirect effect on enhancing plant resistance to pathogenic bacteria. Nanochitosan has been demonstrated to effectively induce a host defense response against plant pathogens and generate systemic resistance [53]. Nanochitosan can induce plant defense mechanisms against a variety of plant pathogens, including plant viruses [54]. It can inhibit phage-induced infection through inactivation at the cellular level and the inhibition of propagation [55]. Nanochitosan has also been shown to upregulate the plant gene functions associated with pathogenicity-related genes, such as PR proteins (PR-1 and PR-2 (β-1,3-glucanase), PR-8 (chitinase), and PR-10) and antioxidant genes. The activation of defense genes leads to the accumulation of various enzymes and stress-specific metabolites. (1) In one study, nanochitosan increased the levels and activities of antifungal (PR-1) and hydrolytic enzymes (β-1,3-glucanases (PR-2) and chitinases (PR-8)) to degrade microbial cell walls [56]. (2) Nanochitosan increased the polyphenol oxidase content, which catalyzes the synthesis of lignin from phenolics. This process contributes to the formation of cell wall structures that serve as a barrier to pathogen penetration [57]. (3) Nanochitosan increased ribosome-inactivating protein (PR-10), which releases adenine residues from both ribosomal and non-ribosomal substrates, thereby inhibiting the pathogenic translation [56]. (4) Nanochitosan initiates complex signal transduction pathways involving reactive oxygen species (ROS) production, such as peroxidase, catalase, and superoxide dismutase in the host plant [57].
Notably, the immunostimulatory activity of nanochitosan may not be mediated by a specific receptor-like molecule but rather by the interaction of its surface cations with negatively charged phospholipids. Furthermore, the pathogen is unlikely to develop resistance to nanochitosan, because the negative charge of microbial cell envelopes is evolutionarily conserved and unlikely to be altered by a single gene mutation. Further evidence is needed to clarify the direct activity of nanochitosan against plant viruses. It is reasonable to assume that the antiviral activity of nanochitosan is dependent on its ability to induce plant immune responses [58]. In summary, nanochitosan can induce the host plant to produce defense-related proteins, enzymes, and secondary metabolites that directly or indirectly degrade pathogens. The mechanism of resistance of nanochitosan to plant pathogens is shown in Figure 4.
Figure 4.
The mechanism of resistance of nanochitosan to plant pathogens.
4.2. The Application of Nanochitosan as an Antimicrobial Agent
Nanochitosan has been demonstrated to have inhibitory effects on bacteria and fungi, making it a promising natural antibacterial agent. Nanochitosan’s broad-spectrum antibacterial properties, good biocompatibility, and natural origin make it an attractive candidate for use as an antimicrobial agent in agriculture. Nanochitosan is used to prevent and mitigate bacterial wilt caused by Ralstonia solanacearum. The prophylactic application of nanochitosan solutions has been demonstrated to effectively reduce the incidence and intensity of bacterial wilt disease among potato and tomato plants. The application of a 200 μg mL−1 nanochitosan solution resulted in a significant increase in the health rate and health grade of potato plants, from 15.38% and 20.87% to 78.93% and 71.85%, respectively. Furthermore, therapeutic spraying demonstrated a degree of efficacy, although it was less pronounced than that observed with preventive spraying [59].
Leaf blight and leaf spot are two bacterial diseases that severely affect rice. Nanochitosan solutions have been demonstrated to exhibit robust antibacterial activity against both of these rice pathogens. Specifically, the application of this type of solution results in a significant reduction in both the incidence and severity of these diseases in rice. Moreover, the activities of phenylalanine ammonia-lyase, peroxidase, and polyphenol oxidase in rice seedlings are significantly increased in the chitosan solution group [60]. The direct antibacterial activity of nanochitosan and its ability to indirectly induce resistance are the primary mechanisms underlying its protective effects against bacterial pathogens in rice. Moreover, the growth of Acidovorax citrulli, the bacterium responsible for bacterial fruit blotch in watermelon, was significantly inhibited. Nanochitosan has also been demonstrated to be an effective agent for combating the common plant pathogen Xanthomonas. A chitosan solution with a concentration of 100 μg mL−1 has been demonstrated to exhibit inhibitory effects on Xanthomonas pathogens from diverse origins. The antibacterial activity of chitosan solutions was enhanced by the addition of sodium chloride, irrespective of the nutrient type or sterilization method. Moreover, the chitosan solution demonstrated robust antibacterial activity against the specific strain R22580 of Xanthomonas axonopodis pv. poinsettiicola within a pH range of 5.5–7.0. This indicates that chitosan has the potential to be utilized as an antimicrobial agent to independently inhibit plant-pathogenic Xanthomonas [61].
Postharvest decay of agricultural products during transportation and storage causes huge economic losses. Considering the problem of pesticide residues, safer alternatives are needed to control postharvest decay. Nanochitosan has gained considerable acceptance as a dietary supplement and has been approved by the FDA [13]. Nanochitosan is employed as a coating additive for fruits, seeds, and vegetables in edible antibacterial films (Figure 5). The antibacterial bioresorbable materials, composed of poly (lactic acid) and chitosan (4% concentration), have been demonstrated to achieve a sterilization rate of 99% when applied to the BTS [62]. A variety of techniques, including direct casting, coating, extrusion, layer-by-layer assembly, and dipping, can be employed to fabricate nanochitosan-based edible films [63]. It is anticipated that nanochitosan will be utilized in the prevention of postharvest diseases affecting various fruits and vegetable, including cucumbers [64], mushrooms [65], fish, tomatoes, mangos, bananas, and pomegranates [66]. Films based on nanochitosan can be further incorporated with functional substances such as antioxidants, plant extracts, essential oils, and other additives to enhance barrier properties and functionality. Nanochitosan-based edible nanomodifiers are used for functionalizing starch/guar gum biocomposites with superior packaging properties, targeting stringent edible food packaging on fresh cuts. The storage quality in terms of microbial growth, pH change, color attributes, and weight loss is better preserved when it is used as an edible coating on cut apple fruits [67].
