Abstract
To assess the role of polymorphonuclear neutrophils (PMNs) in Chlamydia psittaci infection in a pregnant mouse model, pregnant and nonpregnant Swiss OF1 mice were depleted of PMNs by treatment with the RB6-8C5 monoclonal antibody before intraperitoneal infection with C. psittaci serotype 1. Nondepleted mice served as infection controls. Depleted mice aborted earlier and had a much higher mortality rate than nondepleted mice. Bacteriological analysis showed that the number of chlamydiae isolated from the spleens of depleted mice at 5 and 7 days postinfection was 100 times greater than that isolated from nondepleted mice. Histopathological analysis of the placentas of depleted mice showed widespread necrosis of the uteroplacental units, with weak immunoreaction to chlamydial antigen, while the placentas of nondepleted mice showed substantial neutrophil infiltration but no large areas of necrosis, with moderate to strong immunoreaction to chlamydial antigen. The livers of depleted mice showed numerous chlamydial inclusions in the hepatocytes, delayed microgranuloma formation, and in the pregnant animals extensive coagulative periportal necrosis. The livers of nondepleted mice displayed multiple small foci of PMNs and mononuclear cells with microgranuloma formation. Among this group of mice, the pregnant animals always had more hepatic damage than nonpregnant animals. Our results suggest that PMNs play an essential role in the response to C. psittaci primary infection, preventing the uncontrolled multiplication of chlamydiae in the liver and spleen.
Chlamydia psittaci serotype 1 is a gram-negative obligate intracellular bacterium which can colonize many different types of placenta (ruminant, porcine, human, and murine), causing abortion during the last third of gestation. Pronounced neutrophil infiltration and extensive necrosis of the maternal-fetal junctions are characteristics of chlamydial infection of the placenta under natural conditions (4, 37), and they were present in the induced infection of pregnant mice performed in our laboratory (3, 29). Neutrophil infiltration has also been observed in murine placental infection with other intracellular pathogens, such as Brucella abortus (34), Coxiella burnetii (2), and Listeria monocytogenes (21). It has been reported previously that polymorphonuclear neutrophils (PMNs) are able to destroy chlamydiae under in vitro conditions (22, 40). In spite of this, the role of PMNs in chlamydial placental infection is poorly understood, although the extensive neutrophil infiltration of maternal placenta described above may have an adverse effect on gestation outcome, leading to a malfunctioning of the maternal placenta and a premature breaking of the decidua basalis, which could result in a late-term abortion (3).
Neutrophil depletion with the monoclonal antibody (MAb) RB6-8C5 (32), which binds and destroys mature neutrophils and eosinophils, has been widely used to study the role of PMNs in the immune responses of mice to different pathogens (1, 9–11, 30, 33). However, there is no reference in the literature to the use of this depletion model in the study of infection by a placental pathogen. To assess host immunity mechanisms in the placenta, it is very important to study the role of the nonspecific innate immune response and especially the role of PMNs, since the specific immune response in this organ is partially abolished to allow the allogeneic fetus to develop (21) and since PMNs are always the most predominant effector cells recruited to the infectious foci. Furthermore, pregnancy is an event that may favor chlamydial multiplication by two pathways: (i) gestation, which causes a change from a Th1 to a Th2 cytokine response (18) (a Th1 response is necessary to resolve chlamydial infection [20]), and (ii) the production of progesterone (35). Thus, in the present study, we have monitored the evolution of chlamydial infection in pregnant mice with and without neutrophil depletion. In addition, to assess the effect of gestation on the progression of infection, nonpregnant mice were depleted of PMNs and infected for a comparative study. Placenta and liver samples were used for histopathological and immunohistochemical analysis, while spleen samples were used for bacteriological analysis.
MATERIALS AND METHODS
Mice.
Adult Swiss OF1 (outbred) mice, 8 to 10 weeks old, were obtained from Harlan Ibérica (Barcelona, Spain). They were free of common viral and bacterial pathogens according to the results of routine screening procedures performed by the manufacturer. Two groups of mice were used for this study: (i) pregnant mice at the same stage of gestation (10 to 11 days) at challenge and (ii) female nonpregnant mice of the same age (8 weeks) and weighing 26 to 28 g. The pregnant mice, in individual cages, and the nonpregnant mice, in common cages, were given food and water ad libitum and were kept in an environmentally controlled room.
Bacteria.
The abortion-causing C. psittaci strain AB7 (23) was propagated in the yolk sacs of developing chicken embryos. Titers of inocula were determined by enumerating inclusion-forming units (IFU) on McCoy cells as described below, and standardized aliquots were frozen at −80°C until use.
Granulocyte-specific MAb.
The hybridoma producing the RB6-8C5 MAb was provided by R. L. Coffman (DNAX Research Institute, Palo Alto, Calif.). The rat immunoglobulin G2b RB6-8C5 MAb was obtained from the ascites fluid of pristane-primed homozygous nude mice (Harlan) injected with 107 hybridoma cells. The MAb was concentrated by saturated ammonium sulfate precipitation, followed by dialysis against phosphate-buffered saline (PBS), pH 7.2, and filtration with a 0.22-μm-pore-size filter. The MAb was purified by chromatography on a protein G column (Sigma, Madrid, Spain) according to instructions provided by the manufacturer. Protein concentration was estimated by a modified Lowry method by using the bicinchoninic acid 1 procedure (kit from Sigma).
Experimental design.
