Abstract
Viridans streptococci are a heterogeneous group of gram-positive bacteria that are normal inhabitants of the mouth. These organisms are thought to contribute significantly to the etiology of infective endocarditis, although recently they have been implicated in serious infections in other settings. Another group of oral bacteria, gram-negative anaerobes, is associated with chronic dental infections, such as periodontal diseases or endodontic lesion formation. We evaluated the ability of the oral pathogens Streptococcus mutans and Porphyromonas endodontalis to induce a pathogenic response in vivo, with the goal of quantifying the inflammatory response in soft tissue by measuring leukocyte recruitment and hard tissues by measuring osteoclastogenesis. S. mutans induced a strong inflammatory response and was a potent inducer of osteoclast formation, while P. endodontalis was not. To further study the mechanisms by which P. endodontalis and S. mutans elicit significantly different levels of inflammatory responses in vivo, we tested the capacity of each to induce production of cytokines by mononuclear cells in vitro. S. mutans stimulated high levels of interleukin-12 (IL-12), gamma interferon (IFN-γ), and tumor necrosis factor alpha (TNF-α), all of which are associated with inflammation, enhanced monocyte function, and generation of a Th1 response. In contrast, P. endodontalis stimulated production of IL-10 but not of TNF-α, IL-12, or IFN-γ. These results demonstrate that oral pathogens differ dramatically in their abilities to induce inflammatory and immunoregulatory cytokines. Moreover, there is a high degree of correlation between the cytokine profile induced by these bacteria in vitro and their pathogenic capacity in vivo.
Several different bacteria have been associated with oral infections (1, 27, 34). However, the mechanisms by which specific bacteria cause pathogenic lesions are not completely understood. Bacterial contamination of the dental pulp in decayed teeth inevitably leads to pulpitis and necrosis. The pathogens that cause pulpitis are derived from dental plaque, and are, for the most part, gram-positive bacteria such as viridans streptococci. Oral streptococci are also important agents in infective endocarditis and are suspected to contribute to the etiology in over 50% of cases (16, 31). Approximately 20% of viridans streptococcus endocarditis are caused by Streptococcus mutans (5). In addition, viridans group streptococci have been described as responsible for the alpha-streptococcal shock syndrome in neutropenic patients (19). The mechanisms by which viridans streptococci cause bacteremia associated with severe clinical manifestations have not been elucidated. In contrast to oral streptococci, which appear to cause acute or subacute infections, gram-negative anaerobes are primarily associated with chronic oral infections such as periodontal disease or endodontic lesions. For example, Porphyromonas endodontalis has been found in half of chronic endodontic lesions and is almost exclusively found in infections of endodontic origin, suggesting a specific association of P. endodontalis and the dental pulp (4, 32).
The innate immune response associated with infections is linked to the adaptive immune response by cytokine production. For example, bacteria can initiate cell-mediated immunity by stimulating macrophages to produce interleukin-12 (IL-12), which can then promote gamma interferon (IFN-γ) production by natural killer cells. IFN-γ-activated macrophages then secrete more IL-12 as a result of positive feedback, leading to cell-mediated Th1 responses. IFN-γ also activates macrophages to produce tumor necrosis factor alpha (TNF-α), which in turn maintains the activated state of the macrophage (16). TNF-α is a potent proinflammatory cytokine that can stimulate chemokine production by endothelial cells and fibroblasts in vitro (25). In addition to its protective role in the host defense against infectious agents, TNF-α contributes to bone resorptive activity and has been implicated in pathologic bone resorption (11, 21, 28). An effective host defense against bacterial invasion is characterized by the vigorous recruitment and activation of inflammatory cells, which are modulated by the coordinated expression of both pro- and anti-inflammatory cytokines. IL-10, which is produced by monocytes and Th2 lymphocyte subsets, inhibits IFN-γ synthesis by Th1 cells and inhibits the production of proinflammatory cytokines such as IL-12 and TNF-α. The inhibiting effects of IL-10 on cytokine production correlate with their antiinflammatory effects in vivo (9). Therefore, IL-10, IFN-γ, IL-12, and TNF-α have important and cross-regulatory roles in infection (29).
