Abstract
Escherichia coli K1 is the most common gram-negative organism causing neonatal meningitis, but the mechanism by which E. coli K1 crosses the blood-brain barrier is incompletely understood. We have previously described the cloning and molecular characterization of a determinant, ibeA (also called ibe10), from the chromosome of an invasive cerebrospinal fluid isolate of E. coli K1 strain RS218 (O18:K1:H7). Here we report the identification of another chromosomal locus, ibeB, which allows RS218 to invade brain microvascular endothelial cells (BMEC). The noninvasive TnphoA mutant 7A-33 exhibited <1% the invasive ability of the parent strain in vitro in BMEC and was significantly less invasive in the central nervous system in the newborn rat model of hematogenous E. coli meningitis than the parent strain. The TnphoA insert with flanking sequences was cloned and sequenced. A 1,383-nucleotide open reading frame (ORF) encoding a 50-kDa protein was identified and termed ibeB. This ORF was found to be 97% identical to a gene encoding a 50-kDa hypothetical protein (p77211) and located in the 13-min region of the E. coli K-12 genome. However, no homology was observed between ibeB and other known invasion genes when DNA and protein databases in GenBank were searched. Like the TnphoA insertion mutant 7A-33, an isogenic ibeB deletion mutant (IB7D5) was unable to invade BMEC. A 7.0-kb locus containing ibeB was isolated from a LambdaGEM-12 genomic library of E. coli RS218 and subcloned into a pBluescript KS vector (pKS7-7B). pKS7-7B was capable of completely restoring the BMEC invasion of the noninvasive TnphoA mutant 7A-33 and the ibeB deletion mutant IB7D5 to the level of the parent strain. More importantly, the ibeB deletion mutant IB7D5 was fully complemented by pFN476 carrying the ibeB ORF (pFN7C), indicating that ibeB is required for E. coli K1 invasion of BMEC. Taken together, these findings indicate that several E. coli determinants, including ibeA and ibeB, contribute to crossing of the blood-brain barrier.
Bacterial meningitis has remained associated with high mortality and morbidity despite advances in antimicrobial chemotherapy and supportive care (16, 24). A major contributing factor is the incomplete understanding of the pathogenesis and pathophysiology of this disease. For example, most cases of bacterial meningitis develop as a result of hematogenous spread, but it is not completely understood how circulating bacteria cross the blood-brain barrier.
Escherichia coli is the most common gram-negative organism that causes meningitis during the neonatal period. Using E. coli as a paradigm, we have examined how circulating bacteria cross the blood-brain barrier. These studies have become feasible because of the availability of both in vitro and in vivo models of the blood-brain barrier (7, 22). For example, we have successfully isolated and cultivated brain microvascular endothelial cells (BMEC), which constitute the blood-brain barrier (22, 23). The in vivo model of the blood-brain barrier has been established by induction of hematogenous meningitis in infant rats (7, 10). In this experimental meningitis model, bacteria are injected via the subcutaneous or intracardiac route, resulting in bacteremia and subsequent entry of bacteria into the central nervous system (CNS). Since the blood-brain barrier separates the brain and cerebrospinal fluid (CSF) from the intravascular compartment, the entry of bacteria should occur at sites of the blood-brain barrier. The development of techniques for atraumatic collection of blood and CSF specimens has enabled us to use this in vivo model to examine the pathogenic mechanisms involved in crossing of the blood-brain barrier by circulating E. coli (10).
To facilitate the identification of the genes contributing to E. coli invasion of BMEC, we have used transposon TnphoA and generated a collection of noninvasive E. coli mutants (7). TnphoA is a modified transposon engineered by insertion of the phoA gene into one end of Tn5 (12). The gene fusion can be randomly generated by TnphoA insertion into the target gene in the chromosome or plasmid. The TnphoA approach has led to the discovery of critical E. coli determinants involved in the invasion of BMEC in vitro and in vivo. For example, we have previously identified the ibeA (ibe10) locus via TnphoA mutagenesis and screening for loss of invasiveness by use of the in vitro and in vivo models of the blood-brain barrier (7). In addition, we have shown that E. coli OmpA contributes to the invasion of BMEC (15). In the present study, we characterized the noninvasive mutant 7A-33, which was derived from a CSF isolate of E. coli K1 strain RS218 by TnphoA mutagenesis. This mutant was significantly less able than the parent strain to invade BMEC in vitro and to enter the CNS in the newborn rat model of hematogenous meningitis in vivo. Similar findings were obtained with an ibeB deletion mutant. The DNA fragments containing ibeB (invasion of brain endothelial cells) were shown to restore the ability of the noninvasive mutant 7A-33 and the ibeB deletion mutant to invade BMEC.
MATERIALS AND METHODS
Bacterial strains, plasmids, and media.
E. coli RS218 (O18:K1:H7) is a clinical isolate from the CSF of a newborn infant with meningitis (21), and E44 is a spontaneous rifampin-resistant mutant of RS218. DH5α is a host strain for subcloning and preparation of plasmids for DNA sequence determination. Strains containing plasmids were grown at 37°C in L broth (10 g of tryptone, 5 g of NaCl, and 5 g of yeast extract per liter) with ampicillin (50 μg/ml), kanamycin (50 μg/ml), tetracycline (20 μg/ml), or chloramphenicol (100 μg/ml) for positive selection of plasmids (Table 1). Bacteria were cultured in L broth and stored in L broth plus 20% glycerol at −70°C.
TABLE 1.