Figure 5.
The mechanism of antimicrobial nanochitosan films.
Nanochitosan is frequently combined with other functional materials, including biopolymer films, protein-based films, polysaccharide-based films, inorganic material films, synthetic polymer films, extract-based films, and nanochitosan-derivative-based films. The combined utilization of nanochitosan nanoparticles and Zataria multiflora essential oil (ZEO) has been demonstrated to effectively prolong the shelf life of cucumbers. This effect is presumed to be due to the release of bioactive compounds from the nanochitosan. Moreover, the application of a ZEO–nanochitosan coating has been found to enhance the hardness, respiration rate, and DPPH radical scavenging activity of cucumbers, while simultaneously reducing their power. These findings indicate that ZEO–nanochitosan enhances the antioxidant activity of fruits and inhibits microbial growth during storage [64]. One of the most commonly used edible packaging materials is pectin–chitosan film. The effect of the ratio of pectin to nanochitosan on the properties of the film has also been explored. The ratio of pectin to nanochitosan exerts a notable influence on the thickness, mechanical characteristics, water vapor transmission rate, solubility in water, and oxygen permeability of the material. Our findings indicate that when pectin and nanochitosan are combined in a 1:1 ratio, the tensile strength is as high as 8.96 MPa, the solubility in water diminishes to 37.5%, the oxygen permeability is 47.67 cc·mm/m2·day, and the water vapor transmission rate diminishes to 0.2052 g·mm/m2·day·kPa. Moreover, the pectin–chitosan film displays hydrophobic characteristics and exhibits inhibitory effects on the growth of Coccidioides, Escherichia coli, Aspergillus niger, and Saccharomyces cerevisiae [68]. Due to its antimicrobial activity and ability to elicit defense responses, nanochitosan has emerged as a promising treatment option following harvest.
5. The Delivery Properties of Nanochitosan
Nanochitosan has many advantages not only in terms of its source, plant growth promotion, and antimicrobial properties but also in terms of delivery of agrochemicals. The nanodelivery systems can provide the targeted and controlled release of agrochemicals and improve the efficiency and intelligence of agrochemical technologies. A variety of chemicals can be encapsulated in nanochitosan, including plant growth regulators, soil nutrients, and pesticides. The delivery systems based on nanochitosan can avoid the overuse of agrochemicals by controlling the release of active ingredients. These delivery systems may also alleviate the challenge of over-pesticide use [69].
5.1. The Delivery Mechanism of Nanochitosan
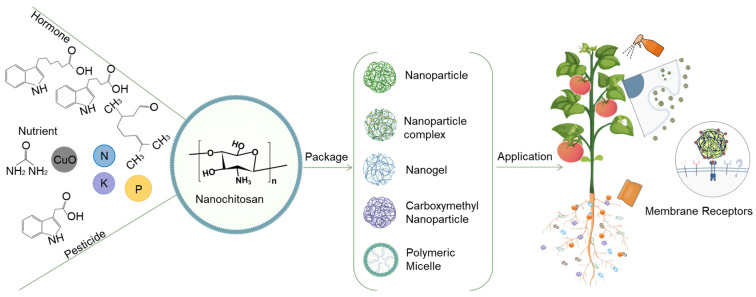
Nanochitosan can function well at the molecular and cellular levels due to its small size, tunable surface chemistry, and high efficiency. Nanochitosan also has analogous structural characteristics to glucosamine, giving it significant advantages as a drug carrier. The accessibility of functional groups in nanochitosan facilitates the formation of nanochitosan with other polymers and metal ions. Nanochitosan exhibits excellent adsorption, film-forming, permeability, fiber-forming, moisture-absorption, and moisture-retention properties (Figure 6). The active ingredients can be encapsulated or embedded in the matrix of nanochitosan polymers by ionic or covalent intermolecular/internal bonds to form an effective nanodelivery system formulation [70].
Figure 6.
The delivery of the nanochitosan and its derivatives.
Nanochitosan, as a sustained-release agent, can control the release of the active ingredient, maintain the concentration within the effective concentration range, and prolong the duration of effective action. Agrochemicals encapsulated in nanochitosan matrices can be triggered by a multitude of biotic and abiotic stress factors, including insect pests, plant pathogens, weeds, pH, drought, flooding, salinity, and temperature, among others. Environmental factors or enzymatic reactions may lead to the degradation or rupture of the capsule matrix. Specifically, these stresses affect the regulation of agrochemical release by modulating pore diffusion, capsule swelling, degradation, and surface desorption. The controlled release of agrochemicals based on stimulus response in nanoformulations allows for the effective and efficient delivery of these chemicals to the target site [71]. Nanochitosan enhances the circulation of agrochemicals and increases their retention time in plant tissue, resulting in prolonged half-lives (t1/2). This control of the release of active ingredients increases their bioavailability [15]. Nevertheless, nanochitosan remains a promising candidate for large-scale agricultural applications, as there are still some challenges to overcome regarding its use as a drug delivery vehicle, such as low solubility, slow drug release, and poor targeting stability.
