Abstract
In previous reports we described a 22-kb Legionella pneumophila chromosomal locus containing 18 genes. Thirteen of these genes (icmT, -R, -Q, -P, -O, -M, -L, -K, -E, -C, -D, -J, and -B) were found to be completely required for intracellular growth and killing of human macrophages. Three genes (icmS, -G, and -F) were found to be partially required, and two genes (lphA and tphA) were found to be dispensable for intracellular growth and killing of human macrophages. Here, we analyzed the requirement of these genes for intracellular growth in the protozoan host Acanthamoeba castellanii, a well-established important environmental host of L. pneumophila. We found that all the genes that are completely required for intracellular growth in human macrophages are also completely required for intracellular growth in A. castellanii. However, the genes that are partially required for intracellular growth in human macrophages are completely required for intracellular growth in A. castellanii. In addition, the lphA gene, which was shown to be dispensable for intracellular growth in human macrophages, is partially required for intracellular growth in A. castellanii. Our results indicate that L. pneumophila utilizes the same genes to grow intracellularly in both human macrophages and amoebae.
Legionella pneumophila, the causative agent of Legionnaires’ disease, is a broad-host-range facultative intracellular pathogen. The bacteria are able to infect, multiply within, and kill human macrophages, as well as free-living amoebae (29, 41). L. pneumophila infection can be divided into several steps that occur in similar ways in both hosts. The bacteria are taken up by regular phagocytosis or by a special mechanism termed “coiling” phagocytosis (12, 27); the bacteria are then found within a specialized phagosome that does not fuse with lysosomes (12, 26). The specialized phagosome undergoes several recruitment events that include association with smooth vesicles, mitochondria, and rough endoplasmic reticulum (1, 25, 49). The bacteria multiply within the specialized phagosome until the cell eventually lyses, releasing bacteria that can start new rounds of infection (29, 41).
Two regions of genes required for human macrophage killing and intracellular multiplication have been discovered in L. pneumophila (reviewed in reference 45). Region I contains 7 genes (icmV, -W, and -X and dotA, -B, -C, and -D) (10, 13, 32, 50), and region II contains 16 genes (icmT, -S, -R, -Q, -P, -O, -M, -L, -K, -E, -G, -C, -D, -J, -B, and -F) (3, 40, 43, 44, 50). The role of these genes in L. pneumophila’s ability to grow intracellularly in amoebae had not previously been determined. In other studies, transposon mutagenesis of the L. pneumophila genome identified mutants defective for intracellular growth and killing of both human macrophages and protozoa (23), as well as other mutants found to be defective for intracellular growth only in human macrophages (24) or only in protozoa (15). However, the genes disrupted in these mutants were not described.
The aim of this study was to determine if the icm genes listed above are also required for intracellular growth in protozoa. One hypothesis is that icm genes are specifically required for intracellular growth in human macrophages. An alternative hypothesis is that icm genes are required for intracellular growth in both human macrophages and protozoan hosts. The results presented here clearly show that icm genes are required for intracellular growth in both hosts, thus supporting the second hypothesis.
MATERIALS AND METHODS
Bacterial strains, plasmids, and media.
Bacterial strains and plasmids used in this work are described in Table 1 and Table 2, respectively. Bacterial media, plates, and antibiotic concentrations were used as described before (44).
TABLE 1.
L. pneumophila strains
| Strain | Genotype and features | Reference |
|---|---|---|
| 25D | Icm− avirulent mutant | 28 |
| GS3001 | JR32 icmS3001::Kan | 44 |
| GS3002 | JR32 icmP3002::Kan | 44 |
| GS3003 | JR32 icmO3003::Kan | 44 |
| GS3005 | JR32 icmO-icmN3005::Kan | 43 |
| GS3006 | JR32 icmN3006::Kan | 43 |
| GS3007 | JR32 icmN3007::Kan | 43 |
| GS3008 | JR32 icmM3008::Kan | 43 |
| GS3009 | JR32 icmL3009::Kan | 43 |
| GS3010 | JR32 icmK3010::Kan | 43 |
| GS3011 | JR32 icmT3011::Kan | 46 |
| JR32 | Salt-sensitive isolate of AM511 | 42 |
| LELA1275 | JR32 icmF1275::Tn903dIIlacZ | 42 |
| LELA3118 | JR32 dotA3118::Tn903dIIlacZ | 42 |
| LELA3244 | JR32 icmD3244::Tn903dIIlacZ | 42 |
| LELA3393 | JR32 icmB3393::Tn903dIIlacZ | 42 |
| LELA3463 | JR32 icmQ3463::Tn903dIIlacZ | 42 |
| LELA3473 | JR32 icmR3473::Tn903dIIlacZ | 42 |
| LELA4004 | JR32 icmX4004::Tn903dIIlacZ | 42 |
| LELA4432 | JR32 icmE4432::Tn903dIIlacZ | 42 |
| MW627 | JR32 tphA627::Kan | 40 |
| MW635 | JR32 icmG635::Kan | 40 |
| MW645 | JR32 icmC645::Kan | 40 |
| MW656 | JR32 icmJ656::Kan | 40 |
TABLE 2.
