Abstract
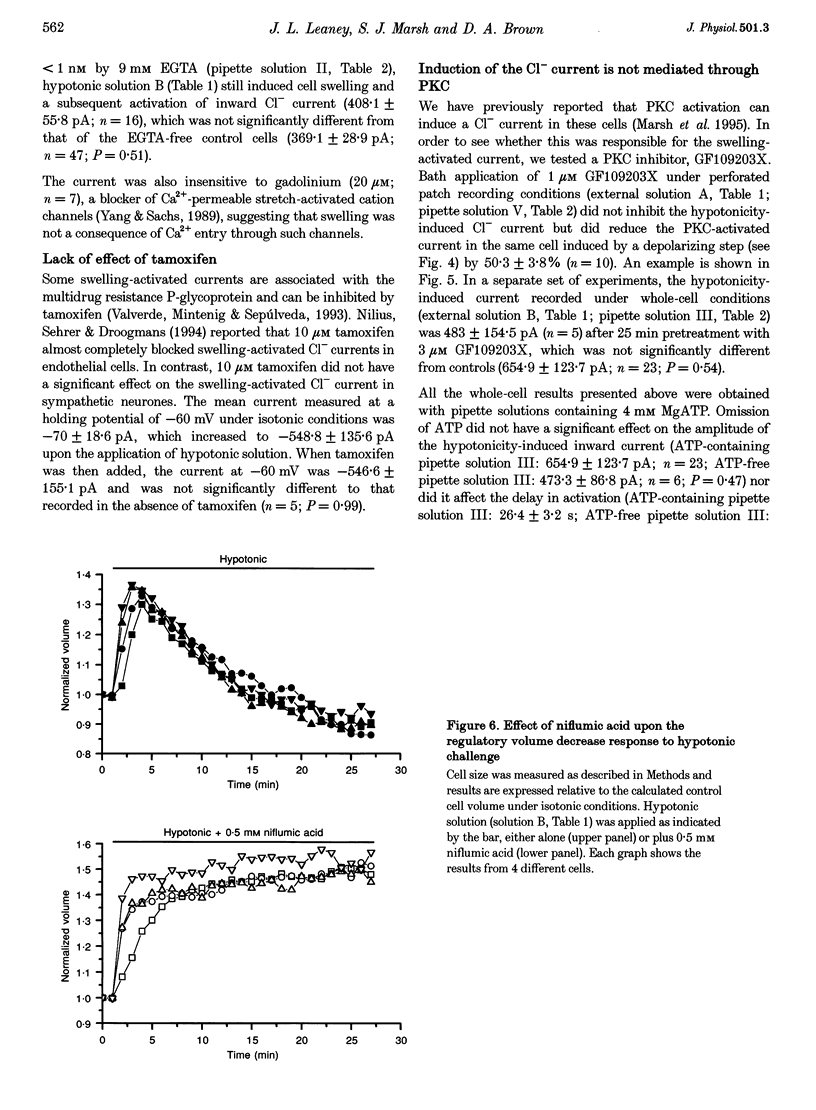
1. We have tested whether neurones show a swelling-induced Cl- current following hypotonic shock, by recording membrane current responses and cell volume changes in voltage clamped isolated rat sympathetic neurones during application of hypotonic solutions. 2. Using both whole-cell and perforated patch recording methods, hypotonic solution caused cell swelling and the activation of an inward Cl- current at -60 mV. This current showed weak outward rectification with no obvious time dependence. It was inhibited by SITS (0.3-1 mM), NPPB (30-300 microM) and niflumic acid (50-200 microM), but not by tamoxifen (10 microM). 3. Hypotonic solution did not cause a rise in intracellular Ca2+ concentration as measured by simultaneous indo-1 fluorescence. Also, neither the volume change nor Cl- current were affected by the removal of external Ca2+ or internal Ca2+ buffering to < or = 1 nM with EGTA. 4. The Cl- current was unaffected by an inhibitor of protein kinase C (PKC; GF109203X, 3 microM) or by omission of ATP from the pipette solution. 5. Cells exhibited a regulatory volume decrease during sustained exposure to hypotonic solution. This was completely inhibited by 0.5 mM niflumic acid. 6. It is concluded that osmotic swelling induces an outwardly rectifying, Ca2(+)- and PKC-independent Cl- current in these nerve cells. It is suggested that this current may be involved in volume regulatory mechanisms.