5.2. The Application of Nanochitosan for Delivery
Nanochitosan and its derivatives are frequently employed in the field of delivery due to their advantageous properties, including biodegradability, biocompatibility, antibacterial properties, and adhesion. Nanochitosan has been demonstrated to be an effective delivery system for hormones, trace elements, and pesticides, and it has been shown to effectively inhibit diseases and promote growth.
Nanochitosan is employed as a hormone delivery carrier to stimulate plant growth. Anderson Espirito Santo Pereira et al. present an alginate–chitosan and chitosan–tripolyphosphate nanoparticle system that effectively encapsulates the plant growth regulator gibberellic acid. This nanoformulation increases leaf area, chlorophyll, and carotenoid levels, demonstrating improved biological activity compared to the free hormone [72]. Serdar Korpayev et al. reported a non-toxic, organic, solvent-free, and stable nanocarrier system for auxin consisting of chitosan and silver nanoparticles. Nanochitosan loaded with the auxins indole-3-acetic acid (IAA) and indole-3-butyric acid (IBA) was successfully synthesized. This approach is more effective and promising than using free IAA or IBA in conjunction with IBA–nanochitosan or IAA–nanochitosan [73].
Deshpande et al. evaluated the suitability of zinc-complexed nanochitosan as a potential “nanocarrier” for foliar fertilization. Zn–nanochitosan was synthesized using tripolyphosphoric acid as a cross-linking agent. In their study, plants were cultivated in a zinc-deficient sand medium. Upon application of Zn-nanochitosan to the leaves of the plants following flowering, a significant increase in zinc content was observed in durum wheat varieties, with an increase of 27% and 42% observed after five weeks [74]. Nanofertilizers are produced by encapsulating nitrogen (N), phosphorus (P), and potassium (K) within nanochitosan. These nanofertilizers have been demonstrated to exhibit remarkable efficacy in enhancing the nutrient absorption, photosynthesis, and overall growth of coffee plants. In comparison to the untreated control plots, the application of these nanofertilizers resulted in a significant increase in nitrogen content by 17.04%, phosphorus content by 16.31%, and potassium content by 67.50% in the treated plots. Moreover, there was a discernible enhancement in the number of leaves, plant height, and leaf area of the coffee seedlings [75]. Kondal et al. reported the effects of biodegradable nanopolymer urea formulations on soil enzyme activity and microbial communities involved in the potato nitrogen cycle. In comparison to the conventional urea treatment, the nanochitosan–urea composite treatment demonstrated a notable increase in the soil’s available potassium, organic carbon content, and dehydrogenase activity. Biodegradable polymer–urea composites exert a significant influence on the microbiota associated with soil nitrogen dynamics [3].
Nanochitosan with copper oxide on three isolates of Fusarium oxysporum infecting cultivated tomato plants was evaluated. The results of the study demonstrated that nanochitosan with copper oxide is capable of controlling fungal spores. Furthermore, the tissue morphology of the treated plants demonstrated no evidence of phytotoxicity [76]. Samar S. Ibrahim et al. employed an ion gel approach to develop a nanocapsule delivery system integrating nanochitosan and nanocellulose. The objective of this system was to precisely control the release of citronella essential oil (CEO) to mitigate the cotton leafworm Spodoptera frugiperda. Insecticidal activity tests indicated that all nanoformulations interfered with the normal development of S. littoralis. The combination of CEO–nanochitosan exhibited encouraging results, significantly extending the larval and pupal stages compared to the untreated control. Furthermore, notable decreases in pupal weight, adult lifespan, and female fecundity were observed, particularly after exposure to CEO–nanochitosan treatments [77].
6. The Challenge of Nanochitosan in Eco-Agriculture
Despite the promising results observed in preliminary nanochitosan studies, the combination of different forms of nanochitosan and their derivatives for the precise protection of the entire plant life cycle, from seed to fruit, for different problems is still a major challenge.
In terms of environmental concerns, there are big research gaps and limitations in nanochitosan applications. Although a large number of articles mention the environmental advantages of nanochitosan, there is a lack of longevity studies and evaluation of the environmental impacts of its long-term, large-scale application, especially its degradation properties and potential ecological risks when it is present in the soil for a long period of time. These are the key points to be studied in the future. The mechanisms by which nanochitosan exerts its effects on plants remain largely unknown, limiting the full range of applications of nanochitosan in plants. It is crucial to reveal and optimize the specific effects of nanochitosan in agrochemical delivery to plants, utilizing advanced molecular biology tools such as transcriptome and proteome profiling.
In terms of concerns about standards and regulations in applications, nanochitosan is approved by the FDA (Food and Drug Administration) for biomedical and food applications. In addition, the quality control of nanochitosan and the cost of production are the main challenges limiting its wider adoption in agriculture. Nanochitosan lacks the ability to respond to environmental changes and stress, as it is susceptible to degradation by factors such as light, heat, and moisture. Advanced synthetic biology, advanced materials, and self-assembly nanotechnology to incorporate nanochitosan formulations will facilitate effective and precise sensing, response, and release capabilities, thereby improving the quality of agricultural products and minimizing the costs. Furthermore, based on a large amount of research, AI technology can explore a more comprehensive understanding of the growth-regulating effects, optimal costs, and stability of nanochitosan at different stages of plant growth [78].
