Abstract
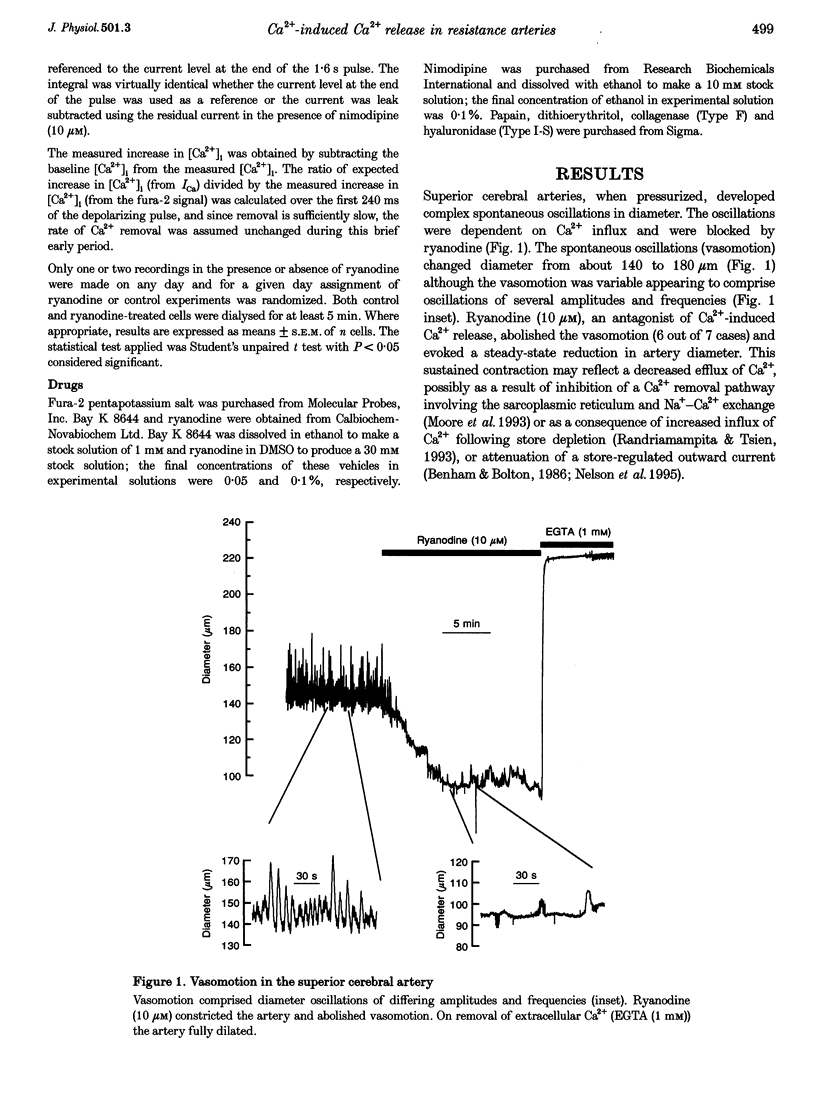
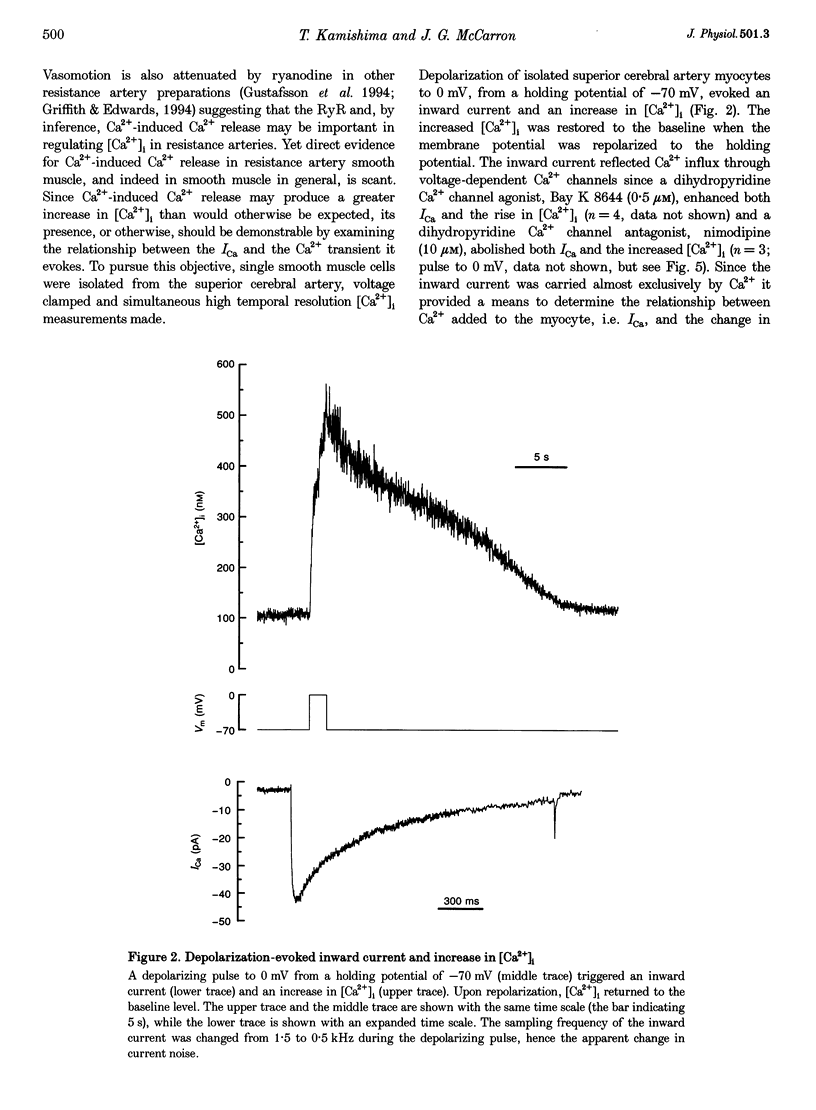
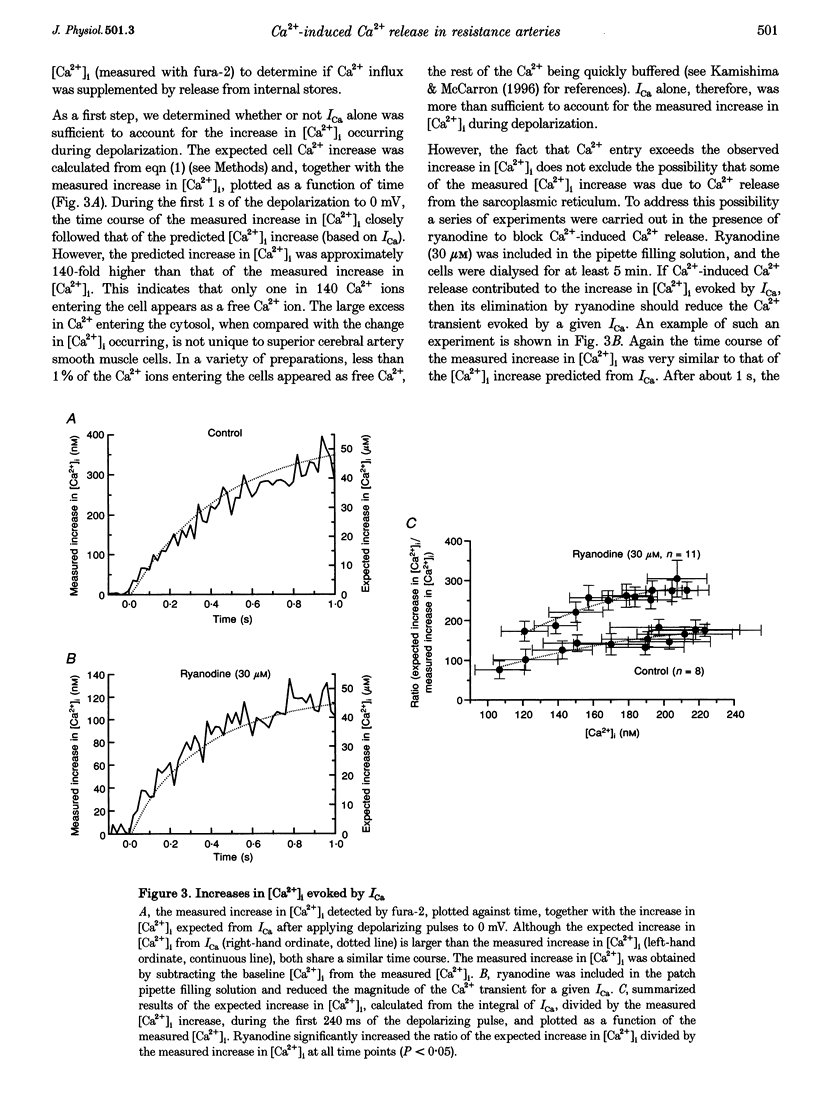
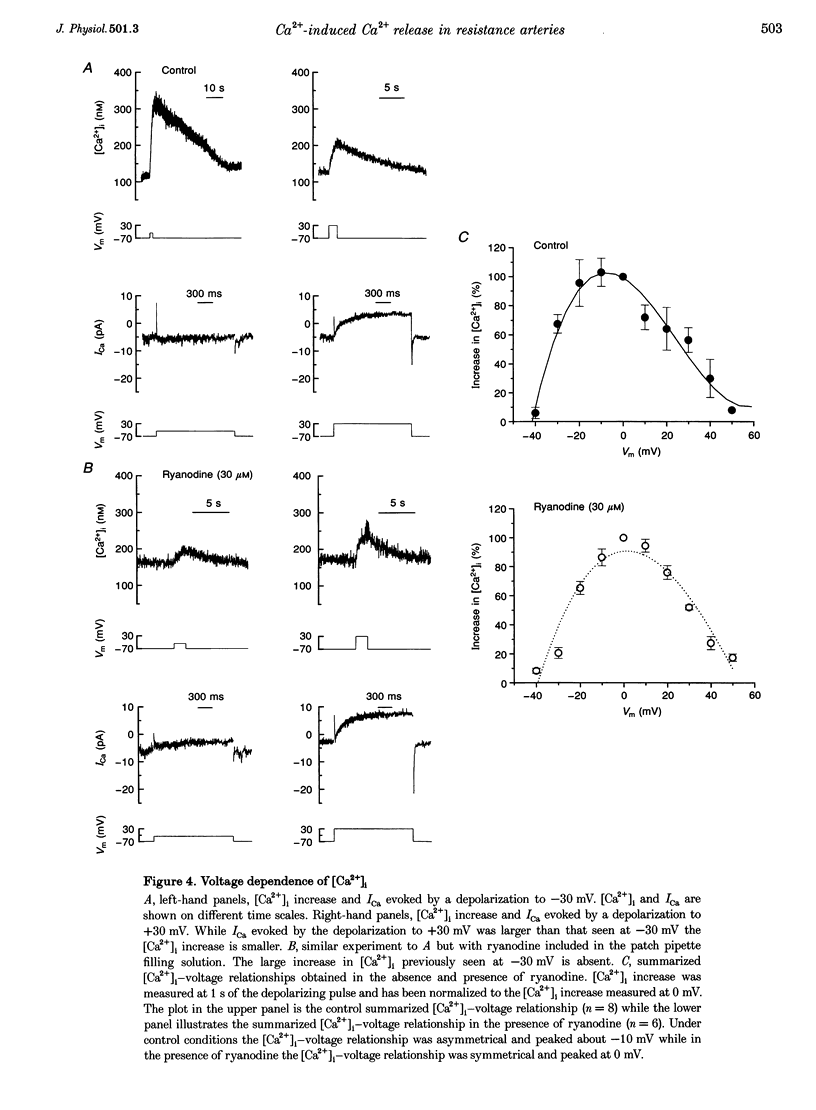
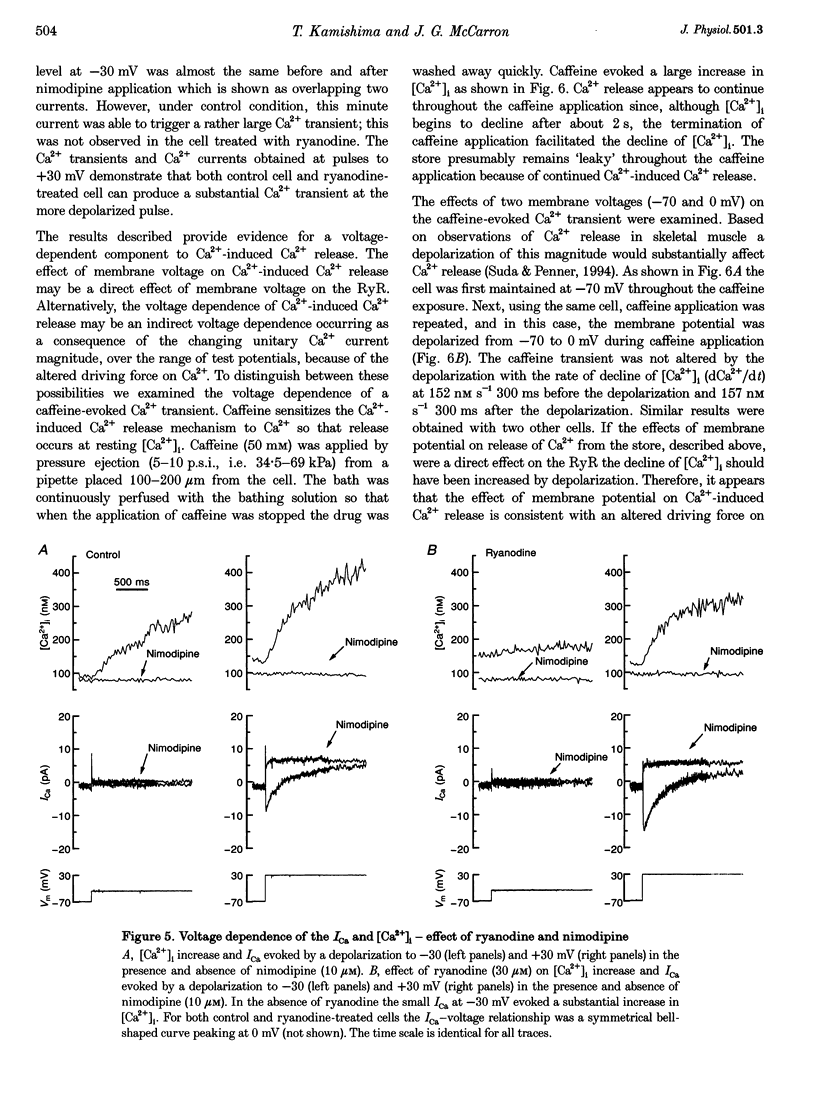
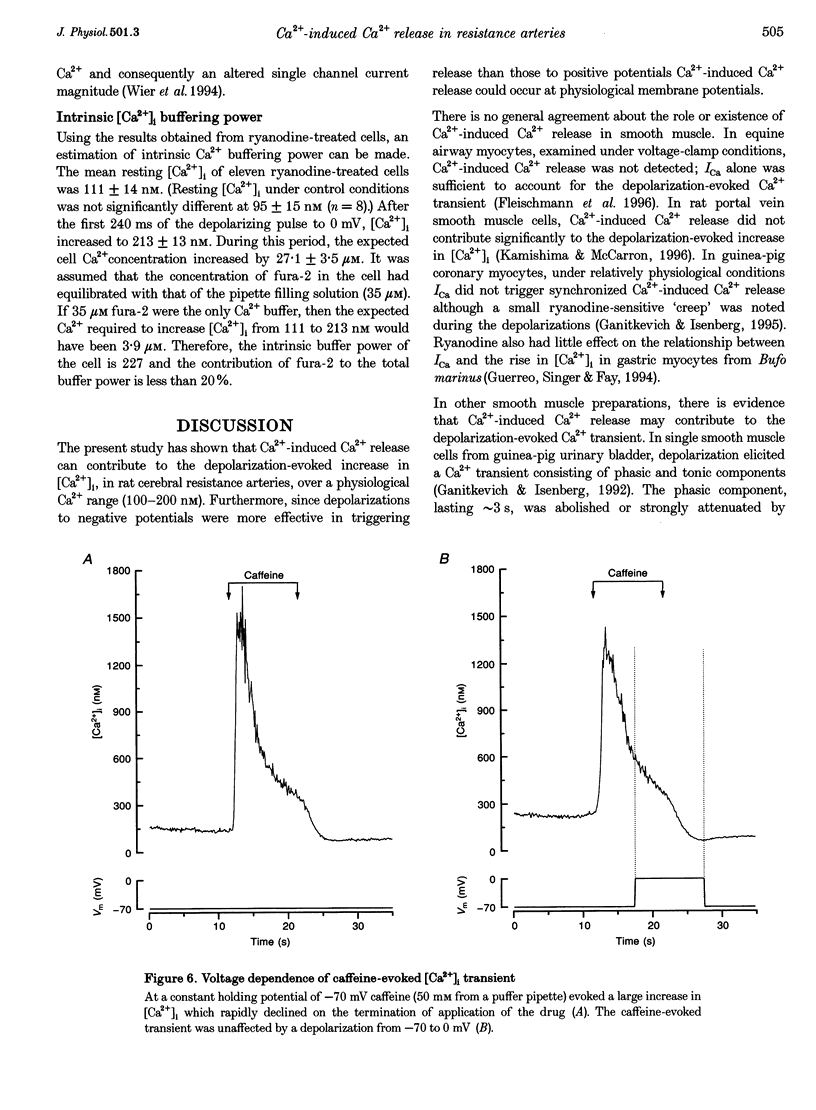
1. There is no general agreement on the presence or role of Ca(2+)-induced Ca2+ release in smooth muscle. In this paper, Ca(2+)-induced Ca2+ release has been investigated in rat resistance-sized superior cerebral arteries to determine its role in regulating the cytosolic Ca2+ concentration ([Ca2+]i). 2. Pressurized superior cerebral arteries developed spontaneous oscillations in diameter. These oscillations were abolished by ryanodine (an inhibitor of Ca(2+)-induced Ca2+ release) and removal of extracellular Ca2+. This suggests, indirectly, that Ca(2+)-induced Ca2+ release may regulate [Ca2+]i in the resistance arteries. 3. To determine if Ca(2+)-induced Ca2+ release could regulate [Ca2+]i, single smooth muscle cells were isolated from the superior cerebral artery, voltage clamped in the whole cell configuration and high temporal resolution [Ca2+]i measurements made. The relationship between the Ca2+ current (ICa) and rise in [Ca2+]i was examined. 4. Depolarization triggered ICa and increased [Ca2+]i. The time course of the measured increase in [Ca2+]i closely followed the increase in [Ca2+]i expected from the time-integrated ICa, although about 140-fold more Ca2+ entered the cytosol than appeared as free Ca2+. When the cells were dialysed with ryanodine (30 microM), the Ca2+ transient evoked by the ICa was substantially reduced indicating that Ca2+ influx triggered Ca2+ release from an internal store. 