Abstract
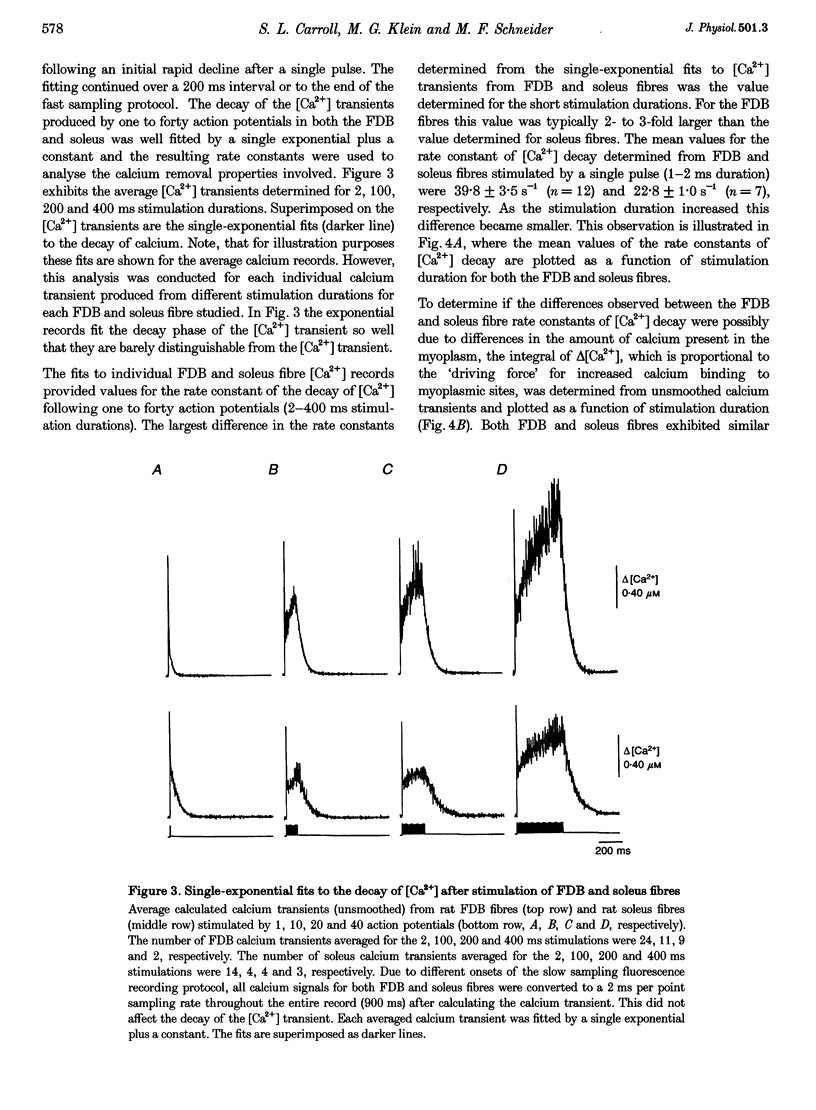
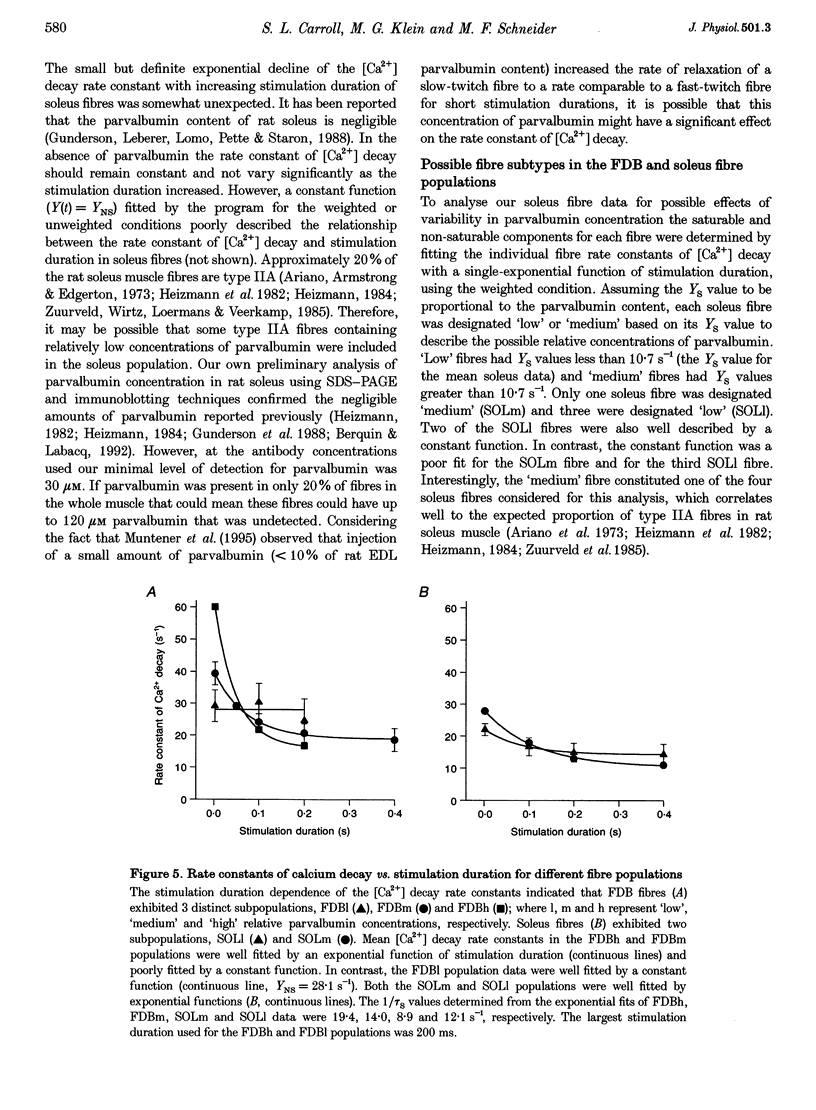
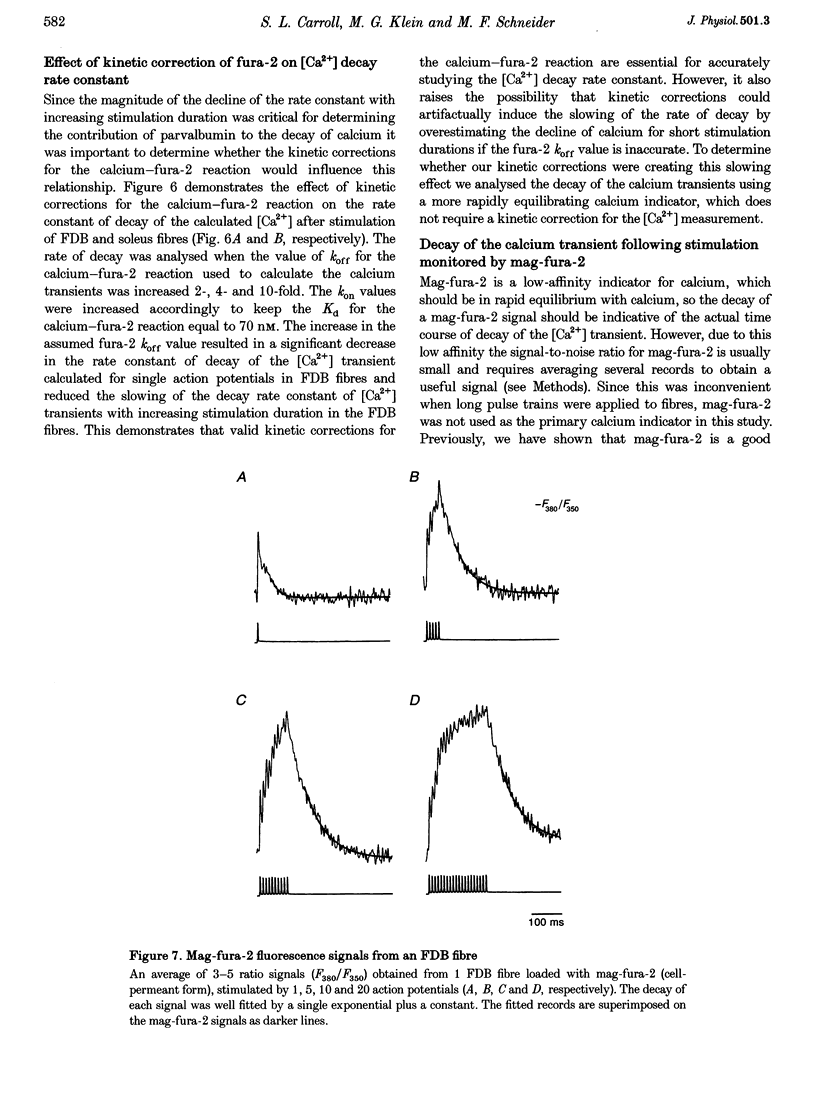
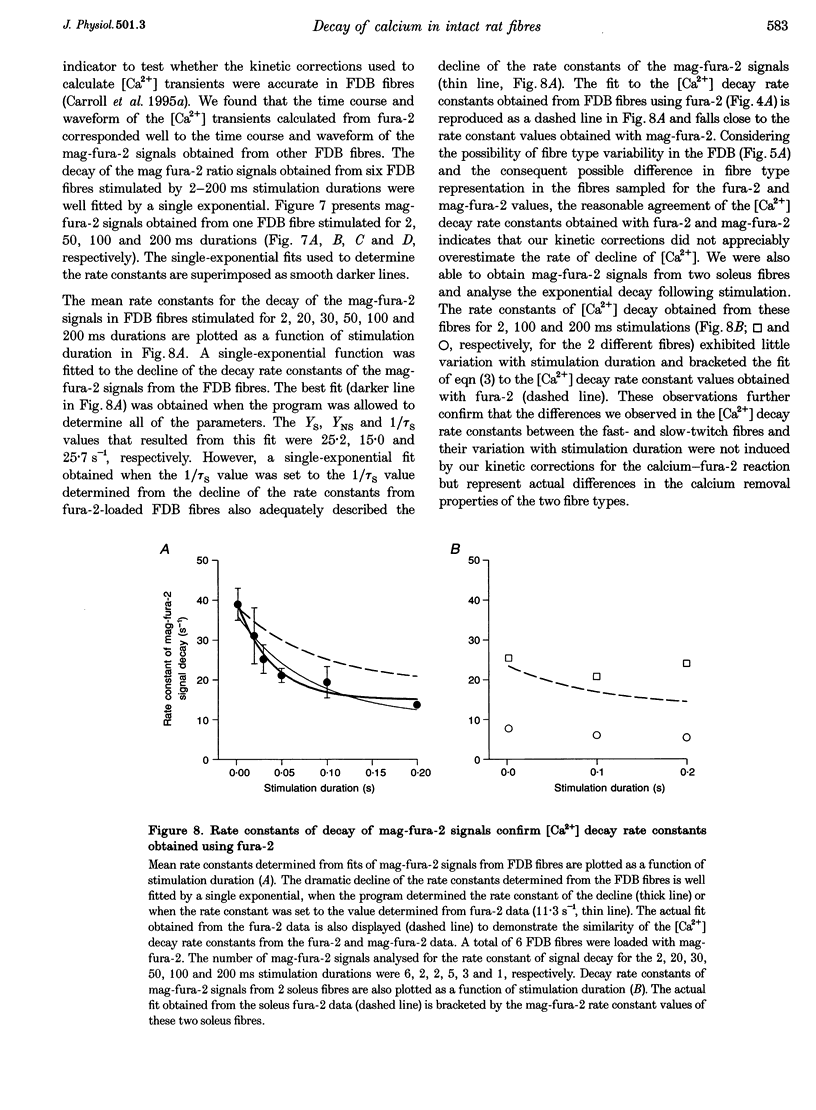
1. Calcium transients were calculated from fura-2 fluorescence signals (corrected for kinetic delays in the Ca(2+)-fura-2 reaction) from single rat skeletal muscle fibres, either fully dissociated from the fast-twitch flexor digitorum brevis (FDB) muscle or in small bundles from the slow-twitch soleus muscle. Fibres or bundles were embedded in agarose gel to inhibit movement and stimulated by single or trains of 1-2 ms electrical pulses (100 Hz, 2-400 ms train duration). 2. The rate constant of decay of [Ca2+] determined from single-exponential fits to the final decay phase of [Ca2+] after a single action potential was considerably faster in FDB fibres than in soleus fibres. As the stimulation duration increased, the rate constant of [Ca2+] decay decreased for both the FDB and soleus fibres, but the effect was greater in FDB than in soleus fibres. 3. Using the magnitude of the decline in the rate constant of [Ca2+] decay with increasing stimulation duration as an index of relative contribution of the saturable Ca2+ binding sites on parvalbumin, subpopulations termed 'high', 'medium' and 'low', referring to estimated parvalbumin content, were determined within each group of FDB and soleus fibres. In fibres assigned to the 'high' and 'medium' groups, parvalbumin was the major contributor (50-73%) to the [Ca2+] decay rate constant after a single action potential. In fibres in the 'low' group, parvalbumin contributed only 0-28% to the rate constant of [Ca2+] decay. 