E. coli strains and plasmids used in this study
| E. coli strain or plasmid | Genotype or characteristic(s) | Reference or source |
|---|---|---|
| Strains | ||
| RS218 | O18:K1:H7 | 21 |
| E44 | Rifr derivative of RS218 | |
| DH5α | F−recA1 hsdR17 thi-1 gyrA96 supE44 endA1 relA1 recA1 deoR Δ(lacZYA-argF)U169 (φ80 lacZM15) | Gibco/BRL |
| SM10λpir | thi thr leu tonA lacY supE recA::RP4-2-Tc::Mu (λpir) pro endA hsdA hsdR supF | 3 |
| 7A-33 | E44 ibeB::TnphoA | This study |
| IB7D5 | ΔibeB derivative of E44 via allelic exchange | This study |
| HB101 | K-12 supE44 ara-14 galK12 rpsL20 (Strr) | 18 |
| Plasmids | ||
| pRT733 | oriR6K mobRP4 TnphoA | 7 |
| pCVD433 | pACYC184 modified by insertion of an MluI linker | 3 |
| pCVD442 | Ampr SacB oriR6K mobRP4 | 4 |
| pCD7D | pCVD442 carrying a 2.3-kb DNA fragment with an in-frame ibeB deletion | This study |
| pCRII | Ampr Kanr oriCol E1 oriF1 | Invitrogen |
| pCD7A | pCVD433 carrying the ibeB locus with a TnphoA insertion | This study |
| pCIB7B | ibeB (0.67 kb) pCRII | This study |
| pCR7C | pCRII carrying the ibeB gene (1.6 kb) | This study |
| pKS7-13K | pBluescript KS carrying a 13.0-kb ibeB locus (13K) | This study |
| pKS7-7B | pBluescript KS carrying a 7.0-kb ibeB locus (7B) | This study |
| pKS7-6N | pBluescript KS carrying a 6.1-kb DNA fragment with an ibeB deletion | This study |
| pKS7-5H | pBluescript KS carrying a 5.7-kb DNA fragment with ibeB deleted (5H) | This study |
| pFN476 | Ampr low copy, T7 promoter | 20 |
| pFN7C | pFN476 carrying the ibeB gene (1.6 kb) | This study |
| pGP1-2 | Kanr, low copy, T7 RNA polymerase gene | 20 |
Chemicals and enzymes.
Restriction endonucleases, T4 DNA ligase, and other enzymes were purchased from New England Biolabs (Beverly, Mass.) unless otherwise noted. Chemicals were purchased from Sigma (St. Louis, Mo.). All isotopes were obtained from New England Nuclear Corp. (Boston, Mass.). Reagents for preparation of DNA sequencing gels were ultrapure quality and were obtained from National Diagnostics (Atlanta, Ga.). Reagents for DNA sequencing reactions with Sequenase and other chemicals were purchased from U.S. Biochemical Corp. (Cleveland, Ohio). DNA sequencing kits with dye terminators were obtained from PE Applied Biosystems (Foster City, Calif.).
Isolation of the noninvasive TnphoA mutant 7A-33 of E. coli K1.
The noninvasive TnphoA mutant 7A-33 was generated as previously described (7). Briefly, strain SM10λpir containing the suicide vector plasmid pRT733 was used as a TnphoA donor, while E. coli K1 strain E44 was used as a recipient. SM10λpir carrying pRT733 was mated with E44 on Luria-Bertani (LB) agar by cross-streaking and then incubation at 37°C for 6 h. The conjugants were selected on LB agar containing kanamycin and rifampin (7). TnphoA mutants were screened for their ability to invade BMEC as described previously (7). Probing of the DNA blots with a 32P-labeled 0.6-kb Kanr gene fragment derived from Tn5 identified noninvasive mutants with a single TnphoA insertion (7).
Tissue cultures and invasion assays.
BMEC were prepared from bovine and human brains (22, 23), and invasion assays were performed as previously described (7). Briefly, brain specimens devoid of large blood vessels were homogenized in Dulbecco minimal essential medium (DMEM) containing 2% bovine calf serum (DMEM-S) and centrifuged in 25% bovine serum albumin for bovine BMEC or in 15% dextran in DMEM-S for human BMEC. The pellets containing crude microvessels were further digested in a solution containing collagenase or dispase (1 mg/ml). Microvascular capillaries were isolated by adsorption to a column of glass beads and were recovered in growth medium (22). The resulting bovine and human BMEC were positive for factor VIII, carbonic anhydrase IV, gamma-glutamyl transpeptidase, and the ability to take up low-density lipoproteins, demonstrating their brain endothelial cell characteristics (22, 23).
Invasion assays were done with approximately 107 bacteria added to confluent monolayers of BMEC at a multiplicity of infection of 50 to 100. The monolayers were incubated for 1.5 h at 37°C to allow invasion to occur. The number of intracellular bacteria was determined after the extracellular bacteria were eliminated by incubation of the monolayers with experimental medium containing gentamicin (100 μg/ml) (7). Results were expressed either as percent invasion [100 × (number of intracellular bacteria recovered/number of bacteria inoculated)] or as relative invasion (invasion as a percentage of the invasion of the parent strain E. coli K1).
Neonatal rat model of hematogenous E. coli K1 meningitis.