In data presented here, we examined the capacities of S. mutans and P. endodontalis to stimulate leukocyte recruitment and osteoclastogenesis in vivo and cytokine production by mononuclear cells in vitro. This was accomplished in an animal model that has recently been shown to be an effective in describing the host response to oral pathogens (35). The results demonstrate that S. mutans stimulates considerable inflammatory cell recruitment and osteoclast formation in vivo and is a strong inducer of proinflammatory cytokine induction in vitro whereas P. endodontalis is not. These results should provide insight into the inflammatory response of S. mutans and its capacity to stimulate the host in oral infections and in bacterial endocarditis.
MATERIALS AND METHODS
Bacteria.
P. endodontalis ATCC 35406 (generously provided by Ann Tanner [Forsyth Dental Center, Boston, Mass.]) was cultured under strict anaerobic conditions (85% N2, 10% H2, and 5% CO2) on Trypticase soy-brain heart infusion-yeast extract-blood agar containing hemin and vitamin K1 (Northwest Laboratory, Waterville, Maine). S. mutans LM7 and OMZ175, grown in Todd-Hewitt broth (Becton Dickinson, Cockeysville, Md.), were generously donated by F. Oppenheim (Boston University Goldman School of Dental Medicine, Boston, Mass.). The bacteria, used at early- to mid-log-phase growth, were heat killed by boiling for 10 min and then washed three times with phosphate-buffered saline (PBS) as described previously (7). The amount of each bacterium was quantitated spectrophotometrically according to a standard curve established by colony formation on bacterial plates.
Mononuclear cell stimulation.
Human peripheral blood was obtained from healthy volunteers by venipuncture followed by the addition of heparin (100 U per 50 ml of blood). Peripheral blood mononuclear cells (PBMC) were isolated from the leukocyte-rich buffy coat following centrifugation over a gradient of Ficoll-Hypaque. Autologous serum was obtained by centrifugation of nonheparinized venous blood. PBMC (3 × 105 per well) were incubated in polypropylene cell culture plates containing the indicated stimulus in a final volume of 250 μl of RPMI tissue culture medium supplemented with 5% autologous serum. All solutions and reagents had nondetectable endotoxin levels (<30 pg/ml) as determined by the Limulus amebocyte lysate assay (Sigma, St. Louis, Mo.). After 24 h, 96 h, or 7 days of stimulation, supernatants were harvested for evaluation of induced cytokine levels by enzyme-linked immunosorbent assay (ELISA).
ELISAs for cytokines.
Antibody pairs for human IL-12 p70 (R&D Systems, Minneapolis, Minn.), IFN-γ, TNF-α, and IL-10 (PharMingen, San Diego, Calif.) were used in the sandwich ELISA. These assays were sensitive to 10 pg/ml of cytokine per ml. Other ELISA reagents were purchased from Kiekegaard & Perry Laboratories, (Gaithersburg, Md.). ELISAs were performed as we described previously (6). Briefly, 96-well plates were incubated with capture monoclonal antibody at 4°C overnight. Nonspecific bind sites were blocked by 1% bovine serum albumin for 1 h at room temperature. After being rinsed with PBS-Tween 20 buffer, the samples were added and incubated at room temperature for 1 h. Biotinylated detecting antibody to the cytokine was added to the samples and incubated for 1 h after the plate was washed with PBS-Tween buffer. Horseradish peroxidase-strepavidin and color metric system (tetramethylenebenzidine) were used as described by the manufacturer (KPL, Kiekegaard & Perry Laboratories). The plates were read at 450 nm within 30 min with an ELISA plate reader (Molecular Devices).
Bacteria inoculation and immunohistology studies.