To evaluate the role of PMNs in serotype 1 C. psittaci infection, we used two mouse models: (i) a pregnant mouse model previously described (3), where the challenge was carried out intraperitoneally with 106 IFU of C. psittaci in 0.2 ml of 0.1 M PBS at days 10 and 11 of pregnancy, and (ii) a nonpregnant mouse model where the challenge was carried out under the same conditions. In both mouse models, the mice were divided into two groups: a PMN-depleted group and a nondepleted control group. Depleted mice received 0.5 mg of RB6-8C5 MAb intravenously 6 h before the challenge and subsequently on days 3 and 5 postinfection (p.i.). Nondepleted mice received rat immunoglobulin G (Sigma) at the same times, by the same route, and at the same dosage. Mice were killed 3, 5, and 7 days p.i. Serum samples were collected from each mouse and stored at −20°C until use. After necropsy, samples from the liver and different areas of the placenta (for pregnant mice) were processed for light microscopy. Finally, the spleens were recovered and frozen at −80°C until bacteriological analysis. Of the 25 pregnant granulocyte-depleted but uninfected mice which served as controls for the pregnant mouse model, 5 were killed 3, 5, and 7 days after the first depletion. The other 10 mice served as controls of the effect of depletion on gestation. Ten uninfected and nondepleted mice served as pregnancy controls. The control group for the nonpregnant mouse model consisted of 15 granulocyte-depleted but uninfected mice, and 5 of them were killed 3, 5, and 7 days after the first RB6-8C5 MAb treatment. May-Grünwald-Giemsa staining was performed on blood smears of sacrificed mice to assess neutrophil depletion. Results are the summary of two independent experiments.
Isolation of chlamydiae from the spleen.
The course of infection was evaluated by counting IFU from the spleens after isolation on McCoy cell monolayers by a modification of a previously described method (17). Briefly, spleens were homogenized and diluted at 1:10 (wt/vol) in sterile PBS containing 0.2 mg of DEAE dextran (Fluka Biochemika, Madrid, Spain) per ml, and 1 ml of each homogenized and diluted specimen was transferred to a 2-ml centrifuge tube. The specimens were centrifuged at 300 × g for 10 min at 4°C, and the supernatant was subsequently diluted in PBS-DEAE dextran from 10−1 to 10−5. Individual wells of 96-well plates (Costar, Sloterweg, The Netherlands) with confluent McCoy cell monolayers were inoculated in triplicate with 25 μl for each specimen dilution and incubated for 2 h at 37°C. The specimens were then centrifuged at 1,400 × g for 30 min at room temperature. The inocula were then removed, and the monolayers were washed with PBS. To each well was added 100 μl of Eagle’s minimal essential medium containing 10% fetal bovine serum, 0.4% glucose, 0.01% sodium pyruvate, 0.1% yeast extract, 50 μg of gentamicin per ml, 2.5 μg of amphotericin B per ml, and 200 mM glutamine (all from Sigma). The plates were incubated at 37°C for 42 h in 5% CO2. The cultures were then fixed with acetone (80% in PBS) at −20°C for 1 h, and chlamydial inclusions were visualized by an indirect immunofluorescence method by an antilipopolysaccharide MAb, as previously described (28). The number of inclusions in McCoy cells within each well was counted under a fluorescent microscope, and the number of IFU per gram of spleen was calculated. The detection limit was 4 × 102 IFU per spleen.
Histopathology and immunohistochemistry.
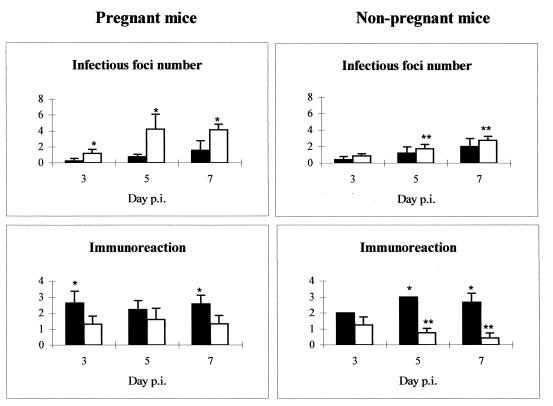
Livers and fetoplacental units (for pregnant mice) were collected and fixed in 10% formaldehyde in PBS. After being dehydrated and embedded in paraffin wax at 56°C, 5-μm sections were cut, stained with hematoxylin and eosin, and analyzed for histopathological changes. To visualize chlamydial antigen in paraffin sections, immunohistochemical staining was carried out with a chlamydial-lipopolysaccharide-specific biotinylated mouse MAb, as previously described (29), by using the avidin-biotin-peroxidase complex (ABC) method according to the instructions of the manufacturer (Vector Laboratories, Burlingame, Calif.). A positive reaction was demonstrated by the precipitation of diaminobenzidine tetrahydrochloride. Sections were subsequently stained with periodic acid-Schiff stain for fetoplacental units or with hematoxylin for livers. In the case of fetoplacental units, each anatomical site from the maternal placenta (metrial gland and decidua basalis) and fetal placenta (labyrinth), as well as the sites of previous placental attachments and the uteri, was assessed independently for histopathological changes or for studying the distribution and intensity of the immunoreaction to C. psittaci antigen. In the evaluation of the liver pathology, inflammatory circular lesions formed by leukocytes were considered infectious foci. The infectious foci were enumerated in 20 fields (×400 magnification) from a section of the same lobe of liver for each mouse. The intensity of the immunoreaction in both the liver and placenta was recorded as negative, weak, moderate, strong, or very strong (see Fig. 5).
FIG. 5.