The noninvasive mutant with a single TnphoA insertion (7A-33) was examined for its ability to enter the CNS in our neonatal rat model of hematogenous E. coli meningitis as described previously (7, 10). Briefly, at 5 days of age, all members of each litter were randomly divided into two groups to receive subcutaneously 1.4 × 104 CFU of the parent strain E44 or 4.4 × 104 CFU of the mutant strain 7A-33. Our pilot experiments revealed that these bacterial inocula for strains E44 and 7A-33 produced nonlethal bacteremia of 105 to 108 CFU/ml of blood in >90% of animals within 18 h of inoculation. At 18 h after bacterial inoculation, blood and CSF specimens were obtained for quantitative cultures as described previously (10). Blood and CSF specimens obtained from animals infected with mutant 7A-33 were cultured in brain heart infusion broth and agar containing kanamycin (40 μg/ml).
Cloning of TnphoA fragments.
MluI-digested genomic DNA from the noninvasive mutant 7A-33 was cloned into the MluI site of pCVD433, which was derived from pACYC184 by insertion of MluI linkers into the EcoRV site (3). Transformation was performed by electroporation of E. coli DH5α in 10% glycerol with 0.1-cm cuvettes and an E. coli gene pulser (Bio-Rad Laboratories, Richmond, Calif.) set at 1.8 kV, 200 Ω, and 25 μF as previously described (7). The kanamycin-resistant transformants were identified as MluI fragments containing TnphoA. The construct carrying a 13-kb MluI fragment of the noninvasive mutant 7A-33 in pCVD433 was designated pCD7A (Table 1).
PCR cloning and analysis of ibeB.
The PCR was performed as described previously (8, 11). Briefly, 0.5 μg of genomic DNA was added to a mixture containing 1× Taq polymerase buffer (Cetus), 1.5 mM MgCl2, 0.5 mM deoxynucleoside triphosphates (Cetus), 50 pmol of each primer, and Taq DNA polymerase in a final volume of 50 μl. Amplification was carried out with a PTC-100 programmed thermal controller (M. J. Research) for 40 cycles: denaturation for 1 min at 94°C, primer annealing for 1 min at 55°C, and primer extension for 3 min at 70°C. The two pairs of primers (primers IB7-3a and IB7-5a and primers IB-5HB and IB7-5a) (Table 2) used for the PCR were synthesized with an Applied Biosystems (Foster City, Calif.) 380B DNA synthesizer. Two DNA fragments, a 0.67-kb fragment carrying the partial ibeB coding sequence and a 1.6-kb fragment containing the complete ibeB open reading frame (ORF), were amplified from genomic DNA of wild-type strain RS218 and subcloned into TA cloning vector pCRII (Invitrogen). The resulting constructs were designated pCIB7B and pCR7C, respectively (Table 1).
TABLE 2.
Oligonucleotides used for cloning and sequencing
| Gene or transposon | Primer | Strand | Sequence | Use(s) |
|---|---|---|---|---|
| TnphoA | Tnp5 | − | 5′-TCGCTAAGAGAATCACGCAGAG-3′ | Sequencing |
| Tn5 | Tnp3 | + | 5′-GCACGATGAAGAGCAGAAG-3′ | Sequencing |
| ibeB | IB-5HB | + | 5′-AGGATCCGAGCCTATGTCTCCTTG-3′ | Cloning, sequencing |
| ibeB | IB7-5a | − | 5′-GTAAAGCGCATGGTCATC-3′ | Cloning, sequencing |
| ibeB | IB7-5b | + | 5′-TTTAATGCCCAGGCTAACG-3′ | Cloning, sequencing |
| ibeB | IB7-3a | + | 5′-GCCAGCAGCGATCTGTCGTC-3′ | Cloning, sequencing |
| ibeB | IB7-31 | − | 5′-CGGCGATATCCAGATTG-3′ | Sequencing |
| ibeB | IB7-32 | − | 5′-TTAGCCGCCATTAACGCGTG-3′ | Sequencing |
| ibeB | IB7-33 | − | 5′-TTACCCAGCGTTTCTTCG-3′ | Sequencing |
| ibeB | IB7-3c | + | 5′-ATCAGCAACTGGCGTATG-3′ | Sequencing |
| ibeB | IB7-34 | − | 5′-TTAACCAGGCCGTTCTGG-3′ | Sequencing |
DNA sequencing and analysis.
The nucleotide sequence of ibeB was determined by the dideoxy chain termination method of Sanger et al. (19) with a Sequenase version 2.0 kit from U.S. Biochemical Corp. and [35S]dATP (1,000 to 1,500 Ci/mmol) from Du Pont, NEN Research Products (Boston, Mass.). To sequence the portion of ibeB proximal to the TnphoA insertion site, the initial DNA sequence was obtained from plasmid pCD7A with the 5′ primer Tnp5 and the 3′ primer Tnp3 (Table 2). The two primers are complementary to the two ends of TnphoA. The remaining DNA sequence of ibeB was determined with primers complementary to the ibeB sequence in pCD7A, pFN7C, and pKS7-7B (Table 1). Both strands of the DNA were resequenced by the automated approach with fluorescence-labeled nucleotides (Applied Biosystems 373A automated sequencer) to ensure accuracy, and the sequence data were analyzed with the DNA analysis program developed by the Genetics Computer Group of the University of Wisconsin. DNA and deduced protein sequences were used to search the DNA and protein databases at the National Center for Biotechnology Information (National Library of Medicine, Washington, D.C.) by use of the BLAST algorithm.
Construction and screening of a genomic library of E. coli RS218.