7. Conclusions
Nanochitosan and its derivatives provide a sustainable alternative to traditional agrochemicals as a combination of biomass chitosan materials and nanotechnology. The reuse of nanochitosan as a waste product of the food industry has important implications for promoting zero waste of resources. This review discussed the sources, applications, mechanisms, and prospects of nanochitosan in agroecology and clarified the necessity for the advancement of agroecology. In this review, we not only focused on advantages in terms of sources, but also gave attention to the attractive potential of nanochitosan for the promotion of plant growth, pest/disease resistance, and drug delivery. We also summarized the mechanism of action and application scenarios of nanochitosan throughout the entire crop cycle, including its effects on crop yield, pesticide activity, and the reduction in agrochemical accumulation. Furthermore, the sources of nanochitosan and its effects on plant growth, antimicrobial properties, and delivery capacity were classified and discussed in detail. Finally, we discussed the challenges and provided an outlook for future nanochitosan research in agroecology, as well as the focus and trends.
Author Contributions
X.W. (Xia Wang): conceptualization, writing—original draft preparation. M.H.: visualization, writing—original draft preparation. X.W. (Xueli Wang): visualization, writing—original draft preparation. S.L.: writing—reviewing and editing. L.L.: visualization. Q.Z.: writing—reviewing draft preparation. Y.W.: writing—reviewing draft preparation. Y.Z.: investigation. Z.Y.: investigation. G.S.: investigation. P.R.: writing—original draft preparation. H.O.: conceptualization, writing—original draft preparation. R.J.: conceptualization, writing—original draft preparation. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This work was supported by a National Natural Science Foundation of China grant (No. 42207050), the Sichuan Science and Technology Program (No. 2024NSFSC0860), and by the Sichuan Province Science and Technology Program (No. 2023NSFSC1979).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Pretty J. Intensification for redesigned and sustainable agricultural systems. Science. 2018;362:eaav0294. doi: 10.1126/science.aav0294. [DOI] [PubMed] [Google Scholar]
- 2.Statistics U. Developmental Science and Sustainable Development Goals for Children and Youth. Volume 439 Springer; Cham, Switzerland: 2019. Global indicator framework for the sustainable development goals and targets of the 2030 agenda for sustainable development. [Google Scholar]
- 3.Kondal R., Kalia A., Krejcar O., Kuca K., Sharma S.P., Luthra K., Dheri G.S., Vikal Y., Taggar M.S., Abd-Elsalam K.A., et al. Chitosan-Urea Nanocomposite for Improved Fertilizer Applications: The Effect on the Soil Enzymatic Activities and Microflora Dynamics in N Cycle of Potatoes (Solanum tuberosum L.) Polymers. 2021;13:2887. doi: 10.3390/polym13172887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jepson P.C., Murray K., Bach O., Bonilla M.A., Neumeister L. Selection of pesticides to reduce human and environmental health risks: A global guideline and minimum pesticides list. Lancet Planet. Health. 2020;4:e56–e63. doi: 10.1016/S2542-5196(19)30266-9. [DOI] [PubMed] [Google Scholar]
- 5.Wang X., Cui Y.X., Zhang X.C., Ju W.L., Duan C.J., Wang Y.Q., Fang L.C. A novel extracellular enzyme stoichiometry method to evaluate soil heavy metal contamination: Evidence derived from microbial metabolic limitation. Sci. Total Environ. 2020;738:139709. doi: 10.1016/j.scitotenv.2020.139709. [DOI] [PubMed] [Google Scholar]
- 6.Wang D., Saleh N.B., Byro A., Zepp R., Sahle-Demessie E., Luxton T.P., Ho K.T., Burgess R.M., Flury M., White J.C., et al. Nano-enabled pesticides for sustainable agriculture and global food security. Nat. Nanotechnol. 2022;17:347–360. doi: 10.1038/s41565-022-01082-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kashyap P.L., Xiang X., Heiden P. Chitosan nanoparticle based delivery systems for sustainable agriculture. Int. J. Biol. Macromol. 2015;77:36–51. doi: 10.1016/j.ijbiomac.2015.02.039. [DOI] [PubMed] [Google Scholar]
- 8.Yadav M., Goswami P., Paritosh K., Kumar M., Pareek N., Vivekanand V. Seafood waste: A source for preparation of commercially employable chitin/chitosan materials. Bioresour. Bioprocess. 2019;6:8. doi: 10.1186/s40643-019-0243-y. [DOI] [Google Scholar]
- 9.Terkula Iber B., Azman Kasan N., Torsabo D., Wese Omuwa J. A Review of Various Sources of Chitin and Chitosan in Nature. J. Renew. Mater. 2022;10:1097–1123. doi: 10.32604/jrm.2022.018142. [DOI] [Google Scholar]