Administration of antigranulocyte MAbs during C. psittaci infection decreases the formation of infectious foci but increases the immunoreactivity in the liver. Shown is the evolution of the infection in the liver in the depleted (black) and nondepleted (white) groups of pregnant and nonpregnant mice. Infectious foci were defined as circular lesions formed by leukocytes and were counted in 20 fields (×400 magnification) from a selection of the same lobe of liver from each mouse. The intensity of the immunoreaction is indicated according to the following classification: 0, negative; 1, weak; 2, moderate; 3, strong; and 4, very strong. ∗, significant differences (P < 0.05) between depleted and nondepleted mice at the same day p.i.; ∗∗, significant differences (P < 0.05) between pregnant and nonpregnant mice at the same day p.i.
TNF-α measurement.
Because previous data have suggested that PMNs are a significant source of tumor necrosis factor alpha (TNF-α) (5, 38) and since TNF-α has frequently been documented as an initiatory cytokine released upon chlamydial invasion of the host (12), the presence of this cytokine in the sera of both depleted and nondepleted mice was determined to establish whether PMNs were the source of TNF-α in our infection model. To determine the presence of TNF-α in response to chlamydial infection, serum samples were collected from mice at different days p.i. and analyzed by a commercial murine enzyme-linked immunosorbent assay kit (R & D Systems Inc., Minneapolis, Minn.) as described in the manufacturer’s instructions.
Statistical analysis.
Data are expressed as means ± standard errors of the means (SEM). Differences between groups of mice were analyzed by Student’s t test, except for the intensity of the immunoreaction, for which a nonparametric test (Mann-Whitney U test) was used. A probability value of less than 0.05 was considered significant.
RESULTS
Clinical signs.
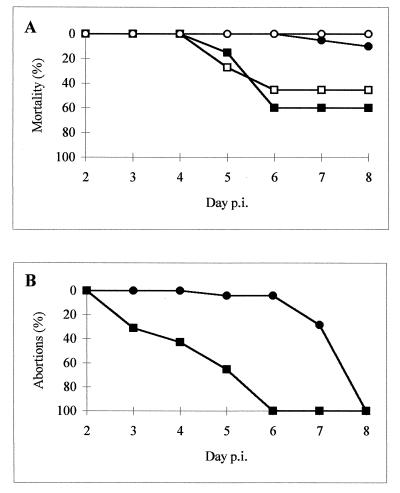
The intraperitoneal inoculation of C. psittaci AB7 induced a feverish syndrome from 2 days p.i. in all infected mice, with lethargy, rough coat, huddling, bilateral conjunctivitis, and subsequently, in the pregnant mice, abortion. Administration of the RB6-8C5 MAb resulted in enhanced mortality in both pregnant and nonpregnant mice (Fig. 1A); numerous animals of these groups (12 of 20 pregnant and 5 of 11 nonpregnant mice) manifested hypothermia and severe conjunctivitis from day 4 p.i. and died on days 5 and 6 p.i. In nondepleted mice, clinical signs were less noticeable, with very low mortality rates (3 of 22) and death occurring at the moment of abortion (Fig. 1A). Nonpregnant mice of this group had only slight lethargy 3 to 4 days p.i. and no signs of conjunctivitis. None of the 14 nonpregnant, nondepleted mice died.
FIG. 1.
Administration of antigranulocyte MAbs during C. psittaci infection increases mortality and advances abortions. The animal groups are represented by squares (depleted mice) or circles (nondepleted mice), black for the pregnant mice and white for the nonpregnant mice.
Abortion occurred earlier (3 to 6 days p.i.) in the pregnant depleted mice (Fig. 1B) than in their nondepleted counterparts (7 to 8 days p.i.). Furthermore, placental retention was observed in most of the depleted mice but not in nondepleted animals. Uninfected depletion control mice receiving the RB6-8C5 MAb had a normal gestation with an average of 12.6 pups per litter. Pregnancy control mice had an average of 13.1 pups per litter.
Bacteriological analysis.
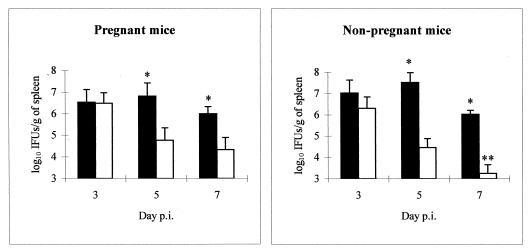
Bacteriological analysis of the spleens (Fig. 2) showed that at day 3 p.i. the numbers of isolated chlamydiae were quite similar in both groups. However, at days 5 and 7 p.i., the chlamydiae were significantly more numerous in depleted mice than in nondepleted mice.
FIG. 2.
Administration of antigranulocyte MAbs increases the growth of C. psittaci in the spleens of infected mice. Shown is the evolution of spleen infection with C. psittaci in neutrophil-depleted (black) and nondepleted (white) groups of pregnant and nonpregnant mice. Quantitative isolation was performed on homogenized tissues from spleens collected at various times p.i. The inclusions were visualized by indirect immunofluorescence. ∗, significant differences (P < 0.05) between depleted and nondepleted mice at the same day p.i.; ∗∗, significant differences (P < 0.05) between pregnant and nonpregnant mice at the same day p.i.
Histopathological and immunohistochemical analysis.