High-molecular-weight chromosomal DNA was purified from E. coli K1 strain RS218 as previously described (7). Genomic DNA was partially digested with Sau3AI (New England Biolabs), which is compatible with BamHI. Partially digested genomic DNA (15 to 23 kb) was partially filled in with dGTP and dATP. This DNA was ligated into LambdaGEM-12 arms with an XhoI half site. Ligation and packaging of recombinant lambda phage were performed according to the manufacturer’s instructions (Promega). The E. coli genomic library was screened by DNA hybridization (7) to identify phage clones that contained ibeB. A 0.67-kb ibeB DNA fragment in pCIB7B was released with EcoRI, purified by preparative agarose electrophoresis and by use of Geneclean (Bio 101), labeled with [α-32P]dCTP by use of an Oligolabeling kit (Pharmacia), and used as a probe for screening (>1 × 108 cpm/μg). The phage plaques were replicated onto nylon filters, UV linked, and hybridized as described previously (7, 9). Plaques hybridizing to the probe were identified by autoradiography and then purified.
Complementation analysis.
A 7.0-kb BamHI-EcoRI subclone of pKS7-13K containing the ibeB ORF (7B) and a 5.7-kb BamHI-HpaI subclone lacking this sequence (5H) were subcloned into the pBluescriptII KS vector (see Fig. 2). The resulting constructs were designated pKS7-7B and pKS7-5H, respectively. A 1.6-kb XbaI-HindIII subclone of pCR7C carrying the complete ibeB ORF was subcloned into pFN476. The resulting construct was designated pFN7C. The ligation mixture was used to transform DH5α, and selection was done on LB plates containing ampicillin, isopropyl-β-d-thiogalactopyranoside (IPTG), and 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal). The white colonies were picked for identification of plasmids containing the insert. Cells of the noninvasive E. coli K1 mutants 7A-33 and IB7D5 were made competent in 10% glycerol as described previously (7). 7A-33 was transformed with the pBluescriptII KS vector and the recombinant plasmids pKS7-7B and pKS7-5H (Table 1). IB7D5 was transformed with pFN7C and pGP1-2. The expression of ibeB in pFN476 is driven by the T7 promoter, and pGP1-2 is a vector carrying the T7 RNA polymerase gene (20). The transformants were tested for their ability to invade BMEC.
FIG. 2.
Restriction map of the DNA fragments containing the ibeB invasion gene cloned from E. coli K1 strain RS218. Relevant restriction sites for BamHI (B), HindIII (H), MluI (M), NsiI (Ns), PstI (P), SacI (Sc), and SmaI (Sm) are shown. The horizontal arrow indicates the transcription direction for the ibeB ORF (box).
Nucleotide sequence accession number.
The nucleotide sequence of ibeB has been deposited in the GenBank nucleotide sequence data library under accession no. AF94824.
RESULTS
Noninvasive phenotype of the TnphoA insertion mutant 7A-33.
We previously showed that the mutant 7A-33, with a single TnphoA insertion, retained the same phenotypic and genotypic characteristics as the parent strain RS218 or E44 (7). When mutant 7A-33 was examined for its ability to invade bovine and human BMEC, compared to that of the parent strain, its invasion capacity was <0.001%, while the parent strain exhibited an invasion frequency of 0.1%.
Prevalence of meningitis in infant rats.
We next examined whether the differences in BMEC invasiveness would be biologically relevant in our well-established infant rat model of experimental hematogenous meningitis. Table 3 summarizes the prevalence of meningitis (defined as positive CSF cultures) in 5-day-old rats infected with the parent strain E44 or its noninvasive mutant with a single TnphoA insertion, 7A-33. As expected, subcutaneous injections of 1.4 × 104 CFU of strain E44 or 4.4 × 104 CFU of mutant 7A-33 resulted in bacteremia of 105 to 108 CFU/ml of blood in 100% of the animals. This level of bacteremia has been shown to be sufficient to allow circulating E. coli to enter the CNS (10). As shown in Table 3, the magnitudes of bacteremia were similar between the two groups. However, the occurrence of meningitis was significantly lower (P, 0.003) in animals receiving mutant strain 7A-33 (4 of 25, or 16%) than in those receiving parent strain E44 (15 of 27, or 56%). These findings support the concept that the TnphoA insertion mutant 7A-33 is truly less invasive both in vitro and in vivo, suggesting that the DNA flanking the transposon insertion in mutant 7A-33 may contain a gene(s) necessary if not sufficient for invasion of BMEC. It is also important to recognize that the BMEC invasion frequency of 0.1% is related to enhanced invasion of the CNS in vivo.
TABLE 3.
Development of bacteremia and meningitis (defined as positive CSF cultures) in two groups of newborn rats receiving E. coli E44 or 7A-33
| E. coli strain | No. of animals | Bacteremia (log CFU/ml of blood [mean ± SD]) | No. (%) of animals with positive CSF |
|---|---|---|---|
| 7A-33 | 25 | 7.01 ± 1.17 | 4 (16)a |
| E44 | 27 | 7.06 ± 1.29 | 15 (56) |
The P value was 0.003 (Fisher’s exact test) for animals receiving 7A-33 compared to animals receiving E44.
Sequence analysis of ibeB.