Plasmids used in this study
| Plasmid | Features | Reference or source |
|---|---|---|
| pGS-Lc-34-14 | icmPO in pMMB207αb-Km-14 | 46 |
| pGS-Lc-34-D1 | icmO in pMMB207αb | 44 |
| pGS-Lc-37-14 | icmTS in pMMB207αb-Km-14 | 46 |
| pGS-Lc-37-D1 | icmS in pMMB207αb | 44 |
| pGS-Lc-47 | icmNMLKEG in pMMB207αb | 43 |
| pGS-Lc-55-14 | icmF and tphA in pMMB207αb-Km-14 | This study |
| pGS-Lc-63-14 | icmGCD in pMMB207-Km-14 | This study |
| pMMB207 | RSF1010 derivative, IncQ lacIq Cmr Ptac oriT | 35 |
| pMMB207-Km-14 | pMMB207 with mobA::Km | This study |
| pMMB207αb-Km-14 | pMMB207αb with mobA::Km | 46 |
| pMW-100 | icmGCDJB, tphA, and icmF in pMMB207 | 40 |
| pMW-275 | icmEGCDJB, tphA, and icmF in pLAFR1 | 43 |
| pMW-560 | icmB in pBC-SK+ | 40 |
| pMW-604 | icmGCD in pMMB207 | 40 |
Plasmid construction.
The cloning vectors pMMB207 (35) and pMMB207αb-Kn-14 (46) were used to construct a new L. pneumophila cloning vector, pMMB207-Kn-14. Both pMMB207 and pMMB207αb-Kn-14 were digested with BspEI and MluI. The BspEI-MluI fragment of pMMB207αb-Kn-14 containing the Kn insertion in mobA was cloned into pMMB207 to generate pMMB207-Kn-14. Both the pMMB207-Kn-14 and pMMB207αb-Kn-14 vectors contain a Kn insertion in mobA.
To construct a mobA-less complementing plasmid for the icmGCD region, a BamHI-EcoRI fragment containing these genes was cloned from pMW604 (40) into the same sites in pMMB207-Kn-14 to generate pGS-Lc-63-14.
The cosmid pMW-275 (43) was used to construct a complementing plasmid for the icmF-tphA region. A XhoI 9-kb fragment was filled in and subcloned into the SmaI site of pMMB207αb-Kn-14 to generate pGS-Lc-55-14; this plasmid contains the icmF and tphA genes and about 4 kb of DNA upstream of icmF.
Intracellular growth in HL-60-derived macrophages.
Intracellular growth assays were performed as previously described (46), with the following modifications. Wells of a 24-well microtiter dish containing 3 × 106 differentiated HL-60-derived macrophages were used for infection. L. pneumophila was added to the wells at a multiplicity of infection of approximately 0.1, and the infected HL-60-derived macrophages were incubated for 1 h at 37°C under CO2 (5%). Then the wells were washed three times with RPMI containing glutamine, and 0.6 ml of RPMI medium, containing 2 mM Gln and 10% normal human serum, was added to the wells. The supernatant of each well was sampled at intervals of about 12 h, and numbers of CFU were determined by plating samples on ACES [N-(2-acetamido)-2-aminoethanesulfonic acid]-buffered charcoal-yeast extract (ABCYE) plates.
Intracellular growth in Acanthamoeba castellanii.
A. castellanii (ATCC 30234) was grown in 30 ml of proteose peptone-yeast extract-glucose medium (PYG) media (34) in a 75-cm2 tissue culture flask at 28°C, as adherent cells, until confluence was reached. Before starting an experiment, the flask was gently shaken and the PYG containing nonadherent amoebae was removed. New PYG was added to the flask and the amoebae were taken off by tapping the flask sharply. The resulting suspension was centrifuged for 10 min at 220 × g, the amoebae were resuspended in PYG at a concentration of 3 × 105 amoebae/ml, and 0.5 ml of the suspension was added to each well of a 48-well plate (1.5 × 105 amoebae/well). The amoebae were incubated for 1 h at 37°C to let the amoebae adhere. Then the PYG was aspirated, the wells were washed once with 0.5 ml of warm (37°C) Ac buffer (34), and 0.5 ml of warm Ac buffer was added to each well. L. pneumophila, in Ac buffer, was added to the wells at a multiplicity of infection of approximately 1. The plate was incubated for 30 min at 37°C, then the Ac buffer was aspirated, the wells were washed three times with 0.5 ml of warm Ac buffer, and 0.6 ml of warm Ac buffer was added to each well. Fifty-microliter samples were taken out at intervals of about 12 h, and numbers of CFU were determined by plating samples on ABCYE plates.
RESULTS
All icm genes completely required for intracellular growth in HL-60-derived macrophages are completely required for intracellular growth in A. castellanii.