5. Voltage pulses to negative membrane potentials were more effective in triggering Ca2+ release than pulses to positive potentials suggesting that the Ca(2+)-induced Ca2+ release was voltage dependent. However, the release of Ca2+ from the internal store triggered by caffeine was voltage independent. These results suggest that the voltage dependence of Ca2+ release is indirect and possibly related to the plasmalemma unitary Ca2+ current magnitude. 6. The results establish that Ca(2+)-induced Ca2+ release contributes to depolarization-evoked increases in [Ca2+]i in rat resistance-sized superior cerebral arteries over the physiological [Ca2+]i range (100-200 nM). Compared with more positive membrane potentials the efficacy of Ca2+ in triggering release is high at physiological membrane potentials.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barcenas-Ruiz L., Wier W. G. Voltage dependence of intracellular [Ca2+]i transients in guinea pig ventricular myocytes. Circ Res. 1987 Jul;61(1):148–154. doi: 10.1161/01.res.61.1.148. [DOI] [PubMed] [Google Scholar]
- Benham C. D., Bolton T. B. Spontaneous transient outward currents in single visceral and vascular smooth muscle cells of the rabbit. J Physiol. 1986 Dec;381:385–406. doi: 10.1113/jphysiol.1986.sp016333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boels P. J., Troschka M., Rüegg J. C., Pfitzer G. Higher Ca2+ sensitivity of triton-skinned guinea pig mesenteric microarteries as compared with large arteries. Circ Res. 1991 Oct;69(4):989–996. doi: 10.1161/01.res.69.4.989. [DOI] [PubMed] [Google Scholar]
- Cannell M. B., Berlin J. R., Lederer W. J. Effect of membrane potential changes on the calcium transient in single rat cardiac muscle cells. Science. 1987 Dec 4;238(4832):1419–1423. doi: 10.1126/science.2446391. [DOI] [PubMed] [Google Scholar]
- Cannell M. B., Cheng H., Lederer W. J. Spatial non-uniformities in [Ca2+]i during excitation-contraction coupling in cardiac myocytes. Biophys J. 1994 Nov;67(5):1942–1956. doi: 10.1016/S0006-3495(94)80677-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannell M. B., Cheng H., Lederer W. J. The control of calcium release in heart muscle. Science. 1995 May 19;268(5213):1045–1049. doi: 10.1126/science.7754384. [DOI] [PubMed] [Google Scholar]
- Carrington W. A., Lynch R. M., Moore E. D., Isenberg G., Fogarty K. E., Fay F. S. Superresolution three-dimensional images of fluorescence in cells with minimal light exposure. Science. 1995 Jun 9;268(5216):1483–1487. doi: 10.1126/science.7770772. [DOI] [PubMed] [Google Scholar]
- Cheng H., Lederer W. J., Cannell M. B. Calcium sparks: elementary events underlying excitation-contraction coupling in heart muscle. Science. 1993 Oct 29;262(5134):740–744. doi: 10.1126/science.8235594. [DOI] [PubMed] [Google Scholar]