- 10.Lee S., Hao L.T., Park J., Oh D.X., Hwang D.S. Nanochitin and Nanochitosan: Chitin Nanostructure Engineering with Multiscale Properties for Biomedical and Environmental Applications. Adv. Mater. 2023;35:2203325. doi: 10.1002/adma.202203325. [DOI] [PubMed] [Google Scholar]
- 11.Hamedi H., Moradi S., Hudson S.M., Tonelli A.E., King M.W. Chitosan based bioadhesives for biomedical applications: A review. Carbohydr. Polym. 2022;282:119100. doi: 10.1016/j.carbpol.2022.119100. [DOI] [PubMed] [Google Scholar]
- 12.Tian B., Liu J. Smart stimuli-responsive chitosan hydrogel for drug delivery: A review. Int. J. Biol. Macromol. 2023;235:123902. doi: 10.1016/j.ijbiomac.2023.123902. [DOI] [PubMed] [Google Scholar]
- 13.Ghosh T.M., Mondal K., Katiyar V. Food Product Optimization for Quality and Safety Control. Apple Academic Press; Williston, VT, USA: 2020. Current Prospects of Bio-Based Nanostructured Materials in Food Safety and Preservation. [Google Scholar]
- 14.El-Araby A., Janati W., Ullah R., Ercisli S., Errachidi F. Chitosan, chitosan derivatives, and chitosan-based nanocomposites: Eco-friendly materials for advanced applications (a review) Front. Chem. 2024;11:1327426. doi: 10.3389/fchem.2023.1327426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maluin F.N., Hussein M.Z., Yusof N.A., Fakurazi S., Maznah Z., Idris A.S., Hilmi N.H.Z., Daim L.D.J. Residual analysis of chitosan-based agronanofungicides as a sustainable alternative in oil palm disease management. Sci. Rep. 2020;10:22323. doi: 10.1038/s41598-020-79335-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abdollahzadeh M., Elhamirad A.H., Shariatifar N., Saeidiasl M., Armin M. Effects of nano-chitosan coatings incorporating with free/nano-encapsulated essential oil of Golpar (Heracleum persicum L.) on quality characteristics and safety of rainbow trout (Oncorhynchus mykiss) Int. J. Food Microbiol. 2023;385:109996. doi: 10.1016/j.ijfoodmicro.2022.109996. [DOI] [PubMed] [Google Scholar]
- 17.Fan Z., Wang L., Qin Y., Li P. Activity of chitin/chitosan/chitosan oligosaccharide against plant pathogenic nematodes and potential modes of application in agriculture: A review. Carbohydr. Polym. 2023;306:120592. doi: 10.1016/j.carbpol.2023.120592. [DOI] [PubMed] [Google Scholar]
- 18.Alsuhaibani A.M., Alayyafi A.A., Albedair L.A., El-Desouky M.G., El-Bindary A.A. Efficient fabrication of a composite sponge for Cr (VI) removal via citric acid cross-linking of metal-organic framework and chitosan: Adsorption isotherm, kinetic studies, and optimization using Box-Behnken design. Mater. Today Sustain. 2024;26:100732. doi: 10.1016/j.mtsust.2024.100732. [DOI] [Google Scholar]
- 19.Zhang W., Khan A., Ezati P., Priyadarshi R., Sani M.A., Rathod N.B., Goksen G., Rhim J.-W. Advances in sustainable food packaging applications of chitosan/polyvinyl alcohol blend films. Food Chem. 2024;443:138506. doi: 10.1016/j.foodchem.2024.138506. [DOI] [PubMed] [Google Scholar]
- 20.Beier S., Bertilsson S. Bacterial chitin degradation—Mechanisms and ecophysiological strategies. Front. Microbiol. 2013;4:149. doi: 10.3389/fmicb.2013.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mahari W.A.W., Waiho K., Azwar E., Fazhan H., Peng W., Ishak S.D., Tabatabaei M., Yek P.N.Y., Almomani F., Aghbashlo M. A state-of-the-art review on producing engineered biochar from shellfish waste and its application in aquaculture wastewater treatment. Chemosphere. 2022;288:132559. doi: 10.1016/j.chemosphere.2021.132559. [DOI] [PubMed] [Google Scholar]
- 22.Sieber V., Hofer M., Brück W.M., Garbe D., Brück T., Lynch C.A. Grand Challenges in Marine Biotechnology. Springer; Cham, Switzerland: 2018. ChiBio: An integrated bio-refinery for processing chitin-rich bio-waste to specialty chemicals; pp. 555–578. [Google Scholar]
- 23.Jin T., Liu T., Lam E., Moores A. Chitin and chitosan on the nanoscale. Nanoscale Horiz. 2021;6:505–542. doi: 10.1039/D0NH00696C. [DOI] [PubMed] [Google Scholar]
- 24.Divya K., Jisha M.S. Chitosan nanoparticles preparation and applications. Environ. Chem. Lett. 2017;16:101–112. doi: 10.1007/s10311-017-0670-y. [DOI] [Google Scholar]
- 25.Perera U.M.S.P., Rajapakse N. Seafood Processing By-Products. Springer; New York, NY, USA: 2014. Chitosan Nanoparticles: Preparation, Characterization, and Applications; pp. 371–387. [Google Scholar]
- 26.Venugopal V. Green processing of seafood waste biomass towards blue economy. Curr. Res. Environ. Sustain. 2022;4:100164. doi: 10.1016/j.crsust.2022.100164. [DOI] [Google Scholar]
- 27.Hernández-Téllez C.N., Plascencia-Jatomea M., Cortez-Rocha M.O. Chitosan in the Preservation of Agricultural Commodities. Elsevier; Amsterdam, The Netherlands: 2016. Chitosan-based bionanocomposites: Development and perspectives in food and agricultural applications; pp. 315–338. [Google Scholar]