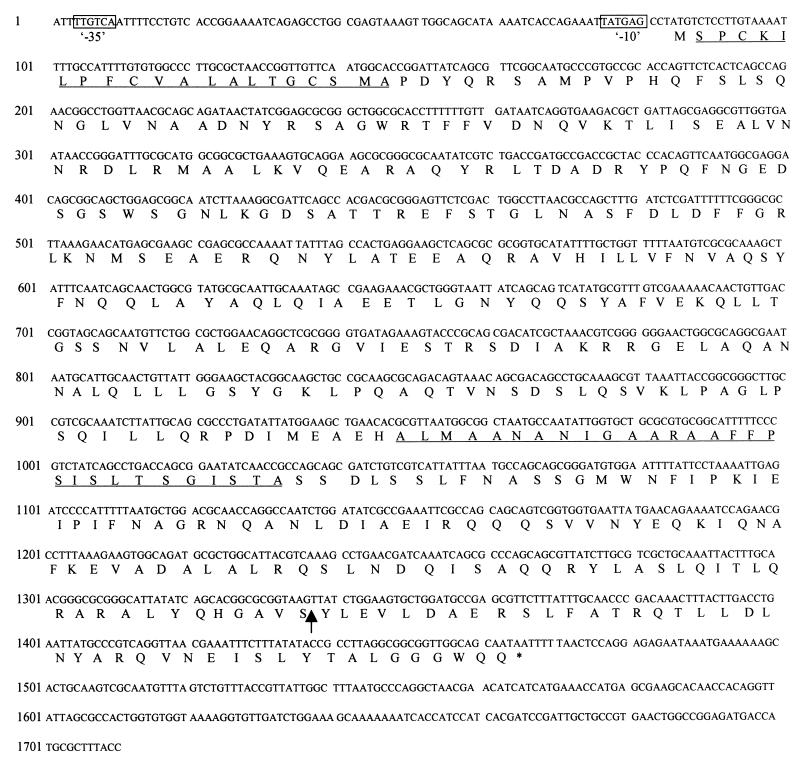
In order to identify the ibeB gene, we sequenced the corresponding region of DNA flanking TnphoA. As shown in Fig. 1, a 1,383-nucleotide open reading frame (ORF) assigned to the ibeB gene coded for a protein with 460 amino acids and a calculated molecular mass of 50 kDa (Fig. 1). This ORF was found to be 97% identical to a gene encoding a 50-kDa hypothetical protein (p77211) and located in the 13-min region of the E. coli K-12 genome (1). However, no homology was observed between ibeB and other known invasion genes when DNA and protein databases in GenBank were searched. Potential −10 (TATGAG) and −35 (TTGTCA) promoter regions were found at the 5′ noncoding region of ibeB. The TnphoA insertion site was identified by sequencing the fusion joint with the 5′ primer Tnp5 and the 3′ primer Tnp3, which are complementary to the two ends of TnphoA. The insertion occurred in the codon corresponding to residue 419.
FIG. 1.
Complete nucleotide sequence and deduced amino acid sequence for the ibeB gene from an E. coli K1 strain. The calculated molecular mass of the full-length protein is 50 kDa. Boxes indicate potential −10 and −35 promoter sites. The arrow indicates a TnphoA insertion site in ibeB. Two putative transmembrane domains are underlined.
A restriction map and the genetic organization of the invasion gene ibeB in the chromosome of E. coli RS218 are shown in Fig. 2. The sequence of the entire ibeB ORF and its upstream and downstream regions is presented in Fig. 1.
Construction of an E. coli RS218 LambdaGEM-12 library and isolation and subcloning of invasion determinants.
E. coli K1 strain RS218 was used as the source of DNA for cloning experiments. This virulent strain is capable of invading human and bovine BMEC and inducing meningitis in newborn rats (7). To clone the invasion determinants from RS218, a genomic library was constructed in LambdaGEM-12. By use of XhoI half sites in the vector and Sau3AI in the genomic inserts for library construction, self-ligation of vector and genomic sequences was eliminated, since only recombinant phages containing a single insert of the appropriate size (9 to 23 kb) were capable of being packaged. Using ibeB (0.67 kb) as a probe, approximately 5 × 105 recombinant phages were screened and seven phage clones for ibeB were identified. The recombinant phage DNAs were purified and digested with NotI. The sizes of the inserts were between 12 and 16 kb. A 13-kb insert containing ibeB was subcloned into the NotI site of pBluescriptII KS (pKS7-13K).
Construction of an isogenic in-frame deletion mutant.
In order to determine whether or not the noninvasive phenotype of the mutant 7A-33 was due to a polar effect of the TnphoA insertion, an ibeB in-frame deletion mutant was generated by integration of the suicide plasmid pCD7D (Table 1). pKS7-6N was constructed by removing the 0.87-kb SmaI-NsiI N-terminal fragment of ibeB from pKS7-7B and religating the plasmid containing part of ibeB with a 30-bp EcoRV-NsiI fragment from plasmid pZerO-2.1 (Invitrogen). A 2.3-kb BglII-EcoRI fragment carrying mutated ibeB from pKS7-6N was converted into blunt ends through a filling-in reaction (18) and subcloned into pCVD442 (4) with SmaI. The resulting construct (pCD7D) was confirmed by PCR and DNA sequencing as having the ibeB in-frame deletion.
The mutants were obtained by mating E44 with SM10λpir carrying pCD7D and selection on LB agar containing ampicillin and rifampin. A single such colony was picked and grown to the late logarithmic phase in LB broth without selection. Dilutions were plated on LB agar plates containing no NaCl and 5% sucrose. Sucrose-resistant colonies were tested for the loss of ampicillin resistance, indicative of the loss of vector sequences.
PCR was used to confirm the deletion in the desired chromosomal ibeB gene in the deletion mutant IB7D5 with a 5′ primer (5′-ATTTCCTCCGCATGTTGC-3′) and a 3′ primer (IB7-31). Amplification was carried out with the following cycle profile: 40 cycles at 94°C for 1 min, 55°C for 1 min, and 70°C for 3 min. The PCR DNA samples were sequenced with primers IB7-31 and IB7-32 to confirm the ibeB in-frame deletion (Table 2).