The host response to bacteria was investigated in both soft and hard tissue, using the calvarial model that was recently shown to be effective in characterizing the response to oral pathogens (35). CD-1 outbred mice, 12 to 14 weeks of age, were obtained from Charles River Laboratories (Boston, Mass.). Mice were inoculated with 108 live P. endodontalis or S. mutans in 50 μl of PBS. Bacteria were applied by supraperiosteal injection in the mid-calvarial region after complete removal of hair with a microshaver (Conair). Vehicle (PBS) alone was injected as a negative control. Mice were sacrificed 2, 5, and 10 days after injection of bacteria. The calvarial samples with attached scalp were harvested, fixed in 4% paraformaldehyde for 24 h at 4°C, and decalcified in EDTA-glycerin-PBS (pH 7.0). After decalcification, specimens were immersed in 30% sucrose–PBS overnight at 4°C, snap frozen, and cut into 5-μm sections with a cryostat according to published procedures (12). The tissue sections were stained with hematoxylin and eosin to count inflammatory cells or tartrate-resistant acid phosphatase (TRAP) to aid in identifying osteoclasts.
Statistical analysis.
Statistical analysis was performed with one-way analysis of variance with Duncan’s post hoc test, to determine the significance of cytokine production between control and bacterium-stimulated samples. Significance was determined at P < 0.05.
RESULTS
The response to P. endodontalis and S. mutans in vivo.
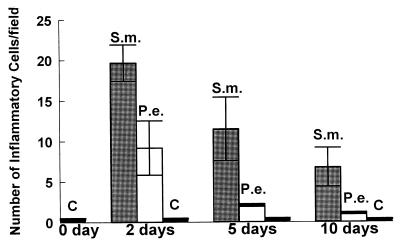
To determine whether different oral bacteria stimulate disparate host responses, P. endodontalis and S. mutans were injected into the mouse calvaria (scalp), adjacent to the periosteum. The number of inflammatory cells recruited and number of osteoclasts formed were quantified. The results demonstrate that S. mutans elicits a pronounced inflammatory infiltrate in mouse calvarial soft tissue, while P. endodontalis elicits a relatively mild inflammatory cell infiltrate (Fig. 1). While both S. mutans and P. endodontalis stimulated a peak of inflammatory cell recruitment at 2 days, S. mutans produced a much more sustained inflammatory infiltrate. At 5 and 10 days, S. mutans enhanced inflammatory cell recruitment 30- and 20-fold, respectively, while P. endodontalis stimulated increases of only 5- and 3-fold, respectively, compared to vehicle alone.
FIG. 1.
Quantitative assessment of leukocyte recruitment following injection of heat-killed S. mutans and P. endodontalis. Heat-killed S. mutans (S.m.) and P. endodontalis (P.e.) (108 bacteria/scalp) were injected into CD-1 wild-type mice. Vehicle alone was injected as a negative control (C). Mice were sacrificed at 0, 2, 5, and 10 days after injection. Sections were stained with hematoxylin and eosin. Image analysis was used to quantify the total number of leukocytes per field at a magnification of ×1,000. Eighteen fields were examined per section. Five specimens were examined per data point.
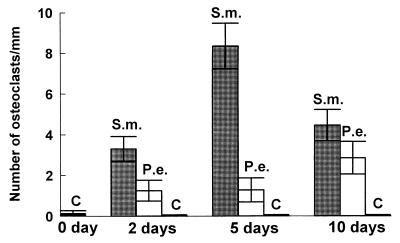
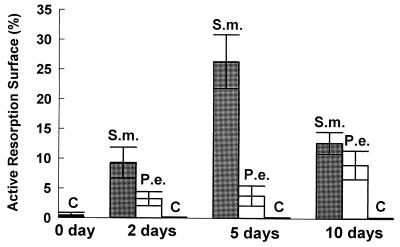
To assess the impact of S. mutans or P. endodontalis infection on hard tissue, we measured osteoclastogenesis and the percent bone surface covered by osteoclasts. S. mutans induced formation of a large number of osteoclasts, which were identified as large multinucleated TRAP-positive cells in lining the bone surface. S. mutans stimulated a sevenfold-greater increase in both the number of osteoclasts and the percent bone surface covered by osteoclasts compared to P. endodontalis at the 5-day time point (Fig. 2 and 3). Thus, the magnitude of the induced osteoclastogenesis agrees well with the results obtained by measuring the recruitment of inflammatory cells, indicating that S. mutans stimulates a more vigorous host response than P. endodontalis. With the negative control (vehicle alone), there was virtually no stimulation of osteoclast formation.
FIG. 2.