Full text
PDF




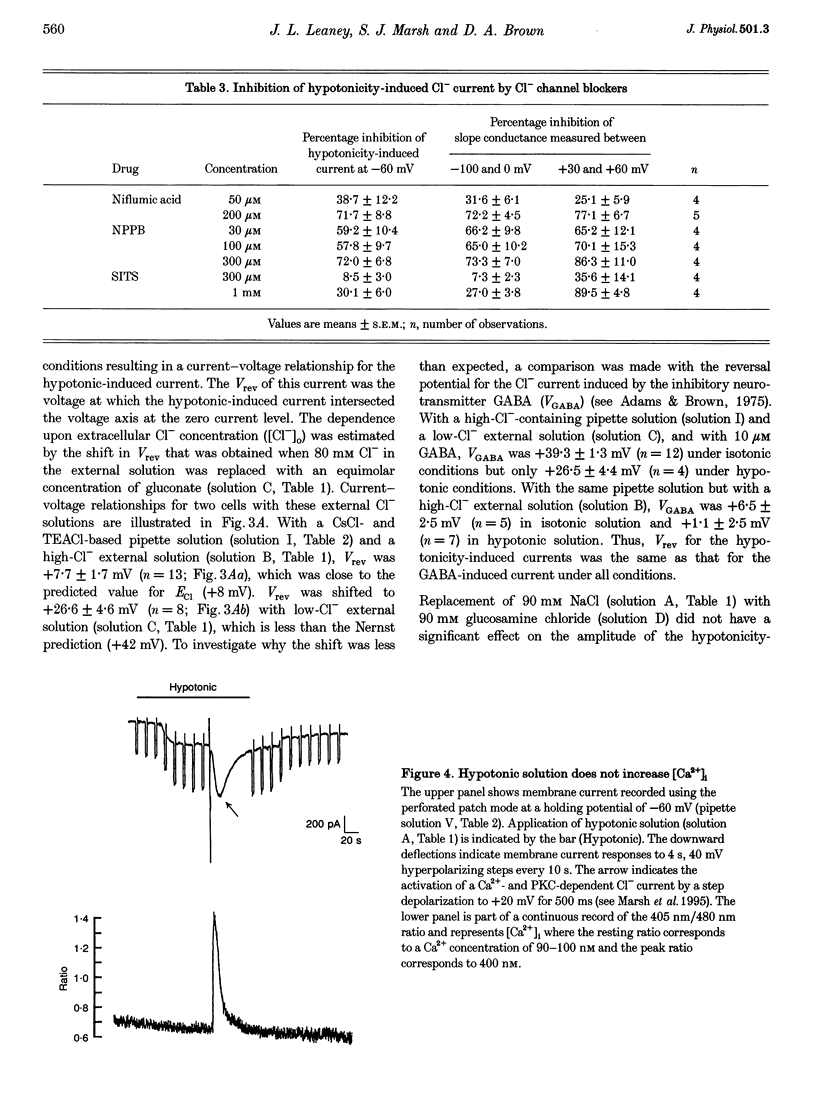



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R., Brown D. A. Actions of gamma-aminobutyric acid on sympathetic ganglion cells. J Physiol. 1975 Aug;250(1):85–120. doi: 10.1113/jphysiol.1975.sp011044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botchkin L. M., Matthews G. Chloride current activated by swelling in retinal pigment epithelium cells. Am J Physiol. 1993 Oct;265(4 Pt 1):C1037–C1045. doi: 10.1152/ajpcell.1993.265.4.C1037. [DOI] [PubMed] [Google Scholar]
- Doroshenko P., Neher E. Volume-sensitive chloride conductance in bovine chromaffin cell membrane. J Physiol. 1992 Apr;449:197–218. doi: 10.1113/jphysiol.1992.sp019082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falke L. C., Misler S. Activity of ion channels during volume regulation by clonal N1E115 neuroblastoma cells. Proc Natl Acad Sci U S A. 1989 May;86(10):3919–3923. doi: 10.1073/pnas.86.10.3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatherazi S., Izutsu K. T., Wellner R. B., Belton C. M. Hypotonically activated chloride current in HSG cells. J Membr Biol. 1994 Nov;142(2):181–193. doi: 10.1007/BF00234940. [DOI] [PubMed] [Google Scholar]
- Gosling M., Poyner D. R., Smith J. W. Effects of arachidonic acid upon the volume-sensitive chloride current in rat osteoblast-like (ROS 17/2.8) cells. J Physiol. 1996 Jun 15;493(Pt 3):613–623. doi: 10.1113/jphysiol.1996.sp021408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Marrion N. V., Smart T. G., Brown D. A. Membrane currents in adult rat superior cervical ganglia in dissociated tissue culture. Neurosci Lett. 1987 Jun 1;77(1):55–60. doi: 10.1016/0304-3940(87)90606-9. [DOI] [PubMed] [Google Scholar]
- Marsh S. J., Trouslard J., Leaney J. L., Brown D. A. Synergistic regulation of a neuronal chloride current by intracellular calcium and muscarinic receptor activation: a role for protein kinase C. Neuron. 1995 Sep;15(3):729–737. doi: 10.1016/0896-6273(95)90160-4. [DOI] [PubMed] [Google Scholar]
- McCarty N. A., O'Neil R. G. Calcium signaling in cell volume regulation. Physiol Rev. 1992 Oct;72(4):1037–1061. doi: 10.1152/physrev.1992.72.4.1037. [DOI] [PubMed] [Google Scholar]
- Nilius B., Sehrer J., Droogmans G. Permeation properties and modulation of volume-activated Cl(-)-currents in human endothelial cells. Br J Pharmacol. 1994 Aug;112(4):1049–1056. doi: 10.1111/j.1476-5381.1994.tb13189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasantes-Morales H., Maar T. E., Morán J. Cell volume regulation in cultured cerebellar granule neurons. J Neurosci Res. 1993 Feb 1;34(2):219–224. doi: 10.1002/jnr.490340209. [DOI] [PubMed] [Google Scholar]