4. Fluorescence recordings using mag-fura-2, a lower-affinity Ca2+ indicator expected to be in equilibrium with myoplasmic Ca2+, gave similar values for both the [Ca2+] decay rate constant after a single action potential and the decrease in this rate constant with increased stimulation duration, as found for the fura-2 [Ca2+] transients from FDB and soleus fibres. Thus, the observed differences in decay rate of Ca2+ were not introduced by kinetic correction of the fura-2 recordings, but are attributed to differences in the Ca2+ binding and transport properties of fast- and slow-twitch mammalian fibres.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ariano M. A., Armstrong R. B., Edgerton V. R. Hindlimb muscle fiber populations of five mammals. J Histochem Cytochem. 1973 Jan;21(1):51–55. doi: 10.1177/21.1.51. [DOI] [PubMed] [Google Scholar]
- Baylor S. M., Chandler W. K., Marshall M. W. Sarcoplasmic reticulum calcium release in frog skeletal muscle fibres estimated from Arsenazo III calcium transients. J Physiol. 1983 Nov;344:625–666. doi: 10.1113/jphysiol.1983.sp014959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berquin A., Lebacq J. Parvalbumin, labile heat and slowing of relaxation in mouse soleus and extensor digitorum longus muscles. J Physiol. 1992 Jan;445:601–616. doi: 10.1113/jphysiol.1992.sp018942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff R. Proliferation of muscle satellite cells on intact myofibers in culture. Dev Biol. 1986 May;115(1):129–139. doi: 10.1016/0012-1606(86)90234-4. [DOI] [PubMed] [Google Scholar]