- Devine C. E., Somlyo A. V., Somlyo A. P. Sarcoplasmic reticulum and excitation-contraction coupling in mammalian smooth muscles. J Cell Biol. 1972 Mar;52(3):690–718. doi: 10.1083/jcb.52.3.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards F. R., Hirst G. D., Silverberg G. D. Inward rectification in rat cerebral arterioles; involvement of potassium ions in autoregulation. J Physiol. 1988 Oct;404:455–466. doi: 10.1113/jphysiol.1988.sp017299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A. Calcium-induced release of calcium from the cardiac sarcoplasmic reticulum. Am J Physiol. 1983 Jul;245(1):C1–14. doi: 10.1152/ajpcell.1983.245.1.C1. [DOI] [PubMed] [Google Scholar]
- Fleischmann B. K., Wang Y. X., Pring M., Kotlikoff M. I. Voltage-dependent calcium currents and cytosolic calcium in equine airway myocytes. J Physiol. 1996 Apr 15;492(Pt 2):347–358. doi: 10.1113/jphysiol.1996.sp021313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganitkevich VYa, Isenberg G. Efficacy of peak Ca2+ currents (ICa) as trigger of sarcoplasmic reticulum Ca2+ release in myocytes from the guinea-pig coronary artery. J Physiol. 1995 Apr 15;484(Pt 2):287–306. doi: 10.1113/jphysiol.1995.sp020665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganitkevich V. Y., Isenberg G. Contribution of Ca(2+)-induced Ca2+ release to the [Ca2+]i transients in myocytes from guinea-pig urinary bladder. J Physiol. 