- Pollard C. E. A volume-sensitive Cl- conductance in a mouse neuroblastoma x rat dorsal root ganglion cell line (F11). Brain Res. 1993 Jun 18;614(1-2):178–184. doi: 10.1016/0006-8993(93)91032-n. [DOI] [PubMed] [Google Scholar]
- Robson L., Hunter M. Role of cell volume and protein kinase C in regulation of a Cl- conductance in single proximal tubule cells of Rana temporaria. J Physiol. 1994 Oct 1;480(Pt 1):1–7. doi: 10.1113/jphysiol.1994.sp020335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkadi B., Parker J. C. Activation of ion transport pathways by changes in cell volume. Biochim Biophys Acta. 1991 Dec 12;1071(4):407–427. doi: 10.1016/0304-4157(91)90005-h. [DOI] [PubMed] [Google Scholar]
- Shuba L. M., Ogura T., McDonald T. F. Kinetic evidence distinguishing volume-sensitive chloride current from other types in guinea-pig ventricular myocytes. J Physiol. 1996 Feb 15;491(Pt 1):69–80. doi: 10.1113/jphysiol.1996.sp021197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange K., Emma F., Jackson P. S. Cellular and molecular physiology of volume-sensitive anion channels. Am J Physiol. 1996 Mar;270(3 Pt 1):C711–C730. doi: 10.1152/ajpcell.1996.270.3.C711. [DOI] [PubMed] [Google Scholar]
- Sánchez-Vives M. V., Gallego R. Calcium-dependent chloride current induced by axotomy in rat sympathetic neurons. J Physiol. 1994 Mar 15;475(3):391–400. doi: 10.1113/jphysiol.1994.sp020080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trouslard J., Marsh S. J., Brown D. A. Calcium entry through nicotinic receptor channels and calcium channels in cultured rat superior cervical ganglion cells. J Physiol. 1993 Aug;468:53–71. doi: 10.1113/jphysiol.1993.sp019759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng G. N. Cell swelling increases membrane conductance of canine cardiac cells: evidence for a volume-sensitive Cl channel. Am J Physiol. 1992 Apr;262(4 Pt 1):C1056–C1068. doi: 10.1152/ajpcell.1992.262.4.C1056. [DOI] [PubMed] [Google Scholar]
- Valverde M. A., Mintenig G. M., Sepúlveda F. V. Differential effects of tamoxifen and I- on three distinguishable chloride currents activated in T84 intestinal cells. Pflugers Arch. 1993 Dec;425(5-6):552–554. doi: 10.1007/BF00374885. [DOI] [PubMed] [Google Scholar]
- Yang X. C., Sachs F. Block of stretch-activated ion channels in Xenopus oocytes by gadolinium and calcium ions. Science. 1989 Feb 24;243(4894 Pt 1):1068–1071. doi: 10.1126/science.2466333. [DOI] [PubMed] [Google Scholar]



