Abstract
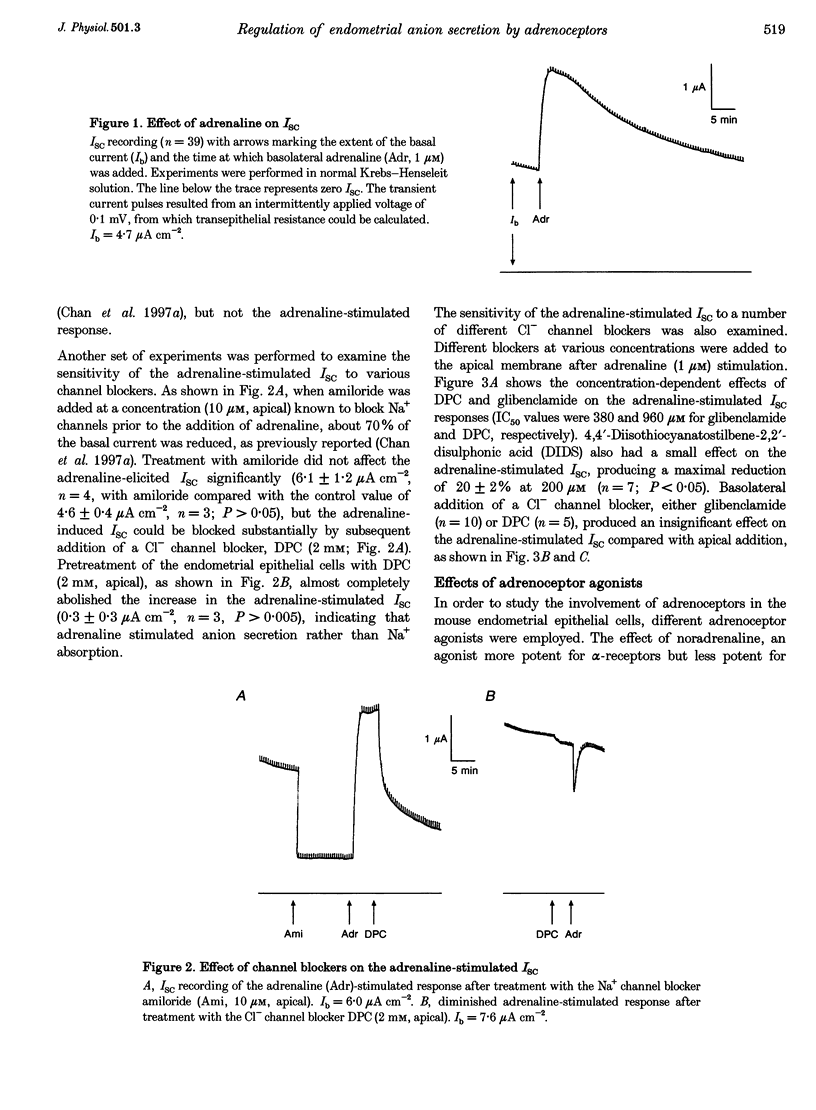
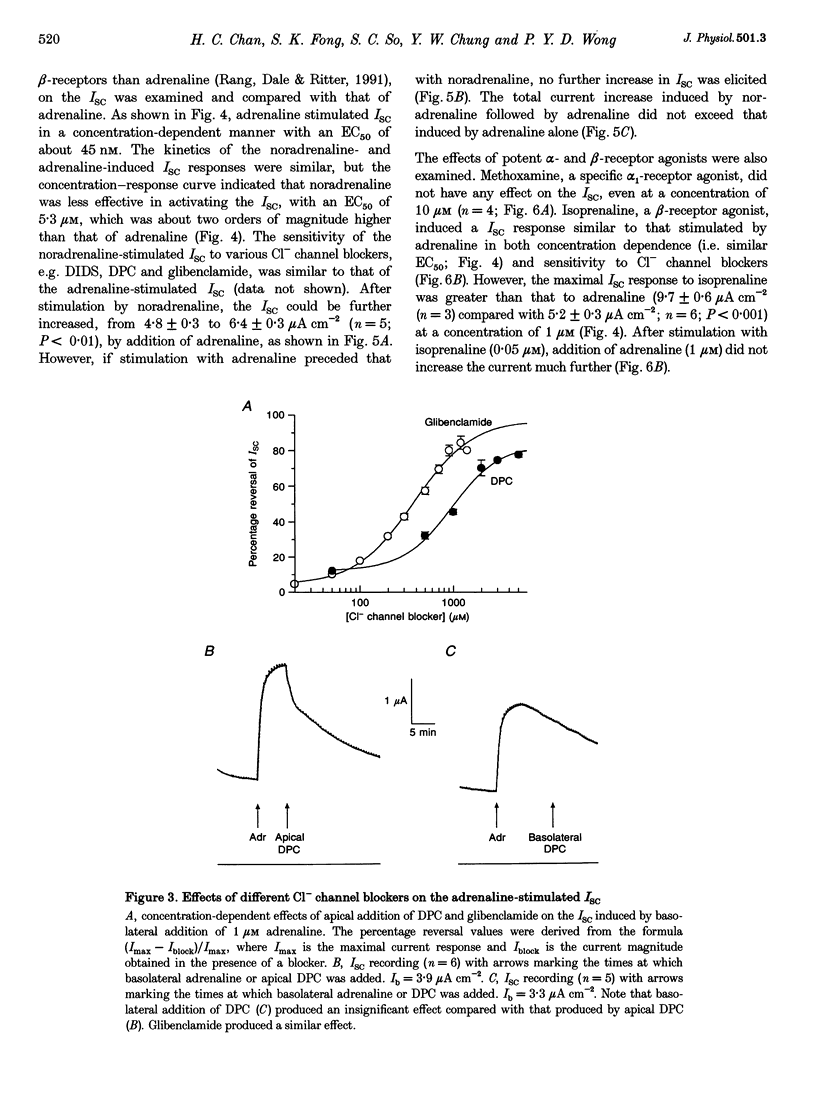
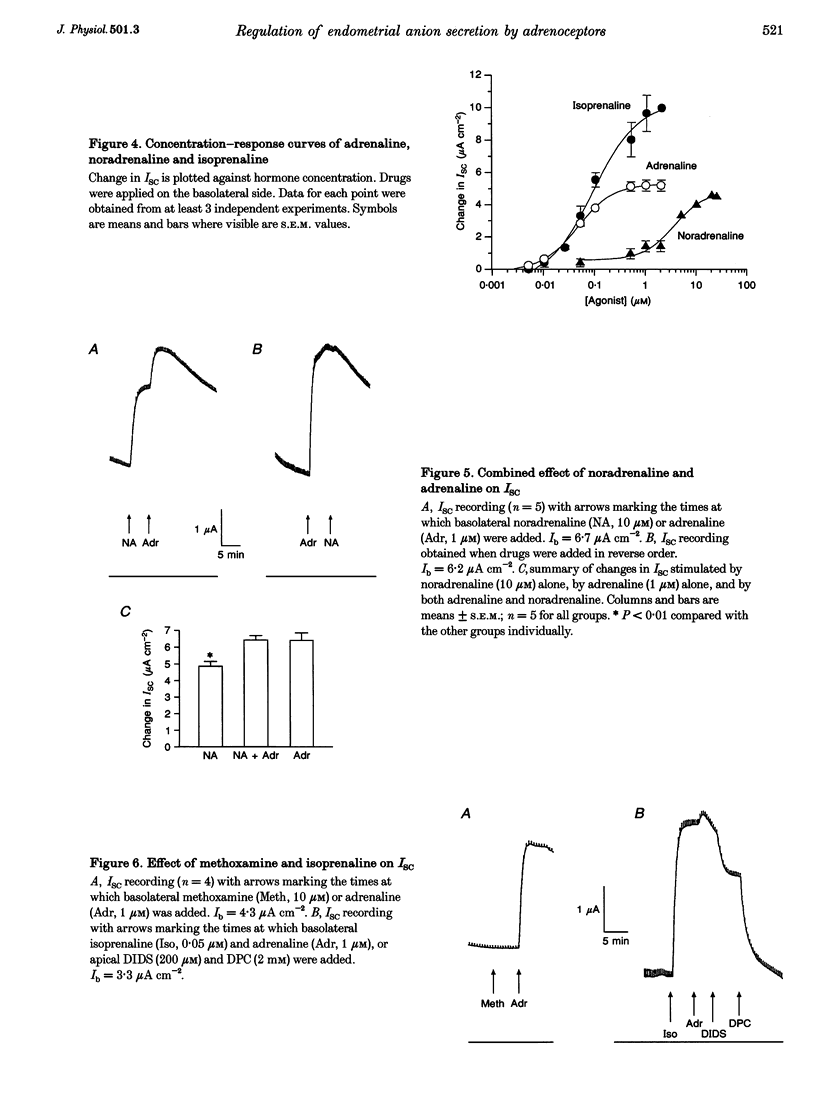
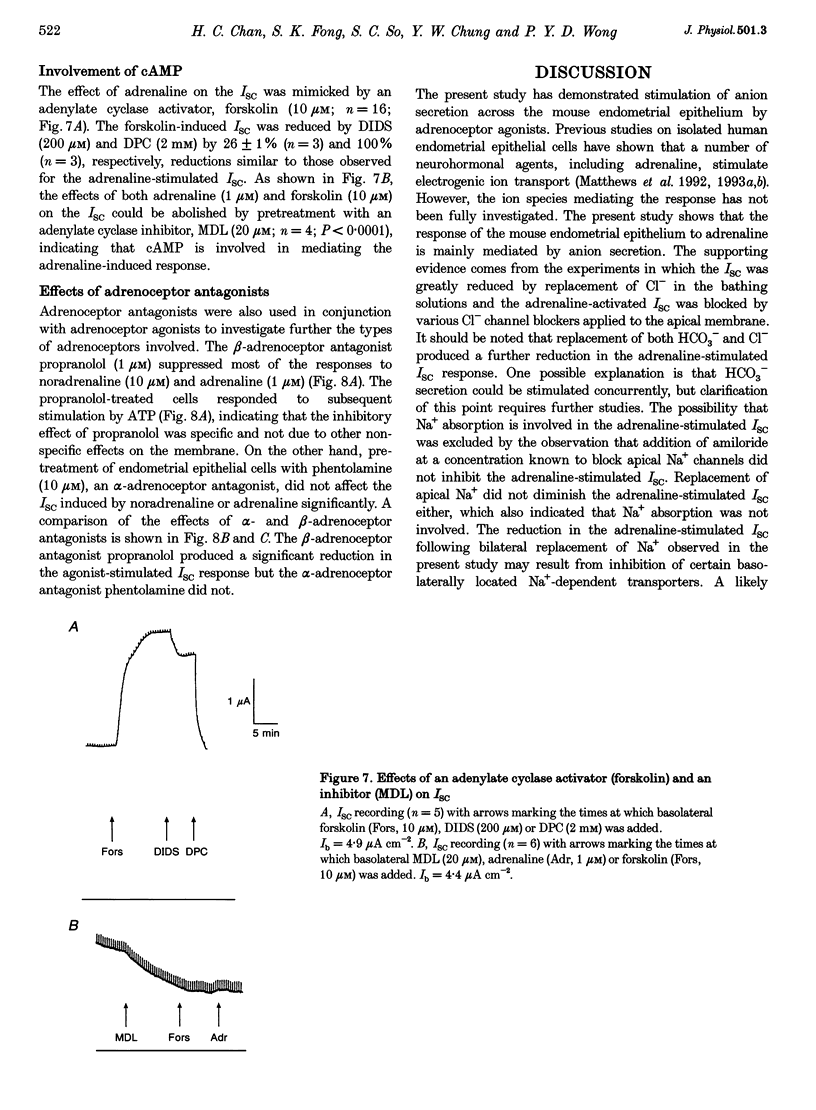
1. Regulation of anion secretion by adrenoceptors in primary culture of mouse endometrial epithelium was investigated using the short circuit current (ISC) technique. 2. Adrenaline stimulated a sustained increase in the ISC in a concentration-dependent manner. The adrenaline-induced ISC could be inhibited by pretreatment with diphenylamine 2,2'-dicarboxylic acid (DPC) or replacement of external Cl- and HCO3-, but not by amiloride or replacement of Na+ in apical solution. 3. The concentration-dependent responses of the adrenaline-induced ISC to the Cl- channel blockers glibenclamide and DPC were examined and exhibited IC50 values of 380 and 960 microM, respectively. 4. The effect of various adrenoceptor agonists on the ISC was examined. The order of potency appeared to be isoprenaline > adrenaline > noradrenaline, while no response was elicited by the alpha-adrenoceptor agonist methoxamine, indicating a predominant involvement of beta-adrenoceptors. 5. The beta-adrenoceptor antagonist propranolol was found to be much more effective than the alpha-adrenoceptor antagonist phentolamine in inhibiting the ISC responses induced by all adrenoceptor agonists examined. 6. The effect of adrenaline on the ISC was mimicked by an adenylate cyclase activator, forskolin, but suppressed by the adenylate cyclase inhibitor MDL 12,330A, indicating the involvement of cAMP. 7. Our results demonstrate that anion secretion by the mouse endometrial epithelium is regulated by beta-adrenoceptors and involves a cAMP-dependent mechanism.