- 28.Jogaiah S., Satapute P., Britto S.D., Konappa N., Udayashankar A.C. Exogenous priming of chitosan induces upregulation of phytohormones and resistance against cucumber powdery mildew disease is correlated with localized biosynthesis of defense enzymes. Int. J. Biol. Macromol. 2020;162:1825–1838. doi: 10.1016/j.ijbiomac.2020.08.124. [DOI] [PubMed] [Google Scholar]
- 29.Upasana A., Parul C., Anita S., Bharti K. Physiological response of maize plants and its rhizospheric microbiome under the influence of potential bioinoculants and nanochitosan. Plant Soil. 2022;474:451–468. doi: 10.1007/s11104-022-05351-2. [DOI] [Google Scholar]
- 30.Li Q., Dunn E.T., Grandmaison E.W., Goosen M.F.A. Applications and Properties of Chitosan. J. Bioact. Compat. Polym. 1992;7:370–397. doi: 10.1177/088391159200700406. [DOI] [Google Scholar]
- 31.Bilir G.S., Sarıahmet M., Ekinci D. Purification and Characterization of Glutathione Reductase Enzyme from Arum Maculatum Leaf. Black Sea J. Agric. 2023;6:269–274. doi: 10.47115/bsagriculture.1247272. [DOI] [Google Scholar]
- 32.Riseh R.S., Vazvani M.G., Kennedy J.F. The application of chitosan as a carrier for fertilizer: A review. Int. J. Biol. Macromol. 2023;252:126483. doi: 10.1016/j.ijbiomac.2023.126483. [DOI] [PubMed] [Google Scholar]
- 33.Ji H., Wang J., Chen F., Fan N., Wang X., Xiao Z., Wang Z. Meta-analysis of chitosan-mediated effects on plant defense against oxidative stress. Sci. Total Environ. 2022;851:158212. doi: 10.1016/j.scitotenv.2022.158212. [DOI] [PubMed] [Google Scholar]
- 34.Ingle P.U., Shende S.S., Shingote P.R., Mishra S.S., Sarda V., Wasule D.L., Rajput V.D., Minkina T., Rai M., Sushkova S. Chitosan nanoparticles (ChNPs): A versatile growth promoter in modern agricultural production. Heliyon. 2022;8:e11893. doi: 10.1016/j.heliyon.2022.e11893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fellet G., Pilotto L., Marchiol L., Braidot E. Tools for nano-enabled agriculture: Fertilizers based on calcium phosphate, silicon, and chitosan nanostructures. Agronomy. 2021;11:1239. doi: 10.3390/agronomy11061239. [DOI] [Google Scholar]
- 36.Samarah N., Wang H., Welbaum G. Pepper (Capsicum annuum) seed germination and vigour following nanochitin, chitosan or hydropriming treatments. Seed Sci. Technol. 2016;44:609–623. doi: 10.15258/sst.2016.44.3.18. [DOI] [Google Scholar]
- 37.Maluin F.N., Hussein M.Z. Chitosan-based agronanochemicals as a sustainable alternative in crop protection. Molecules. 2020;25:1611. doi: 10.3390/molecules25071611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou Y., Yang y., Qi Y., Zhang Z., Wang X., Hu X. Effects of Chitosan on Some Physiological Activity in Germinating Seed of Peanut. J. Peanut Sci. 2002;31:22–25. [Google Scholar]
- 39.Al Masruri M.H.K., Ullah A., Farooq M. Application of nano chitosan-glycinebetaine for improving bread wheat performance under combined drought and heat stresses. J. Soil Sci. Plant Nutr. 2023;23:3482–3499. doi: 10.1007/s42729-023-01265-9. [DOI] [Google Scholar]
- 40.Sathiyabama M., Indhumathi M. Chitosan thiamine nanoparticles intervene innate immunomodulation during Chickpea-Fusarium interaction. Int. J. Biol. Macromol. 2022;198:11–17. doi: 10.1016/j.ijbiomac.2021.12.105. [DOI] [PubMed] [Google Scholar]
- 41.Xiao Z., Ji H., Yue L., Chen F., Yan X.-P., Wang Z., Rasmann S. Nano-chitosan boosts sesame plant anti-herbivore defenses and seed nutritional metabolites. Environ. Sci. Nano. 2024;11:797–811. doi: 10.1039/D3EN00402C. [DOI] [Google Scholar]
- 42.El Amerany F., Meddich A., Wahbi S., Porzel A., Taourirte M., Rhazi M., Hause B. Foliar application of chitosan increases tomato growth and influences mycorrhization and expression of endochitinase-encoding genes. Int. J. Mol. Sci. 2020;21:535. doi: 10.3390/ijms21020535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hawrylak-Nowak B., Dresler S., Rubinowska K., Matraszek-Gawron R. Eliciting effect of foliar application of chitosan lactate on the phytochemical properties of Ocimum basilicum L. and Melissa officinalis L. Food Chem. 2021;342:128358. doi: 10.1016/j.foodchem.2020.128358. [DOI] [PubMed] [Google Scholar]