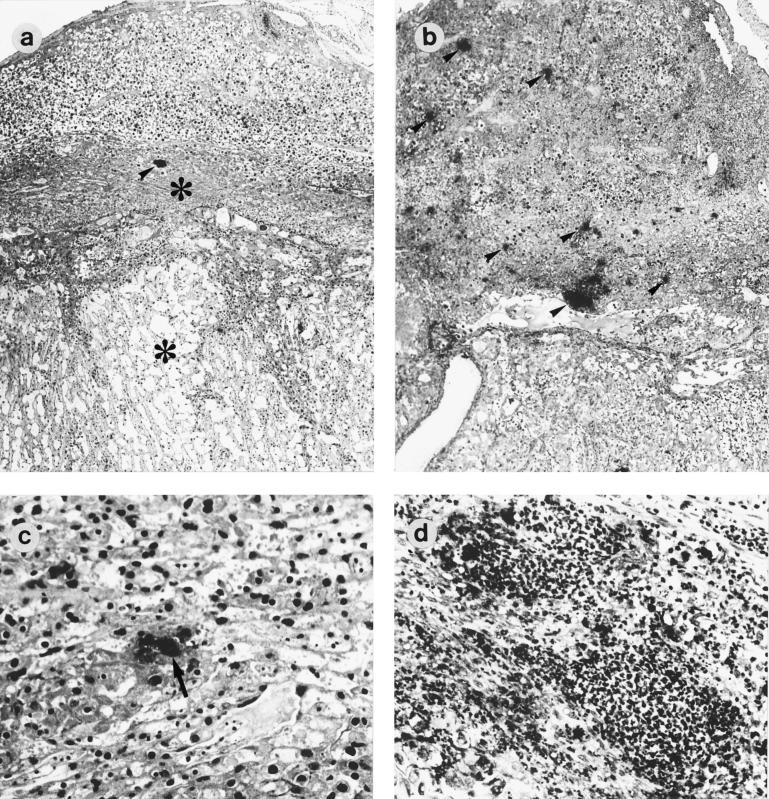
The placentas of depleted mice at day 3 p.i. showed small areas of necrosis in all areas (metrial gland, decidua basalis, and labyrinth). These areas of necrosis were formed by cytoplasma debris with increased acidophilia and clumps of scattered nuclear debris, including pyknotic and karyorrhectic remnants. Immunohistochemical analysis showed that immunoreaction was weak at this stage and located in the metrial gland and decidua basalis. At day 5 p.i., mice which did not abort showed widespread necrosis of the uteroplacental units, especially in the labyrinth, where cells showed eosinophilia in the cytoplasma and pyknosis and karyorrhexis of nuclei. However, the immunoreaction in the metrial gland, decidua basalis, and labyrinth was weak to moderate at this day (Fig. 3a and c). Depleted mice which aborted showed a substantial mononuclear cell infiltrate, with moderate immunoreaction in the sites of previous placental attachment at days 5 and 7 p.i.
FIG. 3.
Histopathology and immunoreaction in the placentas of mice infected with C. psittaci. Paraffin wax sections immunostained by the ABC method show the distribution of positive immunoreactions (arrowheads and arrows) and areas of necrosis (asterisks) in the placentas of depleted (a and c) and nondepleted (b and d) mice at 5 days p.i. (a) Placenta of a depleted mouse showing only one focus of immunoreaction but extensive areas of necrosis in the decidua basalis and labyrinth; (b) placenta of a nondepleted mouse showing immunoreactive foci spread through the metrial gland and decidua basalis; (c) area of necrosis in the labyrinth of a depleted mouse showing a chlamydial inclusion (arrow) (notice the pyknosis of the nuclei in laberynthine cells); (d) substantial neutrophil infiltration in the decidua basalis of a nondepleted mouse. Magnifications, ×40 (a and b) and ×200 (c and d).
The placentas of nondepleted mice showed a weak immunoreaction at day 3 p.i., with small foci disseminated through the metrial gland and the decidua basalis. At days 5 and 7 p.i., the metrial gland and decidua basalis showed a strong immunoreaction, with substantial neutrophil infiltration, small areas of necrosis in the decidua basalis, and numerous decidual and granulated metrial gland cells with chlamydial inclusions (Fig. 3b and d).
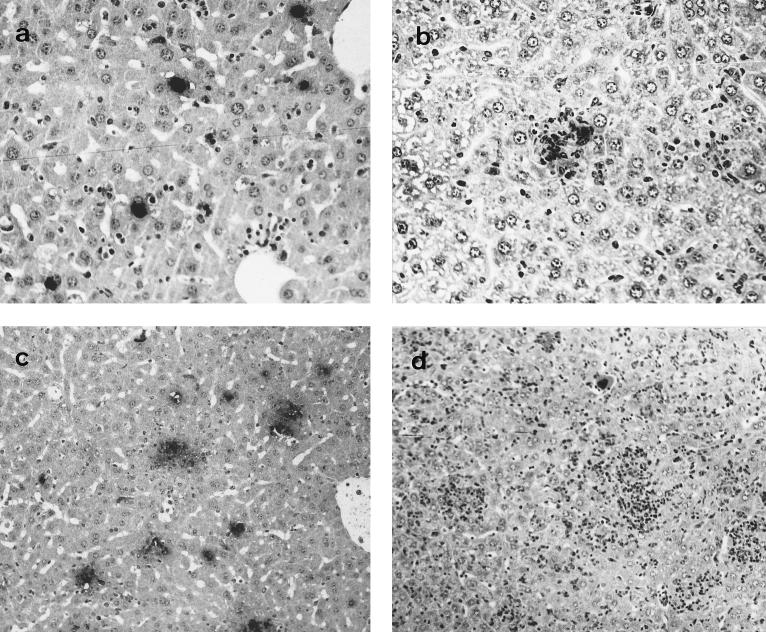
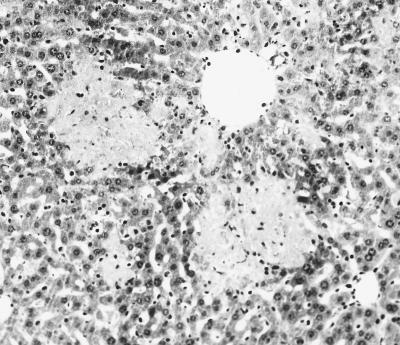
The livers of depleted mice (pregnant and nonpregnant) showed numerous swollen hepatocytes with chlamydial inclusions at day 3 p.i., although very few infectious foci were observed at this time (Fig. 4a and 5). The number of chlamydial inclusions increased at day 5 p.i., when an infiltrate of mononuclear cells forming some infectious foci could be observed. The intensity of the immunoreaction in depleted mice was always greater than in nondepleted mice, both pregnant and nonpregnant (Fig. 5). From day 5 p.i. onward, extensive areas of coagulative periportal necrosis were observed in the pregnant depleted mice (Fig. 6), although the immunohistochemical analysis showed that there was a negative immunoreaction in these areas. At day 7 p.i., surviving mice had numerous infectious foci with a strongly positive immunoreaction (Fig. 4c).