- Briggs F. N., Poland J. L., Solaro R. J. Relative capabilities of sarcoplasmic reticulum in fast and slow mammalian skeletal muscles. J Physiol. 1977 Apr;266(3):587–594. doi: 10.1113/jphysiol.1977.sp011783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd S. K., Bode A. K., Klug G. A. Effects of exercise of varying duration on sarcoplasmic reticulum function. J Appl Physiol (1985) 1989 Mar;66(3):1383–1389. doi: 10.1152/jappl.1989.66.3.1383. [DOI] [PubMed] [Google Scholar]
- Byrd S. K., McCutcheon L. J., Hodgson D. R., Gollnick P. D. Altered sarcoplasmic reticulum function after high-intensity exercise. J Appl Physiol (1985) 1989 Nov;67(5):2072–2077. doi: 10.1152/jappl.1989.67.5.2072. [DOI] [PubMed] [Google Scholar]
- Cannell M. B. Effect of tetanus duration on the free calcium during the relaxation of frog skeletal muscle fibres. J Physiol. 1986 Jul;376:203–218. doi: 10.1113/jphysiol.1986.sp016149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsen R. C., Larson D. B., Walsh D. A. A fast-twitch oxidative-glycolytic muscle with a robust inward calcium current. Can J Physiol Pharmacol. 1985 Aug;63(8):958–965. doi: 10.1139/y85-158. [DOI] [PubMed] [Google Scholar]
- Carroll S. L., Klein M. G., Schneider M. F. Calcium transients in intact rat skeletal muscle fibers in agarose gel. Am J Physiol. 1995 Jul;269(1 Pt 1):C28–C34. doi: 10.1152/ajpcell.1995.269.1.C28. [DOI] [PubMed] [Google Scholar]
- Delbono O., Stefani E. Calcium transients in single mammalian skeletal muscle fibres. J Physiol. 1993 Apr;463:689–707. doi: 10.1113/jphysiol.1993.sp019617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg B. R., Kuda A. M. Discrimination between fiber populations in mammalian skeletal muscle by using ultrastructural parameters. J Ultrastruct Res. 1976 Jan;54(1):76–88. doi: 10.1016/s0022-5320(76)80010-x. [DOI] [PubMed] [Google Scholar]
- Eusebi F., Miledi R., Takahashi T. Calcium transients in mammalian muscles. Nature. 1980 Apr 10;284(5756):560–561. doi: 10.1038/284560a0. [DOI] [PubMed] [Google Scholar]