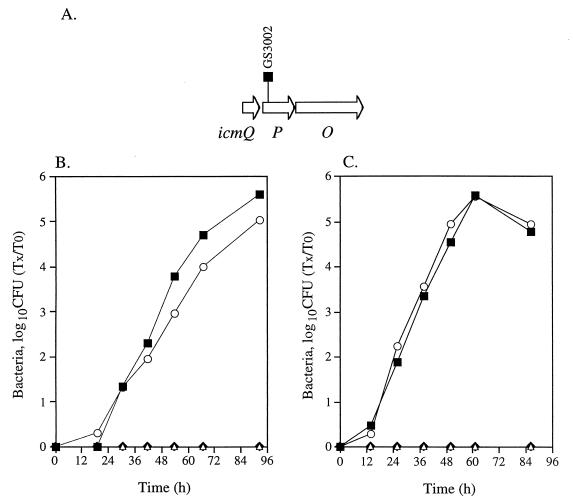
Mutants containing insertions in 13 icm genes (icmT, -R, -Q, -P, -O, -M, -L, -K, -E, -C, -D, -J, and -B) located in region II that were found to be completely required for killing of HL-60-derived macrophages (40, 43, 44) were analyzed for their ability to grow inside HL-60-derived macrophages and A. castellanii. An example of such a mutant that contains an insertion in icmP (GS3002) is presented in Fig. 1. As can be seen in Fig. 1B and C, a mutant containing an insertion in icmP was found to be completely defective for intracellular growth in both hosts, and its growth reached wild-type levels when a plasmid containing the icmP and icmO genes (pGS-Lc-34-14) was introduced into it. A similar analysis was done with mutants with insertions in all the icm genes mentioned (icmT, -R, -Q, -P, -O, -M, -L, -K, -E, -C, -D, -J, and -B), and a result similar to the one presented for the icmP insertion mutant was obtained (the mutants tested are listed in Table 1). All the genes that were found to be completely required for killing of HL-60-derived macrophages were found to be also completely required for intracellular growth in these cells, as well as for intracellular growth in A. castellanii. The mutants with insertions in icmT, icmP, and icmJ (GS3011, GS3002, and MW656, respectively) were also tested for intracellular growth when the downstream genes (icmS, icmO, and icmB, respectively) that probably form one transcriptional unit with them were expressed from a plasmid (pGS-Lc-37-D1, pGS-Lc-34-D1, and pMW-560, respectively). No intracellular growth was observed with these mutants when the downstream gene was supplied, indicating that these genes by themselves are required for intracellular growth. The mutant containing an insertion in icmC (MW645) was only partially complemented when the plasmid pGS-Lc-63-14 was introduced into it (see Fig. 4). Similar results were obtained when mutants containing insertions in icmM, -L, -K, and -E were complemented with the plasmid pGS-Lc-47. The reason for the partial complementation is not known. Two additional genes (icmX and dotA) located in region I (45) that were shown to be required for human macrophage killing (10, 13) were also found to be required for intracellular growth in A. castellanii.
FIG. 1.
Intracellular growth of an icmP insertion mutant in HL-60-derived macrophages and A. castellanii. (A) Chromosomal arrangement of the region surrounding icmP. The location of the insertion in icmP (GS3002) is shown. Intracellular growth in HL-60-derived macrophages (B) and in A. castellanii (C) was tested as described in Materials and Methods; the experiments were done at least three times, and results similar to those shown were obtained. ■, JR32; ⧫, 25D; ▵, GS3002 containing pMMB207αb-Km-14; ○, GS3002 containing pGS-Lc-34-14.
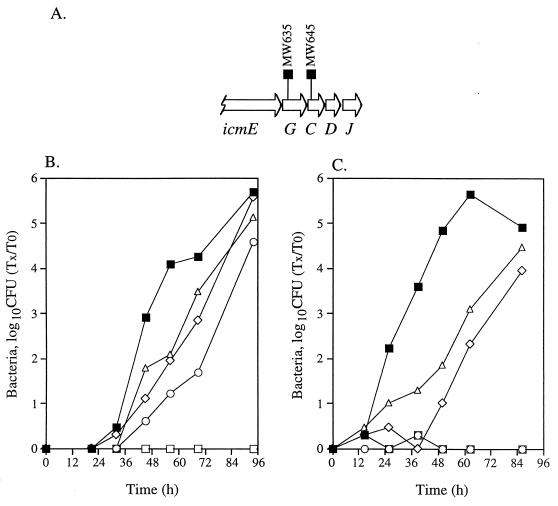
FIG. 4.
Intracellular growth of icmG and icmC insertion mutants in HL-60-derived macrophages and A. castellanii. (A) Chromosomal arrangement of the region surrounding icmG. The locations of the deletion substitutions (MW635 and MW645) are shown. Intracellular growth in HL-60-derived macrophages (B) and in A. castellanii (C) was tested as described in Materials and Methods; the experiments were done at least three times, and results similar to those shown were obtained. ■, JR32; ○, MW635 containing pMMB207-Km-14; ▵, MW635 containing pGS-Lc-63-14; □, MW645 containing pMMB207-Km-14; ◊, MW645 containing pGS-Lc-63-14.
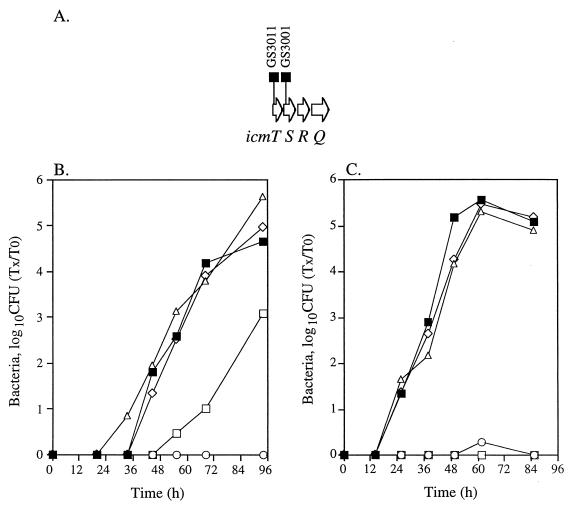
The icmS gene.