FIG. 4.
Histopathology and immunoreaction in the livers of mice infected with C. psittaci. Paraffin wax sections immunostained by the ABC method show the distribution of positive immunoreactions in the livers of depleted (a and c) and nondepleted (b and d) pregnant mice. (a) Liver of a depleted mouse at 3 days p.i. showing several chlamydial inclusions (notice the lack of leukocytes); (b) liver of a nondepleted mouse at 3 days p.i. showing an infectious focus, formed mainly by neutrophils, with a positive immunoreaction (notice that there are not typical chlamydial inclusions); (c) liver of a depleted mouse at 7 days p.i. showing numerous immunoreactive foci; (d) liver of a nondepleted mouse at 7 days p.i. showing extensive leukocyte infiltration (notice the lack of immunoreactivity in the foci). Magnifications, ×200 (a and b) and ×100 (c and d).
FIG. 6.
Histopathology in the livers of mice infected with C. psittaci. This paraffin wax section of the liver of a pregnant depleted mouse, stained with hematoxylin-eosin, at 5 days p.i. shows periportal necrosis (light areas). Magnification, ×100.
There were numerous small foci of intraparenchymal necrosis with PMNs and a moderate immunoreaction in the livers of pregnant nondepleted mice at day 3 p.i. (Fig. 4b and 5). These PMNs were gradually replaced by mononuclear cells at days 5 and 7 p.i., while immunoreaction became weak at day 7 p.i. and numerous infiltrating leukocytes could be seen (Fig. 4d). The numbers of foci in the livers of nonpregnant nondepleted mice were smaller, and the immunoreaction was always weaker in them than in the pregnant nondepleted mice of the same group (Fig. 5), with significant differences (P < 0.05) at days 5 and 7 p.i.
TNF-α measurement.
Levels of TNF-α in the sera of infected mice are shown in Table 1. Individual analysis of the data showed that high levels of TNF-α in depleted mice were always associated with dying (in both pregnant and nonpregnant mice), while surviving mice had lower levels. This fact explains the high SEM found at day 5 p.i., when several dying and aborting animals were analyzed together with convalescent animals that had aborted at days 3 and 4 p.i. The pregnant nondepleted mice showed higher values at day 7 p.i., coinciding with the abortion, while the nonpregnant mice of this group always showed lower TNF-α values, which are consistent with the lack of clinical signs and mild liver pathology observed in this group.
TABLE 1.
Effect of administration of antigranulocyte MAb during C. psittaci infection on TNF-α levels in serum
| Day p.i. | TNF-α concn (pg/ml)a
|
|||
|---|---|---|---|---|
| Pregnant mice
|
Nonpregnant mice
|
|||
| Depleted | Nondepleted | Depleted | Nondepleted | |
| 0 | 17 ± 22.8 | <10 | 13.4 ± 16.2 | <10 |
| 3 | 168.4 ± 93.3 | 152.1 ± 46.7 | 88.8 ± 21.1 | 118.7 ± 49.7 |
| 5 | 841.9 ± 919.7 | 355.1 ± 120.8 | 1,105.6 ± 1,128.4 | 130.2 ± 61.3 |
| 7 | 582.1 ± 339.1 | 1,596.2 ± 1,164.2 | 565.3 ± 223.2 | 264.2 ± 156.1 |
Data are expressed as means ± SEM for duplicate determinations with 8 to 12 mice for each group and were obtained by enzyme-linked immunosorbent assay.
DISCUSSION
The present study shows that PMNs are a critical component of the murine host defense against infection by C. psittaci serotype 1. Mice which had been subjected to neutrophil depletion by the RB6-8C5 MAb treatment aborted earlier and had significantly higher mortality rates than immunocompetent mice. Furthermore, a greater degree of chlamydial multiplication was observed in the livers and spleens. Previous studies (3, 29) reported that PMNs were the main cell population infiltrating the maternal placenta in C. psittaci-infected mice, and abortion in these studies was always observed at the end of gestation, independently of the moment of infection. The possibility that such extensive neutrophil infiltration could have deleterious effects on gestation, either directly, through their lysosomal activity on neighboring decidual cells, or indirectly, since PMNs have recently been reported as a significant source of abortigenic cytokines such as gamma interferon (39) and TNF-α (38), has been the focus of the present study.