- Fitts R. H., Courtright J. B., Kim D. H., Witzmann F. A. Muscle fatigue with prolonged exercise: contractile and biochemical alterations. Am J Physiol. 1982 Jan;242(1):C65–C73. doi: 10.1152/ajpcell.1982.242.1.C65. [DOI] [PubMed] [Google Scholar]
- Garcia J., Schneider M. F. Calcium transients and calcium release in rat fast-twitch skeletal muscle fibres. J Physiol. 1993 Apr;463:709–728. doi: 10.1113/jphysiol.1993.sp019618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis J. M. Relaxation of vertebrate skeletal muscle. A synthesis of the biochemical and physiological approaches. Biochim Biophys Acta. 1985 Jun 3;811(2):97–145. doi: 10.1016/0304-4173(85)90016-3. [DOI] [PubMed] [Google Scholar]
- Gillis J. M., Thomason D., Lefèvre J., Kretsinger R. H. Parvalbumins and muscle relaxation: a computer simulation study. J Muscle Res Cell Motil. 1982 Dec;3(4):377–398. doi: 10.1007/BF00712090. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Gundersen K., Leberer E., Lømo T., Pette D., Staron R. S. Fibre types, calcium-sequestering proteins and metabolic enzymes in denervated and chronically stimulated muscles of the rat. J Physiol. 1988 Apr;398:177–189. doi: 10.1113/jphysiol.1988.sp017037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heizmann C. W., Berchtold M. W., Rowlerson A. M. Correlation of parvalbumin concentration with relaxation speed in mammalian muscles. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7243–7247. doi: 10.1073/pnas.79.23.7243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heizmann C. W. Parvalbumin, an intracellular calcium-binding protein; distribution, properties and possible roles in mammalian cells. Experientia. 1984 Sep 15;40(9):910–921. doi: 10.1007/BF01946439. [DOI] [PubMed] [Google Scholar]