In a previous report (44), we showed that the icmT and icmS genes probably form one transcriptional unit and that the downstream icmR gene (Fig. 2A) is probably transcribed individually. In addition, the mutant containing an insertion in icmS (GS3001) was shown to retain some ability to kill HL-60-derived macrophages. Here, we compared the intracellular growth of the icmS insertion mutant in both HL-60-derived macrophages and A. castellanii, and the results are presented in Fig. 2B and C, respectively. The icmS insertion mutant was found to be only partially defective for intracellular growth in HL-60-derived macrophages (Fig. 2B) but completely defective for intracellular growth in A. castellanii. A mutant containing an insertion in icmT (GS3011) was found to be completely defective for growth in both hosts (Fig. 2B and C). Both insertion mutants (GS3001 and GS3011) attained wild-type levels when supplemented with a plasmid containing the icmT and icmS genes (pGS-Lc-37-14).
FIG. 2.
Intracellular growth of icmT and icmS insertion mutants in HL-60-derived macrophages and A. castellanii. (A) Chromosomal arrangement of the region surrounding icmS. The locations of the deletion substitutions (GS3001 and GS3011) are shown. Intracellular growth in HL-60-derived macrophages (B) and in A. castellanii (C) was tested as described in Materials and Methods; the experiments were done at least three times, and results similar to those shown were obtained. ■, JR32; ○, GS3011 containing pMMB207αb-Km-14; ▵, GS3011 containing pGS-Lc-37-14; □, GS3001 containing pMMB207αb-Km-14; ◊, GS3001 containing pGS-Lc-37-14.
The lphA (icmN) gene.
The lphA gene (lphA stands for lipoprotein homolog) was found to be dispensable for killing of HL-60-derived macrophages (43). Because this gene is located in the middle of a region containing genes required for intracellular growth (Fig. 3A), this result was surprising. Therefore, we used three different insertion mutants (GS3005, GS3006, and GS3007) to analyze this gene (Fig. 3A). Mutants containing an insertion (GS3007) or a deletion substitution (GS3006) in lphA (Fig. 3A) were found to have no defect in intracellular growth in HL-60-derived macrophages (Fig. 3B); this result agreed with the levels of cytotoxicity obtained with these mutants (43). When these mutants were analyzed for their intracellular growth in A. castellanii (Fig. 3C), a moderate defect in intracellular growth was observed. Growth was reduced by a factor of up to 100 at 40 to 50 h postinfection, in comparison to the wild-type strain (JR32). Due to the weak phenotype, we tested an additional mutant containing an insertion in the region between icmO and lphA (GS3005) (Fig. 3A); this mutant was found to be identical to the wild-type strain in its ability to grow intracellularly. Because lphA insertion mutants are defective for intracellular growth in A. castellanii, we renamed this gene icmN.
FIG. 3.
Intracellular growth of icmN and icmM insertion mutants in HL-60-derived macrophages and A. castellanii. (A) Chromosomal arrangement of the region surrounding icmN. The chromosomal arrangements of the mutants tested are shown above the genes. Intracellular growth in HL-60-derived macrophages (B) and in A. castellanii (C) was tested as described in Materials and Methods; the experiments were done at least three times, and results similar to those shown were obtained. ■, JR32; ○, GS3005, □, GS3006, ▵, GS3007; ◊, GS3008.
We tried to complement the icmN mutants with plasmids containing icmN or icmN, -M, -L, -K, and -E, but we were unable to observe complementation. It is very unlikely that the phenotype of the icmN insertion mutants is due to polarity on the downstream gene icmM (Fig. 3A), because a mutant containing an insertion in icmM (GS3008) was found to be completely defective for intracellular growth in both hosts (Fig. 3B and C). We assume that if the phenotype of the icmN mutants was due to polarity on icmM, we would have observed a reduction in the intracellular growth of the icmN mutants in both hosts and not only in A. castellanii.
The icmG gene.
An icmG insertion mutant (MW635) was shown to be moderately defective in killing HL-60-derived macrophages, and the two genes located downstream of icmG (icmC and icmD) (Fig. 4A) were shown to be completely required for killing of HL-60-derived macrophages (40). When the icmG insertion mutant was tested for intracellular growth in HL-60-derived macrophages (Fig. 4B), it was found to have a weak reduction in comparison to the wild-type strain (JR32). When this mutant was tested for its ability to grow inside A. castellanii, no growth was observed (Fig. 4C). When a plasmid containing icmG, -C, and -D (pGS-Lc-63-14) was introduced into this mutant, only partial complementation was observed (Fig. 4B and C). A mutant containing an insertion in icmC (MW645), located downstream from icmG, was found to be completely defective for intracellular growth in both hosts (Fig. 4B and C). As was described for the mutant with the insertion in icmG, the icmC insertion mutant was only partially complemented with pGS-Lc-63-14. No increase in complementation efficiency of either mutant was observed when a plasmid (pMW-100) containing additional downstream genes was used for complementation. The reason for the partial complementation is not known.