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bear C. E., Li C. H., Kartner N., Bridges R. J., Jensen T. J., Ramjeesingh M., Riordan J. R. Purification and functional reconstitution of the cystic fibrosis transmembrane conductance regulator (CFTR). Cell. 1992 Feb 21;68(4):809–818. doi: 10.1016/0092-8674(92)90155-6. [DOI] [PubMed] [Google Scholar]
- Casslén B., Nilsson B. Human uterine fluid, examined in undiluted samples for osmolarity and the concentrations of inorganic ions, albumin, glucose, and urea. Am J Obstet Gynecol. 1984 Dec 1;150(7):877–881. doi: 10.1016/0002-9378(84)90466-6. [DOI] [PubMed] [Google Scholar]
- Chan H. C., Liu C. Q., Fong S. K., Law S. H., Wu L. J., So E., Chung Y. W., Ko W. H., Wong P. Y. Regulation of Cl- secretion by extracellular ATP in cultured mouse endometrial epithelium. J Membr Biol. 1997 Mar 1;156(1):45–52. doi: 10.1007/s002329900186. [DOI] [PubMed] [Google Scholar]
- Fuller C. M., Benos D. J. CFTR! Am J Physiol. 1992 Aug;263(2 Pt 1):C267–C286. doi: 10.1152/ajpcell.1992.263.2.C267. [DOI] [PubMed] [Google Scholar]
- Iritani A., Sato E., Nishikawa Y. Secretion rates and chemical composition of oviduct and uterine fluids in sows. J Anim Sci. 1974 Sep;39(3):582–588. doi: 10.2527/jas1974.393582x. [DOI] [PubMed] [Google Scholar]