Complementation of noninvasive mutants.
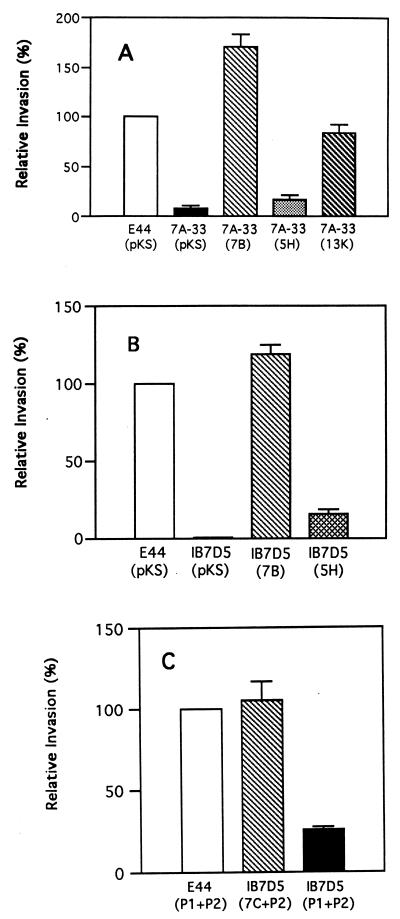
The mutagenesis experiments (TnphoA insertion and isogenic deletion of ibeB) indicated that ibeB was required for invasion of BMEC by strain E44. As shown in Fig. 3, The TnphoA insertion mutant (7A-33) and the ibeB deletion mutant (IB7D5) were significantly less invasive than E44 in BMEC. To demonstrate that the TnphoA insertion and the ibeB deletion were truly responsible for the noninvasive phenotype, we attempted to complement the noninvasive mutants with pKS7-13K, which contained a 13-kb DNA fragment with ibeB in the pBluescriptII KS vector; pKS7-7B, carrying a 7-kb BamHI-EcoRI DNA fragment with ibeB derived from pKS7-13K; and pFN7C, carrying the ibeB gene (1.6 kb). pKS7-7B was capable of completely restoring the invasive phenotype of the TnphoA noninvasive mutant 7A-33 (Fig. 4A) and the ibeB deletion mutant IB7D5 (Fig. 4B). More importantly, pFN7C was able to fully confer invasive capability to IB7D5 (Fig. 4C). However, pKS7-13K was able to partially complement the mutants (Fig. 4A), suggesting that the expression of ibeB may be reduced because of a larger plasmid with a decreased copy number or some unknown repressor elements present in the larger DNA fragment (18). On the contrary, pKS7-5H, carrying a 5.7-kb BamHI-HpaI DNA fragment with an ibeB deletion derived from pKS7-7B, was unable to complement the TnphoA mutant 7A-33 or the ibeB deletion mutant IB7D5 (Fig. 4A and B).
FIG. 3.
BMEC invasion frequencies for E. coli K1 parent strain E44 and its TnphoA and ibeB deletion mutants (7A-33 and IB7D5, respectively). Values are means of four independent assays; error bars indicate standard errors of means.
FIG. 4.
Complementation of the noninvasive mutants of E44 with plasmids carrying the ibeB locus. The invasiveness of the mutants relative to that of the parent strain E44 is shown. Results are means of four separate experiments; error bars represent standard errors of means. (A) Complementation of the TnphoA insertion mutant 7A-33 by pKS7-13K (13K), pKS7-7B (7B), pKS7-5H (5H), and pBluescript KS (pKS). (B) Complementation of the isogenic ibeB deletion mutant IB7D5 by pKS7-7B (7B), pKS7-5H (5H), and pBluescript KS (pKS). (C) Complementation of the isogenic ibeB deletion mutant IB7D5 by combinations of pFN7C (7C), pGP1-2 (P2), and pFN476 (P1).
DISCUSSION
Although most cases of bacterial meningitis develop as a result of hematogenous spread, how circulating bacteria cross the blood-brain barrier is not completely understood. We have previously shown that several E. coli-BMEC interactions contribute to successful crossing of the blood-brain barrier by E. coli; these include E. coli binding to BMEC via S fimbriae (7, 15, 22). However, S fimbria-mediated binding to BMEC glycoproteins and glycolipids was not accompanied by invasion of BMEC (22), suggesting that binding and invasion are separate phenomena for E. coli translocation from blood to the CNS. We have shown that invasion of BMEC is needed for E. coli to cross the blood-brain barrier in vivo. We have recently described the invasion gene locus ibeA (ibe10) from E. coli K1 strain RS218; this locus has been shown to contribute to invasion of BMEC both in vitro and in vivo (7).
In the process of characterizing the noninvasive mutant 7A-33 derived from E. coli K1 strain RS218, we showed that mutant 7A-33 had a single TnphoA insertion and was considerably less invasive for BMEC (the invasion frequency was 0.001%; that for the parent strain was 0.1%) and significantly less invasive for the CNS in the newborn rat model of experimental hematogenous meningitis (4 of 25, or 16%, for the mutant strain versus 15 of 27, or 56%, for the parent strain; P, 0.003). We have previously shown that a high degree of bacteremia is a primary determinant for meningeal invasion by E. coli K1 (10). The magnitudes of bacteremia between the two groups of animals receiving the parent strain and the mutant strain were similar, indicating that a decreased ability of the mutant to enter the CSF was not an artifact of the lack of a sufficient number of circulating bacteria in the bloodstream. Taken together, these findings suggest that the DNA flanking the transposon insertion in the mutant 7A-33 includes a gene(s) necessary if not sufficient for the invasion of BMEC. This gene, derived from mutant 7A-33, was termed ibeB (invasion of brain endothelial cells).