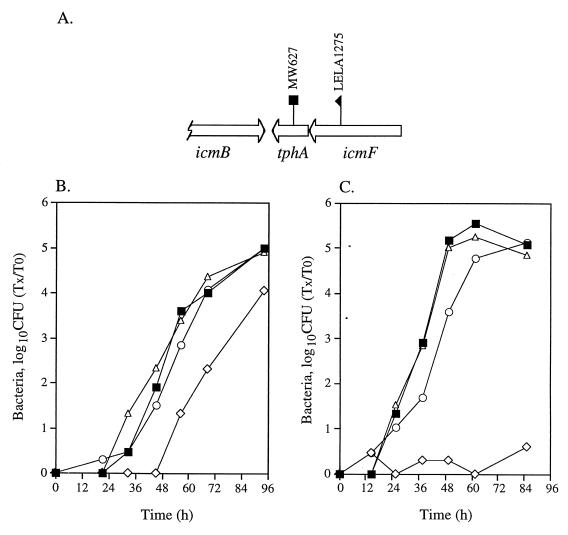
The icmF and tphA genes.
The icmF and tphA genes are located on the opposite strand in relation to the other genes found in region II (Fig. 5A). When mutants containing insertions in icmF and tphA (tphA stands for transport protein homolog) were tested for their ability to kill HL-60-derived macrophages, it was found that a mutant containing an insertion in icmF was partially defective for killing HL-60-derived macrophages, and a mutant containing an insertion in tphA (MW627) was not defective in killing HL-60-derived macrophages (40). Here, we tested mutants with insertions in these genes (LELA1275 for icmF and MW627 for tphA) for intracellular growth in HL-60-derived macrophages (Fig. 5B) and A. castellanii (Fig. 5C). The results obtained with HL-60-derived macrophages are consistent with the observation made with the cytotoxicity assays (40); the icmF insertion mutant was found to be weakly defective for growth inside HL-60-derived macrophages, and the tphA insertion mutant was found to be identical to the wild-type strain. In contrast, the mutant with the insertion in icmF was completely defective for intracellular growth in A. castellanii, and the mutant with the insertion in tphA was indistinguishable from the wild-type strain (Fig. 5C). The mutant with the insertion in icmF (LELA1275) achieved a wild-type level of growth when a plasmid containing icmF and tphA (pGS-Lc-55-14) was introduced into the mutant (Fig. 5B and C).
FIG. 5.
Intracellular growth of icmF and tphA insertion mutants in HL-60-derived macrophages and A. castellanii. (A) Chromosomal arrangement of the region surrounding icmF. The locations of the insertions (LELA1275 and MW627) are shown. Intracellular growth in HL-60-derived macrophages (B) and in A. castellanii (C) was tested as described in Materials and Methods; the experiments were done at least three times, and results similar to those shown were obtained. ■, JR32; ◊, LELA1275 containing pMMB207αb-Km-14; ○, LELA1275 containing pGS-Lc-55-14; ▵, MW627.
DISCUSSION
L. pneumophila is a broad-host-range facultative intracellular pathogen that overcomes many natural host defense mechanisms, enabling it to cause disease in humans. Like Mycobacterium tuberculosis (4), Chlamydia psittaci (22), and Toxoplasma gondii (30), L. pneumophila multiplies within human cells inside a specialized vacuole that does not fuse with secondary lysosomes (26). In nature, L. pneumophila uses a similar mechanism to infect and multiply within free-living amoebae (1, 12, 19).
Besides Legionella, several other bacterial species, such as Mycobacterium avium, Chlamydia pneumoniae, and Listeria monocytogenes, were shown to survive and multiply in amoebae (17, 18, 31). For Legionella, growth within amoebae and ciliated protozoa is probably the main means of survival and multiplication in the environment (5, 6, 19). Legionella has been shown to multiply intracellularly in several species of protozoa, such as Hartmannella, Acanthamoeba, Naegleria, and Tetrahymena (20, 21, 36, 41). During outbreaks of Legionnaires’ disease, the water sources for L. pneumophila were usually found to contain amoebae and/or protozoa (5, 19). Intracellular growth in amoebae, in comparison to growth on artificial media, was shown to affect L. pneumophila in several ways. It was shown to enhance invasion into monocytic cells (34), cause changes in bacterial cell surface properties (8), and increase bacterial resistance to antibiotics (9) and bacterial susceptibility to chemicals (7). In addition, viable but nonculturable L. pneumophila can be resuscitated by coculture with protozoa (47). Moreover, coinfection of mice with L. pneumophila and Hartmannella was shown to cause a more severe respiratory disease than infection with either organism alone (14, 15). The finding that A. castellanii can form respirable vesicles in which L. pneumophila can survive (11) suggests that the vesicles might serve as one of the ways in which L. pneumophila can enter human lungs. These results indicate that the ability of L. pneumophila to multiply within protozoan hosts plays a critical role in its survival in the environment and its ability to cause disease in humans.
L. pneumophila infection of human monocytes and amoebae was studied intensively in the early 1980s by Horwitz and Silverstein (29), and Rowbotham (41). Further studies of L. pneumophila infection revealed that the process of infection occurs in very similar ways in both hosts (1, 12, 26, 27, 49). Several groups compared the requirement of different genes for and the fate of different mutants in intracellular growth in human monocytes and amoebae. Most of the genes tested (gspA, pilEL, hpb, lly, and msp) were found to be dispensable for growth in both hosts (2, 33, 37, 48, 51); the mip gene was shown to be moderately attenuated for growth in both hosts (16). In contrast, mutants that were tested for growth in both hosts can be separated into three groups: A, mutants attenuated for growth in both hosts (23, 38, 39); B, mutants attenuated for growth only in human monocytes (24); and C, mutants attenuated for growth only in amoebae (15). However, the genes disrupted in the mutants from these three groups were not described.