- Ishiguro H., Steward M. C., Wilson R. W., Case R. M. Bicarbonate secretion in interlobular ducts from guinea-pig pancreas. J Physiol. 1996 Aug 15;495(Pt 1):179–191. doi: 10.1113/jphysiol.1996.sp021583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin R. J., Phillips J. C. Rat endometrial bioelectric activity in vivo and in vitro: effects of adrenaline. J Physiol. 1983 Mar;336:465–478. doi: 10.1113/jphysiol.1983.sp014591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews C. J., Thomas E. J., Redfern C. P., Hirst B. H. Ion transport by human endometrial epithelia in vitro. Hum Reprod. 1993 Oct;8(10):1570–1575. doi: 10.1093/oxfordjournals.humrep.a137893. [DOI] [PubMed] [Google Scholar]
- McCormack S. A., Glasser S. R. Differential response of individual uterine cell types from immature rats treated with estradiol. Endocrinology. 1980 May;106(5):1634–1649. doi: 10.1210/endo-106-5-1634. [DOI] [PubMed] [Google Scholar]
- Nilsson B. O., Ljung L. X-ray micro analyses of cations (Na, K, Ca) and anions (S, P, Cl) in uterine secretions during blastocyst implantation in the rat. J Exp Zool. 1985 Jun;234(3):415–421. doi: 10.1002/jez.1402340309. [DOI] [PubMed] [Google Scholar]
- Nordenvall M., Ulmsten U., Ungerstedt U. Influence of progesterone on the sodium and potassium concentrations of rat uterine fluid investigated by microdialysis. Gynecol Obstet Invest. 1989;28(2):73–77. doi: 10.1159/000293518. [DOI] [PubMed] [Google Scholar]