Quantitative assessment of osteoclast formation following injection of heat-killed S. mutans and P. endodontalis. Heat-killed S. mutans (S.m.) and P. endodontalis (P.e.) (108 bacteria/scalp) were injected into CD-1 wild-type mice. Vehicle alone was injected as a negative control (C). Mice were sacrificed at 0, 2, 5, and 10 days after injection. Sections were stained with TRAP. Image analysis was used to quantify the total number of osteoclasts per millimeter at a magnification of ×1,000. Five specimens were examined per data point.
FIG. 3.
Quantitative assessment of osteoclast activity following injection of heat-killed S. mutans (S.m.) and P. endodontalis (P.e.). The results demonstrated in Fig. 2 were analyzed to quantify the percent bone surface covered by osteoclasts at a magnification of ×1,000. Five specimens were examined per data point.
In the experiments described above, we used heat-killed bacteria to simplify interpretation of the experimental results since the confounding influence of bacterial survival in vivo is eliminated. Since it is possible that heat inactivation of the bacteria may had altered their true capacity to induce a host response, we repeated the experiments with live bacteria in vivo. Similar results were obtained, with S. mutans inducing a greater degree of inflammatory response (tissue necrosis and inflammatory cell infiltration) and osteoclast activity (data not shown). Thus, the host response in vivo induced by inoculation with either heat-killed or live S. mutans is dramatically more pronounced than that stimulated by P. endodontalis. Other investigators have found that injection of either heat-killed or live oral bacteria in this model produces similar inflammatory responses (35).
Bacterium-stimulated cytokine production.
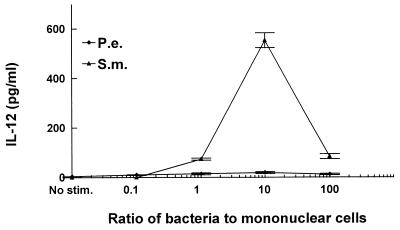
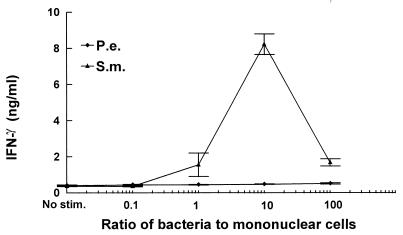
To explore the mechanisms to account for differences in the inflammatory response and osteoclast activation induced by S. mutans or P. endodontalis infection, we examined the capacities of these bacteria to stimulate production of a proinflammatory cytokine profile by mononuclear cells in vitro. Since IL-12 is produced by bacterium-stimulated macrophages and bridges the innate immune response and cell-mediated immune response, we examined IL-12 production stimulated by these two bacteria. The results demonstrate that S. mutans significantly increased IL-12 production compared to unstimulated controls at ratios of 1, 10, and 100 bacteria per PBMC (Fig. 4). The highest levels of production of IL-12 by PBMC were 92-fold above unstimulated control levels at a ratio of 10 bacteria per PBMC. In contrast, P. endodontalis stimulated only a minimal increase in IL-12 production compared to unstimulated controls. Since IFN-γ is a powerful activator for macrophages and IL-12 increases inflammatory responses by enhancing the production of IFN-γ (8, 17), we examined bacterium-stimulated IFN-γ production. Figure 5 shows that S. mutans stimulated high levels of IFN-γ by PBMC. S. mutans stimulated IFN-γ production approximately 22-fold over unstimulated controls at a ratio of 10 bacteria per PBMC, while P. endodontalis induced none.
FIG. 4.
S. mutans stimulates high levels of production of IL-12 by PBMC. PBMC were stimulated by the indicated ratios of bacteria to PBMC. Supernatants were collected after 24 h and analyzed by ELISA for IL-12. The results are representative of three experiments. Each data point is the mean of three triplicate samples. By one-way analysis of variance with Duncan’s post hoc test, S. mutans (S.m.) stimulation significantly increased IL-12 production compared to unstimulated controls (C) (<10 pg/ml) at ratios of 1, 10, and 100 (P < 0.05). P.e., P. endodontalis.
FIG. 5.