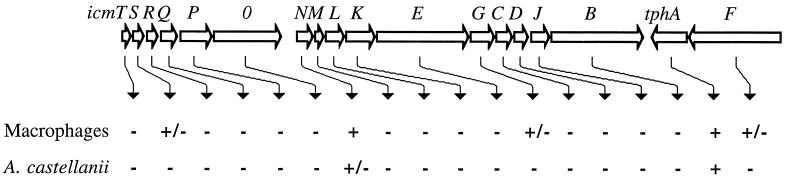
Studies performed in our lab and in the Isberg lab yielded information about several icm and dot genes that were shown to be required for intracellular growth and killing of human macrophages (reviewed in reference 45). The role of these genes in the ability of L. pneumophila to multiply in amoebae has not previously been examined. Here, we present a detailed analysis of the requirement of 18 genes located in icm and dot region II for L. pneumophila intracellular growth in A. castellanii; the data are summarized in Fig. 6. All the genes that were shown to be completely required for intracellular growth in human macrophages were also found to be completely required for intracellular growth in A. castellanii. However, all the genes that were shown to be partially required for intracellular growth in human macrophages were found to be completely required for intracellular growth in A. castellanii. The icmN gene, which was shown to be dispensable for intracellular growth in human macrophages, is partially required for intracellular growth in A. castellanii. Our data indicate that L. pneumophila utilizes the same genes to grow intracellularly in both human macrophages and amoebae, two evolutionarily distinct hosts. The ability of L. pneumophila to infect and multiply inside human macrophages and amoebae in similar ways and by using the same genes indicates that amoebae and human macrophages have many similar properties that allow the bacteria to carry out their infection in both hosts.
FIG. 6.
Intracellular growth requirements for genes in region II. The first line under the genes indicates the growth phenotype in HL-60-derived macrophages, and the second line indicates the growth phenotype in A. castellanii. −, no intracellular growth was observed; +/−, partial intracellular growth was observed; +, intracellular growth was similar to that of the wild-type strain.
ACKNOWLEDGMENTS
The work was supported by a grant from the NIH (AI23549). G. Segal was supported by a long-term fellowship from the EMBO and the Stephen A. Morse Fellowship from Departments of Microbiology and Medicine of Columbia University.
We are grateful to Carmen Rodriguez for excellent technical assistance during this work.
REFERENCES
- 1.Abu Kwaik Y. The phagosome containing Legionella pneumophila within the protozoan Hartmannella vermiformis is surrounded by the rough endoplasmic reticulum. Appl Environ Microbiol. 1996;62:2022–2028. doi: 10.1128/aem.62.6.2022-2028.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abu Kwaik Y, Gao L Y, Harb O S, Stone B J. Transcriptional regulation of the macrophage-induced gene (gspA) of Legionella pneumophila and phenotypic characterization of a null mutant. Mol Microbiol. 1997;24:629–642. doi: 10.1046/j.1365-2958.1997.3661739.x. [DOI] [PubMed] [Google Scholar]
- 3.Andrews H L, Vogel J P, Isberg R R. Identification of linked Legionella pneumophila genes essential for intracellular growth and evasion of the endocytic pathway. Infect Immun. 1998;66:950–958. doi: 10.1128/iai.66.3.950-958.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Armstrong J A, D’Arcy Hart P. Response of cultured macrophages to Mycobacterium tuberculosis with observations on fusion of lysosomes with phagosomes. J Exp Med. 1971;134:713–740. doi: 10.1084/jem.134.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barbaree J M, Fields B S, Feeley J C, Gorman G W, Martin W T. Isolation of protozoa from water associated with a legionellosis outbreak and demonstration of intracellular multiplication of Legionella pneumophila. Appl Environ Microbiol. 1986;51:422–424. doi: 10.1128/aem.51.2.422-424.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barker J, Brown M R. Trojan horses of the microbial world: protozoa and the survival of bacterial pathogens in the environment. Microbiology. 1994;140:1253–1259. doi: 10.1099/00221287-140-6-1253. [DOI] [PubMed] [Google Scholar]
- 7.Barker J, Brown M R W, Collier P J, Farrell I, Gilbert P. Relationship between Legionella pneumophila and Acanthamoeba polyphaga: physiological status and susceptibility to chemical inactivation. Appl Environ Microbiol. 1992;58:2420–2425. doi: 10.1128/aem.58.8.2420-2425.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barker J, Lambert P A, Brown M R W. Influence of intra-amoebic and other growth conditions on the surface properties of Legionella pneumophila. Infect Immun. 1993;61:3503–3510. doi: 10.1128/iai.61.8.3503-3510.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barker J, Scaife H, Brown M R W. Intraphagocytic growth induces an antibiotic-resistant phenotype of Legionella pneumophila. Antimicrob Agents Chemother. 1995;39:2684–2688. doi: 10.1128/aac.39.12.2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berger K H, Merriam J J, Isberg R R. Altered intracellular targeting properties associated with mutations in the Legionella dotA gene. Mol Microbiol. 1994;14:809–822. doi: 10.1111/j.1365-2958.1994.tb01317.x. [DOI] [PubMed] [Google Scholar]