1992 Dec;458:119–137. doi: 10.1113/jphysiol.1992.sp019409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith T. M., Edwards D. H. Fractal analysis of role of smooth muscle Ca2+ fluxes in genesis of chaotic arterial pressure oscillations. Am J Physiol. 1994 May;266(5 Pt 2):H1801–H1811. doi: 10.1152/ajpheart.1994.266.5.H1801. [DOI] [PubMed] [Google Scholar]
- Guerrero A., Singer J. J., Fay F. S. Simultaneous measurement of Ca2+ release and influx into smooth muscle cells in response to caffeine. A novel approach for calculating the fraction of current carried by calcium. J Gen Physiol. 1994 Aug;104(2):395–422. doi: 10.1085/jgp.104.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson H., Bülow A., Nilsson H. Rhythmic contractions of isolated, pressurized small arteries from rat. Acta Physiol Scand. 1994 Oct;152(2):145–152. doi: 10.1111/j.1748-1716.1994.tb09794.x. [DOI] [PubMed] [Google Scholar]
- Györke S., Fill M. Ryanodine receptor adaptation: control mechanism of Ca(2+)-induced Ca2+ release in heart. Science. 1993 May 7;260(5109):807–809. doi: 10.1126/science.8387229. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Iino M. Calcium-induced calcium release mechanism in guinea pig taenia caeci. J Gen Physiol. 1989 Aug;94(2):363–383. doi: 10.1085/jgp.94.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamishima T., McCarron J. G. Depolarization-evoked increases in cytosolic calcium concentration in isolated smooth muscle cells of rat portal vein. J Physiol. 