However, our findings have demonstrated that neutrophil-depleted mice aborted earlier and showed placental retention and increased mortality rates, while histopathological analysis revealed areas of necrosis in the decidua basalis and labyrinth as early as 3 days p.i. The livers of these depleted mice displayed substantial deficiencies in the organization of the host defense, resulting in the delayed formation of infectious foci. The numerous chlamydial inclusions observed in the hepatocytes were probably related to this delayed formation of infectious foci, since few chlamydial inclusions were observed in the livers of nondepleted mice, where the infectious foci were much more numerous. The fact that PMN depletion causes a significant increase in the infection of hepatocytes has been reported for other intracellular bacteria, such as L. monocytogenes (11, 24), Salmonella typhimurium (7), and Francisella tularensis (31). Different mechanisms have been proposed for the way in which PMNs are able to control intracellular bacterial infection in the liver. Some authors have suggested, for example, that neutrophil-mediated lysis of infected hepatocytes is crucial (8); another possibility is that PMNs kill extracellular chlamydiae before infection of the target cells or just after the lysis of infected cells. Finally, it has been suggested that PMNs could have an immunoregulatory function in secreting effector cytokines, such as TNF-α and gamma interferon, or cytokines, such as interleukin-12 (IL-12) (6), that provoke the Th1 lymphocyte response necessary for controlling chlamydial infection (20).
The liver and spleen have previously been reported as the primary target organs in systemic chlamydial infection after intraperitoneal inoculation, with the placenta being colonized later (3). However, while infection was controlled in the liver by the formation of granulomas, chlamydiae found a suitable environment for multiplication in the maternal placenta, especially in the decidua basalis, which is just between the maternal and fetal placenta. In our model of neutrophil depletion, the uncontrolled chlamydia multiplication observed in the liver during the early stages of infection might cause an exacerbated inflammatory cytokine response that in chlamydial infection is endotoxin mediated (16) and which could cause an early abortion before complete chlamydial colonization of the placenta. The placental injury with widespread necrosis found in depleted mice agrees with the existence of such an inflammatory response mediated by macrophages and high levels of TNF-α. Furthermore, we found the highest values of circulating TNF-α in depleted dying animals, while the nondepleted pregnant mice showed high values only at day 7 p.i., just prior to abortion, and nonpregnant nondepleted mice, which had no clinical signs and suffered no mortality, always showed the lowest values. TNF-α is a cytokine that has been related with spontaneous embryo loss in pregnant mice (14, 15), and its increased production has been correlated with the incidence of preterm labor in women (27). Our results suggest that TNF-α-mediated pathology may be an important component of acute chlamydial infection. On the other hand, the extensive periportal necrosis observed in the livers of pregnant depleted mice, mainly at day 5 p.i., was always associated with the necrosis of uteroplacental units or with the existence of placental retention, suggesting that the cause of necrosis could be toxic damage following the placental events, since this kind of liver pathology was not observed in depleted nonpregnant mice.
TNF-α has been described as a cytokine produced in response to chlamydial infection (36). That PMNs are a potential source of this cytokine was demonstrated in an elegant study reported by Marshall and Denkers (19), in which the depletion of PMNs by treatment with the RB6-8C5 MAb prevented the lethal inflammatory cytokine shock induced by Toxoplasma gondii infection. Our findings showed that TNF-α production was not abolished in depleted mice; on the contrary, it was produced earlier than in nondepleted mice, which suggests that PMNs are not a great source of TNF-α in C. psittaci infection.
Our results suggest that gestation favored the development of chlamydial infection. Among the nondepleted mice, pregnant mice displayed increased immunoreaction and a greater number of infectious foci in the liver than nonpregnant mice. It has been reported that in the case of Leishmania major, another intracellular pathogen, pregnancy alters the Th1/Th2 balance toward a Th2 response (18). This could be what happens with C. psittaci. Such differences between pregnant and nonpregnant mice were not observed in the depleted group. Perhaps the depletion of neutrophils overwhelmed the influence imposed by gestation on the response to chlamydial infection.
In conclusion, this study indicates that PMNs act in the liver as a first line of defense against C. psittaci in systemic infection in our mouse abortion model. Some reports have indicated that RB6-8C5 treatment leads to a reduction in the number of CD8+ cells, and cross-reactivity of the antibody with Ly-6C, an antigenic component of the CD8+ cells of some populations, has been described (13). Barteneva et al. (1) postulated that this decrease is probably not due to a direct effect of the MAb itself but to the effects that neutrophil depletion may have on the T-cell immune response. The role of PMNs as regulatory cells of the immune response is slowly being pieced together, and because these cells are able to produce regulatory cytokines, such as IL-10 and IL-12 (6, 25, 26), this aspect will be studied in greater depth in our induced-abortion model.
ACKNOWLEDGMENTS
This work was supported by Comisión Interministeral de Ciencia y Tecnología (CICYT) grant AGF97-0459. A. J. Buendía was the recipient of a predoctoral grant from the Universidad de Murcia, Murcia, Spain. R. Montes de Oca was supported by the Consejo Nacional de Ciencia y Teconología (CONACyT) and the Universidad Autónoma del Estado de México, Toluca, México.
We thank R. L. Coffman for the generous gift of RB6-8C5 hybridoma cells and A. Rodolakis for her helpful suggestions.