- 11.Berk S G, Ting R S, Turner G W, Ashburn R J. Production of respirable vesicles containing live Legionella pneumophila cells by two Acanthamoeba spp. Appl Environ Microbiol. 1998;64:279–286. doi: 10.1128/aem.64.1.279-286.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bozue J A, Johnson W. Interaction of Legionella pneumophila with Acanthamoeba castellanii: uptake by coiling phagocytosis and inhibition of phagosome-lysosome fusion. Infect Immun. 1996;64:668–673. doi: 10.1128/iai.64.2.668-673.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brand B C, Sadosky A B, Shuman H A. The Legionella pneumophila icm locus: a set of genes required for intracellular multiplication in human macrophages. Mol Microbiol. 1994;14:797–808. doi: 10.1111/j.1365-2958.1994.tb01316.x. [DOI] [PubMed] [Google Scholar]
- 14.Brieland J, McClain M, Heath L, Chrisp C, Huffnagle G, LeGendre M, Hurley M, Fantone J, Engleberg C. Coinoculation with Hartmannella vermiformis enhances replicative Legionella pneumophila lung infection in a murine model of Legionnaires’ disease. Infect Immun. 1996;64:2449–2456. doi: 10.1128/iai.64.7.2449-2456.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brieland J, McClain M, LeGendre M, Engleberg C. Intrapulmonary Hartmannella vermiformis: a potential niche for Legionella pneumophila replication in a murine model of legionellosis. Infect Immun. 1997;65:4892–4896. doi: 10.1128/iai.65.11.4892-4896.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cianciotto N P, Fields B S. Legionella pneumophila mip gene potentiates intracellular infection of protozoa and human macrophages. Proc Natl Acad Sci USA. 1992;89:5188–5191. doi: 10.1073/pnas.89.11.5188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cirillo J D, Falkow S, Tompkins L S, Bermudez L E. Interaction of Mycobacterium avium with environmental amoebae enhances virulence. Infect Immun. 1997;65:3759–3767. doi: 10.1128/iai.65.9.3759-3767.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Essig A, Heinemann M, Simnacher U, Marre R. Infection of Acanthamoeba castellanii by Chlamydia pneumoniae. Appl Environ Microbiol. 1997;63:1396–1399. doi: 10.1128/aem.63.4.1396-1399.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fields B S. The molecular ecology of Legionellae. Trends Microbiol. 1996;4:286–290. doi: 10.1016/0966-842x(96)10041-x. [DOI] [PubMed] [Google Scholar]
- 20.Fields B S, Fields S R, Loy J N, White E H, Steffens W L, Shotts E B. Attachment and entry of Legionella pneumophila in Hartmannella vermiformis. J Infect Dis. 1993;167:1146–1150. doi: 10.1093/infdis/167.5.1146. [DOI] [PubMed] [Google Scholar]
- 21.Fields B S, Shotts E B, Jr, Feeley J C, Gorman G W, Martin W T. Proliferation of Legionella pneumophila as an intracellular parasite of the ciliated protozoan Tetrahymena pyriformis. Appl Environ Microbiol. 1984;47:467–471. doi: 10.1128/aem.47.3.467-471.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Friis R R. Interaction of L cells and Chlamydia psittaci: entry of the parasite and host responses to its development. J Bacteriol. 1972;110:706–721. doi: 10.1128/jb.110.2.706-721.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao L-Y, Harb O S, Abu Kwaik Y. Utilization of similar mechanisms by Legionella pneumophila to parasitize two evolutionarily distant host cells, mammalian macrophages and protozoa. Infect Immun. 1997;65:4738–4746. doi: 10.1128/iai.65.11.4738-4746.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gao L-Y, Harb O S, Abu Kwaik Y. Identification of macrophage-specific infectivity loci (mil) of Legionella pneumophila that are not required for infectivity of protozoa. Infect Immun. 1998;66:883–892. doi: 10.1128/iai.66.3.883-892.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Horwitz M A. Formation of a novel phagosome by the Legionnaires’ disease bacterium (Legionella pneumophila) in human monocytes. J Exp Med. 1983;158:1319–1331. doi: 10.1084/jem.158.4.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Horwitz M A. The Legionnaires’ disease bacterium (Legionella pneumophila) inhibits phagosome-lysosome fusion in human monocytes. J Exp Med. 1983;158:2108–2126. doi: 10.1084/jem.158.6.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Horwitz M A. Phagocytosis of the Legionnaires’ disease bacterium (Legionella pneumophila) occurs by a novel mechanism: engulfment within a pseudopod coil. Cell. 1984;36:27–33. doi: 10.1016/0092-8674(84)90070-9. [DOI] [PubMed] [Google Scholar]
- 28.Horwitz M A. Characterization of avirulent mutant Legionella pneumophila that survive but do not multiply within human monocytes. J Exp Med. 1987;166:1310–1328. doi: 10.1084/jem.166.5.1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Horwitz M A, Silverstein S C. Legionnaires’ disease bacterium (Legionella pneumophila) multiplies intracellularly in human monocytes. J Clin Investig. 1980;60:441–450. doi: 10.1172/JCI109874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jones T C, Hirsch J G. The interactions between Toxoplasma gondii and mammalian cells. II. The absence of lysosomal fusion with phagocytic vacuoles containing living parasites. J Exp Med. 1972;136:1173–1184. doi: 10.1084/jem.136.5.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ly T M C, Muller H E. Ingested Listeria monocytogenes survive and multiply in protozoa. J Med Microbiol. 1990;33:51–54. doi: 10.1099/00222615-33-1-51. [DOI] [PubMed] [Google Scholar]