S. mutans stimulates high levels of production of IFN-γ by PBMC. PBMC were stimulated by the indicated ratios of bacteria to PBMC. Supernatants were collected after 24 h and analyzed by ELISA for IFN-γ. The results are representative of three experiments. Each data point is the mean of triplicate samples. By one-way analysis of variance with Duncan’s post hoc test, S. mutans (S.m.) stimulation significantly increased IFN-γ production compared to unstimulated controls (C) (0.4 ng/ml) at ratios of 1, 10, and 100 (P < 0.05). P.e., P. endodontalis.
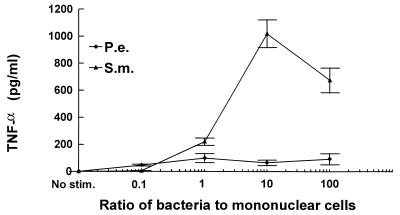
TNF-α is an important proinflammatory cytokine. It can promote inflammatory cell infiltration leading to a vigorous host response and can directly stimulate osteoclast formation and activity (24). Therefore, we examined TNF-α induction by putative endodontic pathogens and found that S. mutans stimulated high levels of TNF-α production by PBMC whereas P. endodontalis was a relatively weak inducer (Fig. 6).
FIG. 6.
S. mutans (S.m.) stimulates high and P. endodontalis (P.e.) stimulates low levels of production of TNF-α. PBMC were stimulated by the indicated ratios of bacteria to PBMC. Supernatants were collected after 24 h and analyzed by ELISA for TNF-α. The results are representative of three experiments. Each data point is the mean of triplicate samples. By one-way analysis of variance with Duncan’s post hoc test, bacterial stimulation significantly increased TNF-α production compared to unstimulated controls (C) at ratios of 1, 10, and 100 (P < 0.05). Unstimulated PBMC secreted undetectable levels (<10 pg/ml) of TNF-α.
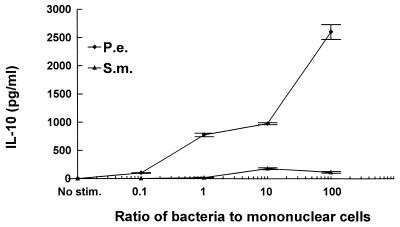
IL-10 inhibits IL-12, IFN-γ, and TNF-α production and is considered an antiinflammatory cytokine. In contrast to the above findings, P. endodontalis stimulated high levels of IL-10 production (2,600 pg/ml) whereas S. mutans stimulated low levels (178 pg/ml) (Fig. 7). Compared to S. mutans, P. endodontalis stimulated 15-fold more IL-10 production.
FIG. 7.
P. endodontalis (P.e.) stimulates high levels of production of IL-10 by PBMC. PBMC were stimulated by the indicated ratios of bacteria to PBMC. Supernatants were collected after 24 h and analyzed by ELISA. The results are representative of three experiments. Each data point is the mean of triplicate samples. By one-way analysis of variance with Duncan’s post hoc test, bacterial stimulation significantly increased IL-10 production compared to unstimulated controls at ratios of 1, 10, and 100 (P < 0.05). Unstimulated controls secreted undetectable levels of (<10 pg/ml) of IL-10. S.m. S. mutans.