- 44.Chaudhary P., Khati P., Gangola S., Kumar A., Kumar R., Sharma A. Impact of nanochitosan and Bacillus spp. on health, productivity and defence response in Zea mays under field condition. 3 Biotech. 2021;11:237. doi: 10.1007/s13205-021-02790-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cheng Y., Wang Y., Han Y., Li D., Zhang Z., Zhu X., Tan J., Wang H. The stimulatory effects of nanochitin whisker on carbon and nitrogen metabolism and on the enhancement of grain yield and crude protein of winter wheat. Molecules. 2019;24:1752. doi: 10.3390/molecules24091752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mola Ali Abasiyan S., Dashbolaghi F., Mahdavinia G.R. Chitosan cross-linked with κ-carrageenan to remove cadmium from water and soil systems. Environ. Sci. Pollut. Res. 2019;26:26254–26264. doi: 10.1007/s11356-019-05488-1. [DOI] [PubMed] [Google Scholar]
- 47.Shirkhani Z., Chehregani Rad A., Mohsenzadeh F. Improving Cd-phytoremediation ability of Datura stramonium L. by Chitosan and Chitosan nanoparticles. Biologia. 2021;76:2161–2171. doi: 10.1007/s11756-021-00758-1. [DOI] [Google Scholar]
- 48.Allan C.R., Hadwiger L.A. The fungicidal effect of chitosan on fungi of varying cell wall composition. Exp. Mycol. 1979;3:285–287. doi: 10.1016/S0147-5975(79)80054-7. [DOI] [Google Scholar]
- 49.Xing K., Zhu X., Peng X., Qin S. Chitosan antimicrobial and eliciting properties for pest control in agriculture: A review. Agron. Sustain. Dev. 2015;35:569–588. doi: 10.1007/s13593-014-0252-3. [DOI] [Google Scholar]
- 50.Ke Y., Ding B., Zhang M., Dong T., Fu Y., Lv Q., Ding W., Wang X. Study on inhibitory activity and mechanism of chitosan oligosaccharides on Aspergillus flavus and Aspergillus fumigatus. Carbohydr. Polym. 2022;275:118673. doi: 10.1016/j.carbpol.2021.118673. [DOI] [PubMed] [Google Scholar]
- 51.Pelgrift R.Y., Friedman A.J. Nanotechnology as a therapeutic tool to combat microbial resistance. Adv. Drug Deliv. Rev. 2013;65:1803–1815. doi: 10.1016/j.addr.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 52.Mao S., Sun W., Kissel T. Chitosan-based formulations for delivery of DNA and siRNA. Adv. Drug Deliv. Rev. 2010;62:12–27. doi: 10.1016/j.addr.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 53.Narasimhamurthy K., Udayashankar A.C., De Britto S., Lavanya S.N., Abdelrahman M., Soumya K., Shetty H.S., Srinivas C., Jogaiah S. Chitosan and chitosan-derived nanoparticles modulate enhanced immune response in tomato against bacterial wilt disease. Int. J. Biol. Macromol. 2022;220:223–237. doi: 10.1016/j.ijbiomac.2022.08.054. [DOI] [PubMed] [Google Scholar]
- 54.Terry L.A., Joyce D.C. Elicitors of induced disease resistance in postharvest horticultural crops: A brief review. Postharvest Biol. Technol. 2004;32:1–13. doi: 10.1016/j.postharvbio.2003.09.016. [DOI] [Google Scholar]
- 55.Helmy Y.A., Taha-Abdelaziz K., Hawwas H.A.E.-H., Ghosh S., AlKafaas S.S., Moawad M.M., Saied E.M., Kassem I.I., Mawad A.M. Antimicrobial Resistance and Recent Alternatives to Antibiotics for the Control of Bacterial Pathogens with an Emphasis on Foodborne Pathogens. Antibiotics. 2023;12:274. doi: 10.3390/antibiotics12020274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chun S.-C.C., Chandrasekaran M. Chitosan and chitosan nanoparticles induced expression of pathogenesis-related proteins genes enhances biotic stress tolerance in tomato. Int. J. Biol. Macromol. 2019;125:948–954. doi: 10.1016/j.ijbiomac.2018.12.167. [DOI] [PubMed] [Google Scholar]
- 57.Roychoudhury A., Datta K., Tagore R. Role of Chitosan and Chitosan-Based Nanomaterials in Plant Sciences. Academic Press; Cambridge, MA, USA: 2022. Influence of chitosan and chitosan based nanoparticles against abiotic stress in plants; pp. 297–320. [Google Scholar]
- 58.Kumaraswamy R., Kumari S., Choudhary R.C., Pal A., Raliya R., Biswas P., Saharan V. Engineered chitosan based nanomaterials: Bioactivities, mechanisms and perspectives in plant protection and growth. Int. J. Biol. Macromol. 2018;113:494–506. doi: 10.1016/j.ijbiomac.2018.02.130. [DOI] [PubMed] [Google Scholar]
- 59.Khairy A.M., Tohamy M.R., Zayed M.A., Mahmoud S.F., El-Tahan A.M., El-Saadony M.T., Mesiha P.K. Eco-friendly application of nano-chitosan for controlling potato and tomato bacterial wilt. Saudi J. Biol. Sci. 2022;29:2199–2209. doi: 10.1016/j.sjbs.2021.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li B., Liu B., Shan C., Ibrahim M., Lou Y., Wang Y., Xie G., Li H.y., Sun G. Antibacterial activity of two chitosan solutions and their effect on rice bacterial leaf blight and leaf streak. Pest Manag. Sci. 2013;69:312–320. doi: 10.1002/ps.3399. [DOI] [PubMed] [Google Scholar]
- 61.Wang Y., Li L., Li B., Wu G., Tang Q., Ibrahim M., Li H., Xie G., Sun G. Action of chitosan against Xanthomonas pathogenic bacteria isolated from Euphorbia pulcherrima. Molecules. 2012;17:7028–7041. doi: 10.3390/molecules17067028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ouyang H., Li Z., Gu M., Hu Y., Xu L., Jiang D., Cheng S., Zou Y., Deng Y., Shi B. A bioresorbable dynamic pressure sensor for cardiovascular postoperative care. Adv. Mater. 2021;33:2102302. doi: 10.1002/adma.202102302. [DOI] [PubMed] [Google Scholar]