1996 Apr 1;492(Pt 1):61–74. doi: 10.1113/jphysiol.1996.sp021289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-López J. R., Shacklock P. S., Balke C. W., Wier W. G. Local calcium transients triggered by single L-type calcium channel currents in cardiac cells. Science. 1995 May 19;268(5213):1042–1045. doi: 10.1126/science.7754383. [DOI] [PubMed] [Google Scholar]
- Moore E. D., Etter E. F., Philipson K. D., Carrington W. A., Fogarty K. E., Lifshitz L. M., Fay F. S. Coupling of the Na+/Ca2+ exchanger, Na+/K+ pump and sarcoplasmic reticulum in smooth muscle. Nature. 1993 Oct 14;365(6447):657–660. doi: 10.1038/365657a0. [DOI] [PubMed] [Google Scholar]
- Nelson M. T., Cheng H., Rubart M., Santana L. F., Bonev A. D., Knot H. J., Lederer W. J. Relaxation of arterial smooth muscle by calcium sparks. Science. 1995 Oct 27;270(5236):633–637. doi: 10.1126/science.270.5236.633. [DOI] [PubMed] [Google Scholar]
- Niggli E., Lederer W. J. Voltage-independent calcium release in heart muscle. Science. 1990 Oct 26;250(4980):565–568. doi: 10.1126/science.2173135. [DOI] [PubMed] [Google Scholar]
- Poenie M. Alteration of intracellular Fura-2 fluorescence by viscosity: a simple correction. Cell Calcium. 1990 Feb-Mar;11(2-3):85–91. doi: 10.1016/0143-4160(90)90062-y. [DOI] [PubMed] [Google Scholar]
- Quayle J. M., Bonev A. D., Brayden J. E., Nelson M. T. Calcitonin gene-related peptide activated ATP-sensitive K+ currents in rabbit arterial smooth muscle via protein kinase A. J Physiol. 