REFERENCES
- 1.Barteneva N, Theodor I, Peterson E M, de la Maza L M. Role of neutrophils in controlling early stages of a Chlamydia trachomatis infection. Infect Immun. 1996;64:4830–4833. doi: 10.1128/iai.64.11.4830-4833.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baumgärtner W, Bachmann S. Histological and immunocytochemical characterization of Coxiella burnetii-associated lesions in the murine uterus and placenta. Infect Immun. 1992;60:5232–5241. doi: 10.1128/iai.60.12.5232-5241.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buendía A J, Sánchez J, Martínez M C, Cámara P, Navarro J A, Rodolakis A, Salinas J. Kinetics of infection and effects on placental cell populations in a murine model of Chlamydia psittaci-induced abortion. Infect Immun. 1998;66:2128–2134. doi: 10.1128/iai.66.5.2128-2134.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buxton D, Barlow R M, Finlayson J, Anderson I E, Mackellar A. Observations on the pathogenesis of Chlamydia psittaci infection of pregnant sheep. J Comp Pathol. 1990;102:221–237. [PubMed] [Google Scholar]
- 5.Cassatella M A. The production of cytokines by polymorphonuclear neutrophils. Immunol Today. 1995;16:21–26. doi: 10.1016/0167-5699(95)80066-2. [DOI] [PubMed] [Google Scholar]
- 6.Cassatella M A, Meda L, Gasperini S, D’Andrea A, Ma X, Trinchieri G. Interleukin-12 production by human polymorphonuclear leukocytes. Eur J Immunol. 1995;25:1–10. doi: 10.1002/eji.1830250102. [DOI] [PubMed] [Google Scholar]
- 7.Conlan J W. Critical roles of neutrophils in host defense against experimental systemic infections of mice by Listeria monocytogenes, Salmonella typhimurium, and Yersinia enterocolitica. Infect Immun. 1997;65:630–635. doi: 10.1128/iai.65.2.630-635.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conlan J W, North R J. Neutrophil-mediated dissolution of infected host cells as a defense strategy against a facultative intracellular bacterium. J Exp Med. 1991;174:741–744. doi: 10.1084/jem.174.3.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Conlan J W, North R J. Early pathogenesis of infection in the liver with the facultative intracellular bacteria Listeria monocytogenes, Francisella tularensis, and Salmonella typhimurium involves lysis of infected hepatocytes by leukocytes. Infect Immun. 1992;60:5164–5171. doi: 10.1128/iai.60.12.5164-5171.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conlan J W, North R J. Neutrophils are essential for early anti-Listeria defense in the liver, but not in the spleen or peritoneal cavity, as revealed by a granulocyte-depleting monoclonal antibody. J Exp Med. 1994;179:259–268. doi: 10.1084/jem.179.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Czuprynski C J, Brown J F, Marosheck N, Wagner R D, Steinberg H. Administration of anti-granulocyte mAb RB6-8C5 impairs the resistance of mice to Listeria monocytogenes infection. J Immunol. 1994;152:1836–1846. [PubMed] [Google Scholar]
- 12.Darville T, Laffoon K K, Kishen L R, Rank R G. Tumor necrosis factor alpha activity in genital tract secretions of guinea pigs infected with chlamydiae. Infect Immun. 1995;63:4675–4681. doi: 10.1128/iai.63.12.4675-4681.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fleming T J, Fleming M L, Malek T R. Selective expression of Ly-6G on myeloid lineage cells in mouse bone marrow. RB6-8C5 mAb to granulocyte-differentiation antigen (Gr-1) detects members of the Ly-6 family. J Immunol. 1993;151:2399–2408. [PubMed] [Google Scholar]
- 14.Gorivodsky M, Zemlyak Y, Orenstein H, Savion S, Fein A, Torchinsky A, Toder V. TNF-α messenger RNA and protein expression in the uteroplacental unit of mice with pregnancy loss. J Immunol. 1998;160:4280–4288. [PubMed] [Google Scholar]
- 15.Haddad E K, Duclos A J, Lapp W S, Baines M G. Early embryo loss is associated with the prior expression of macrophage activation markers in the decidua. J Immunol. 1997;158:4886–4892. [PubMed] [Google Scholar]
- 16.Ingalls R R, Rice P A, Qureshi N, Takayama K, Lin J S, Golenbock D T. The inflammatory cytokine response to Chlamydia trachomatis infection is endotoxin mediated. Infect Immun. 1995;63:3125–3130. doi: 10.1128/iai.63.8.3125-3130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kelly K A, Robinson E A, Rank R G. Initial route of antigen administration alters the T-cell cytokine profile produced in response to the mouse pneumonitis biovar of Chlamydia trachomatis following genital infection. Infect Immun. 1996;64:4976–4983. doi: 10.1128/iai.64.12.4976-4983.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krishnan L, Guilbert L J, Russell A S, Wegmann T G, Mosmann T R, Belosevic M. Pregnancy impairs resistance of C57BL/6 mice to Leishmania major infection and causes decreased antigen-specific IFN-gamma response and increased production of T helper 2 cytokines. J Immunol. 1996;156:644–652. [PubMed] [Google Scholar]
- 19.Marshall A J, Denkers E Y. Toxoplasma gondii triggers granulocyte-dependent cytokine-mediated lethal shock in d-galactosamine-sensitized mice. Infect Immun. 1998;66:1325–1333. doi: 10.1128/iai.66.4.1325-1333.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perry L L, Feilzer K, Caldwell H D. Immunity to Chlamydia trachomatis is mediated by T helper 1 cells through IFN-γ-dependent and -independent pathways. J Immunol. 1997;158:3344–3352. [PubMed] [Google Scholar]