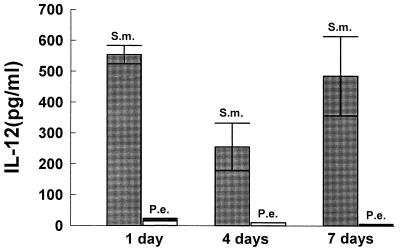
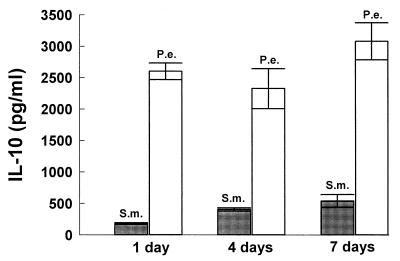
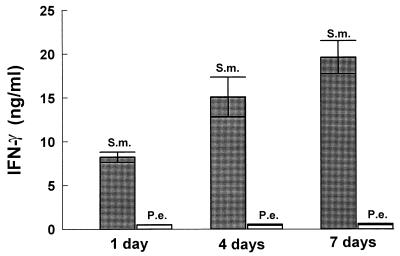
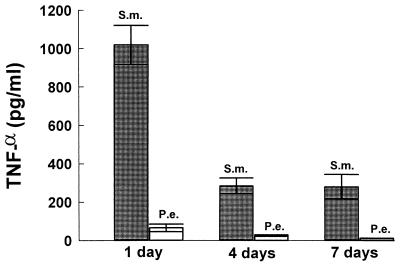
To further investigate the capacity of each of the two bacteria to stimulate production of an inflammatory cytokine profile, time-dependent cytokine induction was assessed over periods of 1, 4, and 7 days. This would take into account potential cell-to-cell interactions that occur from different leukocyte subsets found in mononuclear cell populations. Figures 8 to 11 demonstrate that the patterns of cytokine induction by both bacteria are consistent with time. S. mutans stimulated high levels of IL-12 (Fig. 8) and IFN-γ (Fig. 9) at 1-, 4-, and 7-day time points, while P. endodontalis did not. The levels of TNF-α production decreased with time for both bacteria but kept the same order of potency. Thus, S. mutans was a more efficacious stimulator than P. endodontalis (Fig. 10). For IL-10 induction, the order was reversed, as P. endodontalis stimulated high levels and S. mutans stimulated low levels of IL-10 (Fig. 11). These findings are not restricted to the strain of S. mutans used here (OMZ175), since similar results were obtained with another strain, LM7 (data not shown).
FIG. 8.
Bacterium-stimulated production of IL-12 by PBMC at 1, 4, and 7 days. PBMC were stimulated by 10 bacteria per PBMC. Supernatants were collected at the indicated times and analyzed by ELISA for IL-12. The results are representative of two experiments. Each data point is the mean of three triplicate samples. By one-way analysis of variance with Duncan’s post hoc test, S. mutans (S.m.) stimulation significantly increased IL-12 production compared to P. endodontalis (P.e.) at all time points (P < 0.05).
FIG. 11.
Bacterium-stimulated production of IL-10 by PBMC at 1, 4, and 7 days. PBMC were stimulated by 100 bacteria per PBMC. Supernatants were collected at the indicated times and analyzed by ELISA. The results are representative of two experiments. Each data point is the mean of triplicate samples. By one way analysis of variance with Duncan’s post hoc test, P. endodontalis (P.e.) stimulation significantly increased IL-10 production compared to S. mutans (S.m.) at all time points (P < 0.05).
FIG. 9.
Bacterium-stimulated production of IFN-γ by PBMC at 1, 4, and 7 days. PBMC were stimulated by 10 bacteria per PBMC. Supernatants were collected at the indicated times and analyzed by ELISA for IFN-γ. The results are representative of two experiments. Each data point is the mean of triplicate samples. By one-way analysis of variance with Duncan’s post hoc test, S. mutans (S.m.) stimulation significantly increased IFN-γ production compared to P. endodontalis (P.e.) at all time points (P < 0.05).
FIG. 10.
Bacterium-stimulated production of TNF-α by PBMC at 1, 4, and 7 days. PBMC were stimulated by 10 bacteria per PBMC. Supernatants were collected at the indicated times and analyzed by ELISA for TNF-α. The results are representative of two experiments. Each data point is the mean of triplicate samples. By one-way analysis of variance with Duncan’s post hoc test, S. mutans (S.m.) stimulation significantly increased TNF-α production compared to P. endodontalis (P.e.) at all time points (P < 0.05).
DISCUSSION
We demonstrate here that two putative oral pathogens are considerably different in their capacities to stimulate human PBMC to produce inflammatory and immunoregulatory cytokines. Interestingly, S. mutans, which stimulates high levels of Th1 cytokines such as IL-12 and IFN-γ, also stimulates high levels of the proinflammatory cytokine TNF-α. In contrast, P. endodontalis, which induces little or none of the above cytokines, appears to be potent in inducing IL-10, an anti-inflammatory cytokine. It is possible that IL-10 induced in response to P. endodontalis inhibits expression of other cytokines by the mononuclear cells. Regardless of the mechanism, it is clear that the inherent properties of an invading bacterium influence the cytokine profile that is ultimately produced.