1994 Feb 15;475(1):9–13. doi: 10.1113/jphysiol.1994.sp020045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quayle J. M., Dart C., Standen N. B. The properties and distribution of inward rectifier potassium currents in pig coronary arterial smooth muscle. J Physiol. 1996 Aug 1;494(Pt 3):715–726. doi: 10.1113/jphysiol.1996.sp021527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randriamampita C., Tsien R. Y. Emptying of intracellular Ca2+ stores releases a novel small messenger that stimulates Ca2+ influx. Nature. 1993 Aug 26;364(6440):809–814. doi: 10.1038/364809a0. [DOI] [PubMed] [Google Scholar]
- Suda N., Penner R. Membrane repolarization stops caffeine-induced Ca2+ release in skeletal muscle cells. Proc Natl Acad Sci U S A. 1994 Jun 7;91(12):5725–5729. doi: 10.1073/pnas.91.12.5725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trafford A. W., O'Neill S. C., Eisner D. A. Factors affecting the propagation of locally activated systolic Ca transients in rat ventricular myocytes. Pflugers Arch. 1993 Oct;425(1-2):181–183. doi: 10.1007/BF00374521. [DOI] [PubMed] [Google Scholar]
- Wier W. G., Egan T. M., López-López J. R., Balke C. W. Local control of excitation-contraction coupling in rat heart cells. J Physiol. 1994 Feb 1;474(3):463–471. doi: 10.1113/jphysiol.1994.sp020037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasui K., Palade P., Györke S. Negative control mechanism with features of adaptation controls Ca2+ release in cardiac myocytes. Biophys J. 1994 Jul;67(1):457–460. doi: 10.1016/S0006-3495(94)80501-6. [DOI] [PMC free article] [PubMed] [Google Scholar]