We have previously shown, using the hematogenous E. coli meningitis model, that the K1 capsule is a critical determinant needed for E. coli to cross the blood-brain barrier as live bacteria (10). We have also shown that OmpA contributes to the invasion of BMEC by E. coli K1 (15). Both the parent strain RS218 and the mutant strain 7A-33 were found to possess the K1 capsule and OmpA. In addition, the EcoRV-MluI TnphoA fragment of the mutant 7A-33 did not hybridize to the probes for the K1 capsule and OmpA. These findings suggest that the noninvasive property of 7A-33 is not likely to be the result of a polar effect of TnphoA on the other known genes involved in invasion (e.g., those for the K1 capsule and OmpA). This concept was also supported by our demonstration that the isogenic ibeB deletion mutant IB7D5 was unable to invade BMEC, and its inability to invade BMEC was fully complemented by the ibeB ORF.
Nucleotide sequence analysis of the ibeB gene showed a single ORF encoding a protein of 460 amino acids and having a predicted molecular mass of 50 kDa (Fig. 1). The deduced protein displayed the characteristics of an outer membrane protein with two transmembrane domains (Fig. 1). This ORF was found to be 97% identical to a gene encoding a 50-kDa hypothetical protein (p77211) and located in the 13-min region of the E. coli K-12 genome. However, no homology was found with genes for any other recognized invasion proteins, suggesting that E. coli K1 ibeB results in a novel phenotype, i.e., E. coli invasion of BMEC. It is not clear whether the ibeB homologue from E. coli K-12 will result in the same phenotype in E. coli K1 strains.
The invasion of BMEC by E. coli represents a unique mechanism used by bacteria to gain entry into the CNS. We have previously shown that the characteristics of invasion of endothelial cells by E. coli K1 are specific to BMEC and that no such invasion characteristics are observed for endothelial cells of nonbrain origin, e.g., human umbilical vein endothelial cells (15). It is of interest that human BMEC have been shown to form a continuous lining of endothelial cells and to exhibit a transendothelial electrical resistance of 100 to 600 Ω · cm (14, 17), a unique property of the BMEC monolayer (compared to the systemic vascular endothelium). It is important to recognize that the frequency of invasion of BMEC by the parent strain RS218 (approximately 0.1%) is considerably lower than the reported frequency of invasion of epithelial cells by other gram-negative bacteria, such as Shigella and Salmonella species (usually 1 to 10%). However, as shown here and in our previous publication (7), the BMEC invasion frequency of approximately 0.1% contributes to enhanced bacterial penetration through the blood-brain barrier in vivo and thus is biologically relevant.
We have recently shown that the invasion gene locus ibeA contributes to E. coli invasion of human BMEC (7). Here we report another chromosomal invasion locus, ibeB, contributing to E. coli invasion of BMEC. The genetic locus ibeB was capable of completely restoring the ability of the ibeB deletion and TnphoA insertion mutants to invade BMEC. However, E. coli K-12 strain HB101 was not complemented by the ibeB locus (data not shown), suggesting that multiple determinants contribute to E. coli invasion of BMEC. Our demonstration that multiple chromosomal genes are required for E. coli invasion of BMEC is conceptually similar to the reported requirements for different determinants in the attachment and entry of epithelial cells by other meningitis-causing bacteria (2, 13, 25). It remains to be determined how different invasive loci of E. coli contribute to crossing of the blood-brain barrier.
In conclusion, we cloned and characterized the chromosomal gene locus that allows E. coli K1 strain RS218 to invade BMEC both in vitro and in vivo. This gene, termed ibeB, encoded a 50-kDa potential membrane protein. A 7-kb DNA fragment (7B) containing ibeB as well as the ibeB ORF was able to restore the ability of the in-frame ibeB deletion mutant to invade BMEC. Studies are in progress to define the mechanisms by which the ibeB locus exerts its invasive phenotype.
ACKNOWLEDGMENTS
We thank Jin Liu for restriction enzyme mapping of ibeB clones.
This study was supported by a research career development award from Childrens Hospital Los Angeles Research Institute (to S.-H.H.), a grant-in-aid from the American Heart Association Great Los Angeles Affiliate (to S.-H.H.), and USPHS grants R29AI40635 (to S.-H.H.) and R01NS26310 (to K.S.K.).