- Hollingworth S., Zhao M., Baylor S. M. The amplitude and time course of the myoplasmic free [Ca2+] transient in fast-twitch fibers of mouse muscle. J Gen Physiol. 1996 Nov;108(5):455–469. doi: 10.1085/jgp.108.5.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou T. T., Johnson J. D., Rall J. A. Effect of temperature on relaxation rate and Ca2+, Mg2+ dissociation rates from parvalbumin of frog muscle fibres. J Physiol. 1992 Apr;449:399–410. doi: 10.1113/jphysiol.1992.sp019092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou T. T., Johnson J. D., Rall J. A. Parvalbumin content and Ca2+ and Mg2+ dissociation rates correlated with changes in relaxation rate of frog muscle fibres. J Physiol. 1991 Sep;441:285–304. doi: 10.1113/jphysiol.1991.sp018752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein M. G., Simon B. J., Szucs G., Schneider M. F. Simultaneous recording of calcium transients in skeletal muscle using high- and low-affinity calcium indicators. Biophys J. 1988 Jun;53(6):971–988. doi: 10.1016/S0006-3495(88)83178-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y. S., Ondrias K., Duhl A. J., Ehrlich B. E., Kim D. H. Comparison of calcium release from sarcoplasmic reticulum of slow and fast twitch muscles. J Membr Biol. 1991 Jun;122(2):155–163. doi: 10.1007/BF01872638. [DOI] [PubMed] [Google Scholar]
- Liu Y., Carroll S. L., Klein M. G., Schneider M. F. Calcium transients and calcium homeostasis in adult mouse fast-twitch skeletal muscle fibers in culture. Am J Physiol. 1997 Jun;272(6 Pt 1):C1919–C1927. doi: 10.1152/ajpcell.1997.272.6.C1919. [DOI] [PubMed] [Google Scholar]