The proinflammatory cytokine profile that was induced in human PBMC corresponded well with both quantitative and qualitative measures of the inflammatory response observed in vivo in mice. That human and murine leukocytes would behave similarly is not surprising given the large body of evidence indicating similarities in various immune responses (15, 23). When injected in vivo, S. mutans induced formation of a dense inflammatory cell infiltrate and bone resorptive activity compared to P. endodontalis. This finding is consistent with the production of TNF-α and IFN-γ stimulated by S. mutans and the relative lack of their expression induced by P. endodontalis.
S. mutans gained notoriety in the 1960s as a cariogenic pathogen; its virulence factors related to caries formation have been extensively studied (2). Bacteriemias of S. mutans are capable of causing significant morbidity and mortality through bacterial endocarditis or other complications such as respiratory distress syndrome (14, 22, 30). The mechanisms by which S. mutans produce an inflammatory response and causes tissue damage have not been elucidated. Our results demonstrated that S. mutans is able to elicit cell-mediated Th1 responses by stimulating expression of IL-12, IFN-γ, and TNF-α in mononuclear cells. We postulate that the exuberant production of these cytokines stimulated by S. mutans may contribute to pathological process of the infection since Th1 cytokines can exacerbate inflammatory pathology by enhancing chemokine expression and recruitment of inflammatory cells (3, 10, 18, 20, 26).
In contrast to S. mutans, P. endodontalis (which is associated with chronic infections of the dental pulp) has been found to be of low virulence in experimental monoinfections but seems to play an essential role in anaerobic mixed infection (33). The reasons for this interesting phenomenon have not been explained. In the present study, we demonstrate that P. endodontalis induces production of high levels of IL-10 but low levels of IL-12, IFN-γ, and TNF-α. Consistent with this cytokine profile, in vivo, P. endodontalis elicits mild inflammatory infiltration and bone resorption activity. It is possible that the cytokine profile induced by P. endodontalis (i.e., IL-10) contributes to its low virulence in monoinfections. However, in mixed infections, the “stealth” property of this microorganism may help itself and other bacteria to invade the host without causing strong host responses, so that the pathogens can reside in infected sites for long periods and grow to large numbers. The stealth properties of P. endodontalis are consistent with finding that a bacterium of the same genus, Porphyromonas gingivalis, has similar properties. P. gingivalis is poorly recognized by cells of the innate host defense system (13), and over 1,000 times more bacteria are required to stimulate high levels of chemokine production compared to S. mutans (6). Thus, both P. endodontalis and P. gingivalis seem to possess stealth properties, which may provide a selective advantage to the bacteria by enabling them to avoid host defense mechanisms. It is possible that this represents an important aspect of their pathologic potential.
From our results, we conclude that specific bacteria can induce the synthesis of a profile that is consistent with up-regulation of the host response or one that is not. Thus, the type of host response generated is considerably different for specific bacteria and is reflected by the cytokine profile induced in vitro. This conclusion is supported by results demonstrating that the nature of the cytokine profile induced in vitro is consistent with the capacity of the bacteria to produce an inflammatory infiltrate and stimulate pathologic osteoclast activity in vivo.
ACKNOWLEDGMENTS
We thank John Richardson for help in the histologic analysis of inflammatory cell infiltrates.
This study was partially supported by American Association of Endodontists.