- 21.Redline R W, Lu C Y. Specific defects in the anti-listerial immune response in discrete regions of the murine uterus and placenta account for susceptibility to infection. J Immunol. 1988;140:3947–3955. [PubMed] [Google Scholar]
- 22.Register K B, Morgan P A, Wyrick P B. Interaction between Chlamydia spp. and human polymorphonuclear leukocytes in vitro. Infect Immun. 1986;52:664–670. doi: 10.1128/iai.52.3.664-670.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rodolakis A, Bernard F, Lantier F. Mouse models for evaluation of virulence of Chlamydia psittaci isolated from ruminants. Res Vet Sci. 1989;46:34–39. [PubMed] [Google Scholar]
- 24.Rogers H W, Unanue E R. Neutrophils are involved in acute, nonspecific resistance to Listeria monocytogenes in mice. Infect Immun. 1993;61:5090–5096. doi: 10.1128/iai.61.12.5090-5096.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Romani L, Mencacci A, Cenci E, Del Sero G, Bistoni F, Puccetti P. An immunoregulatory role for neutrophils in CD4+ T helper subset selection in mice with candidiasis. J Immunol. 1997;158:2356–2362. [PubMed] [Google Scholar]
- 26.Romani L, Mencacci A, Cenci E, Spaccapelo R, Del Sero G, Nicoletti I, Trinchieri G, Bistoni F, Puccetti P. Neutrophil production of IL-12 and IL-10 in candidiasis and efficacy of IL-12 therapy in neutropenic mice. J Immunol. 1997;158:5349–5356. [PubMed] [Google Scholar]
- 27.Romero R, Manogue K H, Mitchell M D, Wu Y K, Oyarzun E, Robins J C, Cerami A. Infection and labor. IV. Cachectin-tumor necrosis factor in the amniotic fluid of women with intraamniotic infection and preterm labor. Am J Obstet Gynecol. 1989;161:336–344. doi: 10.1016/0002-9378(89)90515-2. [DOI] [PubMed] [Google Scholar]
- 28.Salinas J, Souriau A, Cuello F, Rodolakis A. Antigenic diversity of ruminant Chlamydia psittaci strains demonstrated by the indirect microimmunofluorescent test with monoclonal antibodies. Vet Microbiol. 1995;43:219–226. doi: 10.1016/0378-1135(94)00100-b. [DOI] [PubMed] [Google Scholar]
- 29.Sánchez J, Buendía A J, Salinas J, Bernabé A, Rodolakis A, Stewart I J. Murine granulated metrial gland cells are susceptible to Chlamydia psittaci infection in vivo. Infect Immun. 1996;64:3897–3900. doi: 10.1128/iai.64.9.3897-3900.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schito M L, Barta J R. Nonspecific immune responses and mechanisms of resistance to Eimeria papillata infections in mice. Infect Immun. 1997;65:3165–3170. doi: 10.1128/iai.65.8.3165-3170.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sjöstedt A, Conlan J W, North R J. Neutrophils are critical for host defense against primary infection with the facultative intracellular bacterium Francisella tularensis in mice and participate in defense against reinfection. Infect Immun. 1994;62:2779–2783. doi: 10.1128/iai.62.7.2779-2783.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tepper R I, Coffman R L, Leder P. An eosinophil-dependent mechanism for the antitumor effect of interleukin-4. Science. 1992;240:516–518. doi: 10.1126/science.1636093. [DOI] [PubMed] [Google Scholar]
- 33.Thomas J, Gangappa S, Kanangat S, Rouse B T. On the essential involvement of neutrophils in the immunopathologic disease: herpetic stromal keratitis. J Immunol. 1997;158:1383–1391. [PubMed] [Google Scholar]
- 34.Tobias L, Cordes D O, Schurig G G. Placental pathology of the pregnant mouse inoculated with Brucella abortus strain 2308. Vet Pathol. 1993;30:119–129. doi: 10.1177/030098589303000204. [DOI] [PubMed] [Google Scholar]
- 35.Tuffrey M, Taylor-Robinson D. Progesterone is a key factor in the development of a mouse model for genital-tract infection with Chlamydia trachomatis. FEMS Microbiol Lett. 1981;12:111–115. [Google Scholar]
- 36.Williams D M, Magee D M, Bonewald L F, Smith J G, Bleicker C A, Byrne G I, Schachter J. A role in vivo for tumor necrosis factor alpha in host defense against Chlamydia trachomatis. Infect Immun. 1990;58:1572–1576. doi: 10.1128/iai.58.6.1572-1576.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wong S Y, Gray E S, Buxton D, Finlayson J, Johnson F W A. Acute placentitis and spontaneous abortion caused by Chlamydia psittaci: a histological and ultrastructural study. J Clin Pathol. 1985;38:707–711. doi: 10.1136/jcp.38.6.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xing Z, Kirpalani H, Torry D, Jordana M, Gauldie J. Polymorphonuclear leukocytes as a significant source of tumor necrosis factor-α in endotoxin-challenged lung tissue. Am J Pathol. 1993;143:1009–1015. [PMC free article] [PubMed] [Google Scholar]
- 39.Yeaman G R, Collins J E, Currie J K, Guyre P M, Wira C R, Fanger M W. IFN-γ is produced by polymorphonuclear neutrophils in human uterine endometrium and by cultured peripheral blood polymorphonuclear neutrophils. J Immunol. 1998;160:5145–5153. [PubMed] [Google Scholar]
- 40.Yong E C, Chi E Y, Chen W-J, Kuo C-C. Degradation of Chlamydia trachomatis in human polymorphonuclear leukocytes: an ultrastructural study of perioxidase-positive phagolysosomes. Infect Immun. 1986;53:427–431. doi: 10.1128/iai.53.2.427-431.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]