REFERENCES
- 1.Blattner F R, Plunkett III G, Bloch C A, Perna N T, Burland V, Riley M, Collado-Vides J, Glasner J D, Rode C K, Mayhew G F, Gregor J, Davis N W, Kirkpatrick H A, Goeden M A, Rose D J, Mau B, Shao Y. The complete genome sequence of Escherichia coli K-12. Science. 1997;277:1453–1462. doi: 10.1126/science.277.5331.1453. [DOI] [PubMed] [Google Scholar]
- 2.de Vries F P, van Der Ende A, van Putten J P, Dankert J. Invasion of primary nasopharyngeal epithelial cells by Neisseria meningitidis is controlled by phase variation of multiple surface antigens. Infect Immun. 1996;64:2998–3006. doi: 10.1128/iai.64.8.2998-3006.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Donnenberg M S, Calderwood S B, Donohue-Rolfe A, Keusch G T, Kaper J B. Construction and analysis of TnphoA mutants of enteropathogenic Escherichia coli that invade HEp-2 cells. Infect Immun. 1990;58:1565–1571. doi: 10.1128/iai.58.6.1565-1571.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Donnenberg M S, Kaper J B. Construction of an eae deletion mutant of enteropathogenic Escherichia coli by using a positive-selection suicide vector. Infect Immun. 1991;59:4310–4317. doi: 10.1128/iai.59.12.4310-4317.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Donnenberg M S, Yu J, Kaper J B. A second chromosomal gene necessary for intimate attachment of enteropathogenic Escherichia coli to epithelial cells. J Bacteriol. 1993;175:4670–4680. doi: 10.1128/jb.175.15.4670-4680.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hartman A B, Venkatesan M, Oaks E V, Buysse J M. Sequence and molecular characterization of a multicopy invasion plasmid antigen gene, ipaH, of Shigella flexneri. J Bacteriol. 1990;172:1905–1915. doi: 10.1128/jb.172.4.1905-1915.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang S H, Wass C A, Fu Q, Prasadarao N A, Stins M F, Kim K S. Escherichia coli invasion of brain microvascular endothelial cells in vitro and in vivo: molecular cloning and characterization of invasion gene ibe10. Infect Immun. 1995;63:4470–4475. doi: 10.1128/iai.63.11.4470-4475.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang S H. Inverse polymerase chain reaction: application in cDNA cloning. Methods Mol Biol. 1997;67:287–294. [Google Scholar]
- 9.Huang S H, Tang A, Drisco B, Zhang S-Q, Seeger R, Ching Li C, Jong A Y. Human dTMP kinase: gene expression and enzyme activity coinciding with cell cycle progression and cell growth. DNA Cell Biol. 1994;13:461–471. doi: 10.1089/dna.1994.13.461. [DOI] [PubMed] [Google Scholar]
- 10.Kim K S, Itabashi H, Gemski P, Warren R L, Cross A S. The K1 capsule is the critical determinant in the development of E. coli meningitis in the rat. J Clin Investig. 1992;90:897–905. doi: 10.1172/JCI115965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kolmodin L A, Williams J F. PCR: basic principles and routine practice. Methods Mol Biol. 1997;67:1–15. doi: 10.1385/0-89603-483-6:3. [DOI] [PubMed] [Google Scholar]
- 12.Manoil C, Beckwith J. TnphoA: a transposon probe for protein export signals. Proc Natl Acad Sci USA. 1985;82:8129–8133. doi: 10.1073/pnas.82.23.8129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Merz A J, So M. Attachment of piliated, Opa− and Opc− gonococci and meningococci to epithelial cells elicits cortical actin rearrangements and clustering of tyrosine-phosphorylated proteins. Infect Immun. 1997;65:4341–4349. doi: 10.1128/iai.65.10.4341-4349.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nizet V, Kim K S, Stins M, Jonas M, Chi E Y, Nguyen D, Rubens C E. Invasion of brain microvascular endothelial cells by group B streptococci. Infect Immun. 1997;65:5074–5081. doi: 10.1128/iai.65.12.5074-5081.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prasadarao N V, Wass C A, Weiser J N, Stins M F, Huang S H, Kim K S. Outer membrane protein A of Escherichia coli contributes to invasion of brain microvascular endothelial cells. Infect Immun. 1996;64:146–151. doi: 10.1128/iai.64.1.146-153.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quagliarello V, Scheld W M. Bacterial meningitis: pathogenesis, pathophysiology, and progress. N Engl J Med. 1992;327:864–872. doi: 10.1056/NEJM199209173271208. [DOI] [PubMed] [Google Scholar]
- 17.Ring A, Weiser J N, Tuomanen E I. Pneumococcal trafficking across the blood-brain barrier: molecular analysis of a novel bidirectional pathway. J Clin Investig. 1998;102:347–360. doi: 10.1172/JCI2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 19.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sankar P, Hutton M E, VanBogelen R A, Clark R L, Neidhardt F C. Expression analysis of cloned chromosomal segments of Escherichia coli. J Bacteriol. 1993;175:5145–5152. doi: 10.1128/jb.175.16.5145-5152.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Silver R P, Aaronson W, Sutton A, Schneerson R. Comparative analysis of plasmids and some metabolic characteristics of Escherichia coli K1 from diseased and healthy individuals. Infect Immun. 1980;29:200–206. doi: 10.1128/iai.29.1.200-206.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stins M F, Prasadarao N V, Ibric L, Wass C A, Luckett P, Kim S K. Binding characteristics of S fimbriated Escherichia coli to isolated brain microvascular endothelial cells. Am J Pathol. 1994;145:1228–1236. [PMC free article] [PubMed] [Google Scholar]
- 23.Stins M F, Gilles F, Kim K S. Selective expression of adhesion molecules on human brain microvascular endothelial cells. J Neuroimmunol. 1997;76:81–90. doi: 10.1016/s0165-5728(97)00036-2. [DOI] [PubMed] [Google Scholar]
- 24.Tuomanen E. Entry of pathogens into the central nervous system. FEMS Microbiol Rev. 1996;18:289–299. doi: 10.1111/j.1574-6976.1996.tb00245.x. [DOI] [PubMed] [Google Scholar]
- 25.Virji M, Makepeace K, Ferguson D J, Achtman M, Moxon E R. Meningococcal Opa and Opc proteins: their role in colonization and invasion of human epithelial and endothelial cells. Mol Microbiol. 1993;10:499–510. doi: 10.1111/j.1365-2958.1993.tb00922.x. [DOI] [PubMed] [Google Scholar]