- Sheppard D. N., Welsh M. J. Effect of ATP-sensitive K+ channel regulators on cystic fibrosis transmembrane conductance regulator chloride currents. J Gen Physiol. 1992 Oct;100(4):573–591. doi: 10.1085/jgp.100.4.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tizzano E. F., Chitayat D., Buchwald M. Cell-specific localization of CFTR mRNA shows developmentally regulated expression in human fetal tissues. Hum Mol Genet. 1993 Mar;2(3):219–224. doi: 10.1093/hmg/2.3.219. [DOI] [PubMed] [Google Scholar]
- Trezise A. E., Linder C. C., Grieger D., Thompson E. W., Meunier H., Griswold M. D., Buchwald M. CFTR expression is regulated during both the cycle of the seminiferous epithelium and the oestrous cycle of rodents. Nat Genet. 1993 Feb;3(2):157–164. doi: 10.1038/ng0293-157. [DOI] [PubMed] [Google Scholar]
- USSING H. H., ZERAHN K. Active transport of sodium as the source of electric current in the short-circuited isolated frog skin. Acta Physiol Scand. 1951 Aug 25;23(2-3):110–127. doi: 10.1111/j.1748-1716.1951.tb00800.x. [DOI] [PubMed] [Google Scholar]
- VISHWAKARMA P. The pH and bicarbonate-ion content of the oviduct and uterine fluids. Fertil Steril. 1962 Sep-Oct;13:481–485. doi: 10.1016/s0015-0282(16)34633-7. [DOI] [PubMed] [Google Scholar]
- Vetter A. E., O'Grady S. M. Mechanisms of electrolyte transport across the endometrium. I. Regulation by PGF2 alpha and cAMP. Am J Physiol. 1996 Feb;270(2 Pt 1):C663–C672. doi: 10.1152/ajpcell.1996.270.2.C663. [DOI] [PubMed] [Google Scholar]
- Welsh M. J. Electrolyte transport by airway epithelia. Physiol Rev. 1987 Oct;67(4):1143–1184. doi: 10.1152/physrev.1987.67.4.1143. [DOI] [PubMed] [Google Scholar]