- 32.Marra A, Blander S J, Horwitz M A, Shuman H A. Identification of a Legionella pneumophila locus required for intracellular multiplication in human macrophages. Proc Natl Acad Sci USA. 1992;89:9607–9611. doi: 10.1073/pnas.89.20.9607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moffat J F, Edelstein P H, Regula D P J, Cirillo J D, Tompkins L S. Effects of an isogenic Zn-metalloprotease-deficient mutant of Legionella pneumophila in a guinea-pig pneumonia model. Mol Microbiol. 1994;12:693–705. doi: 10.1111/j.1365-2958.1994.tb01057.x. [DOI] [PubMed] [Google Scholar]
- 34.Moffat J F, Tompkins L S. A quantitative model of intracellular growth of Legionella pneumophila in Acanthamoeba castellanii. Infect Immun. 1992;60:296–301. doi: 10.1128/iai.60.1.296-301.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morales V M, Backman A, Bagdasarian M. A series of wide-host-range low-copy-number vectors that allow direct screening for recombinants. Gene. 1991;97:39–47. doi: 10.1016/0378-1119(91)90007-x. [DOI] [PubMed] [Google Scholar]
- 36.Newsome A L, Baker R L, Miller R D, Arnold R R. Interactions between Naegleria fowleri and Legionella pneumophila. Infect Immun. 1985;50:449–452. doi: 10.1128/iai.50.2.449-452.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O’Connell W A, Hickey E K, Cianciotto N P. A Legionella pneumophila gene that promotes hemin binding. Infect Immun. 1996;64:842–848. doi: 10.1128/iai.64.3.842-848.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pope C D, O’Connell W A, Cianciotto N P. Legionella pneumophila mutants that are defective for iron acquisition and assimilation and intracellular infection. Infect Immun. 1996;64:629–636. doi: 10.1128/iai.64.2.629-636.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pruckler J M, Benson R F, Moyenuddin M, Martin W T, Fields B S. Association of flagellum expression and intracellular growth of Legionella pneumophila. Infect Immun. 1995;63:4928–4932. doi: 10.1128/iai.63.12.4928-4932.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Purcell M, Shuman H A. The Legionella pneumophila icmGCDJBF genes are required for killing of human macrophages. Infect Immun. 1998;66:2245–2255. doi: 10.1128/iai.66.5.2245-2255.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rowbotham T J. Preliminary report on the pathogenicity of Legionella pneumophila for freshwater and soil amoebae. J Clin Pathol. 1980;33:1179–1183. doi: 10.1136/jcp.33.12.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sadosky A B, Wiater L A, Shuman H A. Identification of Legionella pneumophila genes required for growth within and killing of human macrophages. Infect Immun. 1993;61:5361–5373. doi: 10.1128/iai.61.12.5361-5373.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Segal G, Purcell M, Shuman H A. Host cell killing and bacterial conjugation require overlapping sets of genes within a 22-kb region of the Legionella pneumophila genome. Proc Natl Acad Sci USA. 1998;95:1669–1674. doi: 10.1073/pnas.95.4.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Segal G, Shuman H A. Characterization of a new region required for macrophage killing by Legionella pneumophila. Infect Immun. 1997;65:5057–5066. doi: 10.1128/iai.65.12.5057-5066.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Segal G, Shuman H A. How is the intracellular fate of the Legionella pneumophila phagosome determined? Trends Microbiol. 1998;6:253–255. doi: 10.1016/s0966-842x(98)01308-0. [DOI] [PubMed] [Google Scholar]
- 46.Segal G, Shuman H A. Intracellular multiplication and human macrophage killing by Legionella pneumophila are inhibited by conjugal components on IncQ plasmid RSF1010. Mol Microbiol. 1998;30:197–208. doi: 10.1046/j.1365-2958.1998.01054.x. [DOI] [PubMed] [Google Scholar]
- 47.Steinert M, Emödy L, Amann R, Hacker J. Resuscitation of viable but nonculturable Legionella pneumophila Philadelphia JR32 by Acanthamoeba castellanii. Appl Environ Microbiol. 1997;63:2047–2053. doi: 10.1128/aem.63.5.2047-2053.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stone B J, Abu Kwaik Y. Expression of multiple pili by Legionella pneumophila: identification and characterization of a type IV pilin gene and its role in adherence to mammalian and protozoan cells. Infect Immun. 1998;66:1768–1775. doi: 10.1128/iai.66.4.1768-1775.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Swanson M S, Isberg R R. Association of Legionella pneumophila with the macrophage endoplasmic reticulum. Infect Immun. 1995;63:3609–3620. doi: 10.1128/iai.63.9.3609-3620.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vogel J P, Andrews H L, Wong S K, Isberg R R. Conjugative transfer by the virulence system of Legionella pneumophila. Science. 1998;279:873–876. doi: 10.1126/science.279.5352.873. [DOI] [PubMed] [Google Scholar]
- 51.Wintermeyer E, Flügel M, Ott M, Steinert M, Rdest U, Mann K-H, Hacker J. Sequence determination and mutational analysis of the lly locus of Legionella pneumophila. Infect Immun. 1994;62:1109–1117. doi: 10.1128/iai.62.3.1109-1117.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]