- 63.Nair M.S., Tomar M., Punia S., Kukula-Koch W., Kumar M. Enhancing the functionality of chitosan-and alginate-based active edible coatings/films for the preservation of fruits and vegetables: A review. Int. J. Biol. Macromol. 2020;164:304–320. doi: 10.1016/j.ijbiomac.2020.07.083. [DOI] [PubMed] [Google Scholar]
- 64.Mohammadi A., Hashemi M., Hosseini S.M. Postharvest treatment of nanochitosan-based coating loaded with Zataria multiflora essential oil improves antioxidant activity and extends shelf-life of cucumber. Innov. Food Sci. Emerg. Technol. 2016;33:580–588. doi: 10.1016/j.ifset.2015.10.015. [DOI] [Google Scholar]
- 65.Huang Q., Qian X., Jiang T., Zheng X. Effect of chitosan and guar gum based composite edible coating on quality of mushroom (Lentinus edodes) during postharvest storage. Sci. Hortic. 2019;253:382–389. doi: 10.1016/j.scienta.2019.04.062. [DOI] [Google Scholar]
- 66.Devlieghere F., Vermeulen A., Debevere J. Chitosan: Antimicrobial activity, interactions with food components and applicability as a coating on fruit and vegetables. Food Microbiol. 2004;21:703–714. doi: 10.1016/j.fm.2004.02.008. [DOI] [Google Scholar]
- 67.Ghosh T., Katiyar V. Nanochitosan functionalized hydrophobic starch/guar gum biocomposite for edible coating application with improved optical, thermal, mechanical, and surface property. Int. J. Biol. Macromol. 2022;211:116–127. doi: 10.1016/j.ijbiomac.2022.05.079. [DOI] [PubMed] [Google Scholar]
- 68.Ngo T.M.P., Nguyen T.H., Dang T.M.Q., Tran T.X., Rachtanapun P. Characteristics and antimicrobial properties of active edible films based on pectin and nanochitosan. Int. J. Mol. Sci. 2020;21:2224. doi: 10.3390/ijms21062224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sharma A., Sood K., Kaur J., Khatri M. Agrochemical loaded biocompatible chitosan nanoparticles for insect pest management. Biocatal. Agric. Biotechnol. 2019;18:101079. doi: 10.1016/j.bcab.2019.101079. [DOI] [Google Scholar]
- 70.Del Prado-Audelo M.L., Caballero-Florán I.H., Sharifi-Rad J., Mendoza-Muñoz N., González-Torres M., Urbán-Morlán Z., Florán B., Cortes H., Leyva-Gómez G. Chitosan-decorated nanoparticles for drug delivery. J. Drug Deliv. Sci. Technol. 2020;59:101896. doi: 10.1016/j.jddst.2020.101896. [DOI] [Google Scholar]
- 71.Muthukrishnan S., Murugan I., Selvaraj M. Chitosan nanoparticles loaded with thiamine stimulate growth and enhances protection against wilt disease in Chickpea. Carbohydr. Polym. 2019;212:169–177. doi: 10.1016/j.carbpol.2019.02.037. [DOI] [PubMed] [Google Scholar]
- 72.Pereira A.E.S., Silva P.M., Oliveira J.L., Oliveira H.C., Fraceto L.F. Chitosan nanoparticles as carrier systems for the plant growth hormone gibberellic acid. Colloids Surf. B Biointerfaces. 2016;150:141–152. doi: 10.1016/j.colsurfb.2016.11.027. [DOI] [PubMed] [Google Scholar]
- 73.Korpayev S., Karakeçili A., Dumanoğlu H., Ibrahim Ahmed Osman S. Chitosan and silver nanoparticles are attractive auxin carriers: A comparative study on the adventitious rooting of microcuttings in apple rootstocks. Biotechnol. J. 2021;16:2100046. doi: 10.1002/biot.202100046. [DOI] [PubMed] [Google Scholar]
- 74.Deshpande P., Dapkekar A., Oak M.D., Paknikar K.M., Rajwade J.M. Zinc complexed chitosan/TPP nanoparticles: A promising micronutrient nanocarrier suited for foliar application. Carbohydr. Polym. 2017;165:394–401. doi: 10.1016/j.carbpol.2017.02.061. [DOI] [PubMed] [Google Scholar]
- 75.Ha N.M.C., Nguyen T.H., Wang S.-L., Nguyen A.D. Preparation of NPK nanofertilizer based on chitosan nanoparticles and its effect on biophysical characteristics and growth of coffee in green house. Res. Chem. Intermed. 2019;45:51–63. doi: 10.1007/s11164-018-3630-7. [DOI] [Google Scholar]
- 76.Mosa M.A., El-Abeid S.E. Chitosan-Loaded Copper Oxide Nanoparticles: A Promising Antifungal Nanocomposite against Fusarium Wilt Disease of Tomato Plants. Sustainability. 2023;15:14295. doi: 10.3390/su151914295. [DOI] [Google Scholar]
- 77.Ibrahim S.S., Abou-Elseoud W.S., Elbehery H.H., Hassan M.L. Chitosan-cellulose nanoencapsulation systems for enhancing the insecticidal activity of citronella essential oil against the cotton leafworm Spodoptera littoralis. Ind. Crop. Prod. 2022;184:115089. doi: 10.1016/j.indcrop.2022.115089. [DOI] [Google Scholar]
- 78.Burke M., Driscoll A., Lobell D.B., Ermon S. Using satellite imagery to understand and promote sustainable development. Science. 2021;371:eabe8628. doi: 10.1126/science.abe8628. [DOI] [PubMed] [Google Scholar]