- Melzer W., Ríos E., Schneider M. F. The removal of myoplasmic free calcium following calcium release in frog skeletal muscle. J Physiol. 1986 Mar;372:261–292. doi: 10.1113/jphysiol.1986.sp016008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müntener M., Käser L., Weber J., Berchtold M. W. Increase of skeletal muscle relaxation speed by direct injection of parvalbumin cDNA. Proc Natl Acad Sci U S A. 1995 Jul 3;92(14):6504–6508. doi: 10.1073/pnas.92.14.6504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Permiakov E. A., Ostrovskii A. V., Kalinichenko L. P., Deikus G. Iu. Kinetika dissotsiatsii kompleksov parval'bumina s ionami kal'tsiia i magniia. Mol Biol (Mosk) 1987 Jul-Aug;21(4):1017–1022. [PubMed] [Google Scholar]
- Westerblad H., Allen D. G. Relaxation, [Ca2+]i and [Mg2+]i during prolonged tetanic stimulation of intact, single fibres from mouse skeletal muscle. J Physiol. 1994 Oct 1;480(Pt 1):31–43. doi: 10.1113/jphysiol.1994.sp020338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerblad H., Lännergren J. Slowing of relaxation during fatigue in single mouse muscle fibres. J Physiol. 1991 Mar;434:323–336. doi: 10.1113/jphysiol.1991.sp018472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubrzycka-Gaarn E., Korczak B., Osinska H., Sarzala M. G. Studies on sarcoplasmic reticulum from slow-twitch muscle. J Muscle Res Cell Motil. 1982 Jun;3(2):191–212. doi: 10.1007/BF00711942. [DOI] [PubMed] [Google Scholar]
- Zuurveld J. G., Wirtz P., Loermans H. M., Veerkamp J. H. Postnatal growth and differentiation in three hindlimb muscles of the rat. Characterization with biochemical and enzyme-histochemical methods. Cell Tissue Res. 1985;241(1):183–192. doi: 10.1007/BF00214640. [DOI] [PubMed] [Google Scholar]