REFERENCES
- 1.Baumgartner J, Falkler W J. Bacteria in the apical 5mm of infected root canals. J Endodont. 1991;17:380–383. doi: 10.1016/s0099-2399(06)81989-8. [DOI] [PubMed] [Google Scholar]
- 2.Burne R A. Oral streptococci…products of their environment. J Dent Res. 1998;77:445–452. doi: 10.1177/00220345980770030301. [DOI] [PubMed] [Google Scholar]
- 3.Danforth J, Strieter R, Kunkel S, Arenberg D, VanOtteren G, Standiford T. Macrophage inflammatory protein-1 alpha expression in vivo and in vitro: the role of lipoteichoic acid. Clin Immunol Immunopathol. 1995;74:77–83. doi: 10.1006/clin.1995.1011. [DOI] [PubMed] [Google Scholar]
- 4.Haapasalo M. Black-pigmented gram-negative anaerobes in endodontic infections. FEMS Immunol Med Microbiol. 1993;6:213–217. doi: 10.1111/j.1574-695X.1993.tb00329.x. [DOI] [PubMed] [Google Scholar]
- 5.Horaud T, Delbos F. Viridans streptococci in infective endocarditis: species distribution and susceptibility to antibiotics. Eur Heart J. 1984;5(Suppl. C):39–44. doi: 10.1093/eurheartj/5.suppl_c.39. [DOI] [PubMed] [Google Scholar]
- 6.Jiang Y, Russell T R, Graves D, Cheng H, Nong S, Levitz S. Monocyte chemoattractant protein 1 and interleukin-8 production in mononuclear cells stimulated by oral microorganisms. Infect Immun. 1996;64:4450–4455. doi: 10.1128/iai.64.11.4450-4455.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiang Y, Zhu J, Luscinskas W, Graves D. MCP-1-stimulated monocyte attachment to laminin is mediated by B2-integrins. Am J Physiol. 1994;267:C1112–C1118. doi: 10.1152/ajpcell.1994.267.4.C1112. [DOI] [PubMed] [Google Scholar]
- 8.Lau A, Sigaroudinia M, Yeung M, Kohl S. Interleukin-12 induces interferon-gamma expression and natural killer cytotoxicity in cord blood mononuclear cells. Pediatr Res. 1996;39:150–155. doi: 10.1203/00006450-199601000-00023. [DOI] [PubMed] [Google Scholar]
- 9.Li L, Elliott J, Mosmann T. IL-10 inhibits cytokine production, vascular leakage, and swelling during T helper 1 cell-induced delayed-type hypersensitivity. J Immunol. 1994;153:3967–3978. [PubMed] [Google Scholar]
- 10.Pearlman E, Lass J, Bardenstein D, Diaconu E, Hazlett F J, Albright J, Higgins A, Kazura J. IL-12 exacerbates helminth-mediated corneal pathology by augmenting inflammatory cell recruitment and chemokine expression. J Immunol. 1997;158:827–833. [PubMed] [Google Scholar]
- 11.Pfizenmaiener K, Himmler A, Schütze S, Scheurich P, Krönke M. TNF receptors and TNF signal transduction. In: Beutler B, editor. Tumor necrosis factors: the molecules and their emerging role in medicine. New York, N.Y: Raven Press; 1993. pp. 439–472. [Google Scholar]
- 12.Rahimi P, Wang C, Stashenko P, Lee S, Lorenzo J, Graves D. MCP-1 expression and monocyte recruitment in osseous inflammation. Endocrinology. 1995;136:2752–2759. doi: 10.1210/endo.136.6.7750500. [DOI] [PubMed] [Google Scholar]
- 13.Reife R, Shapiro R, Bamber B, Berry K, Mick G, Darveau R. Porphyromonas gingivalis lipopolysaccharide is poorly recognized by molecular components of innate host defense in a mouse model of early inflammation. Infect Immun. 1995;63:4686–4694. doi: 10.1128/iai.63.12.4686-4694.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robbins N, Szilagyi G, Tanowitz H, Luftschein S, Baum S. Infective endocarditis caused by Streptococcus mutans. A complication of idiopathic hypertrophic subaortic stenosis. Arch Intern Med. 1977;137:1171–1174. [PubMed] [Google Scholar]
- 15.Romagnani S. Human TH1 and TH2 subsets: “eppur si muove.”. Eur Cytokine Netw. 1994;5:7–12. [PubMed] [Google Scholar]
- 16.Samaranayake L. Essential microbiology for dentistry. New York, N.Y: Churchill Livingstone; 1996. [Google Scholar]
- 17.Sartori A, Ma X, Gri G, Showe L, Benjamin D, Trinchieri G. Interleukin-12: an immunoregulatory cytokine produced by B cells and antigen-presenting cells. Methods. 1997;11:116–127. doi: 10.1006/meth.1996.0395. [DOI] [PubMed] [Google Scholar]
- 18.Schwarz M, Radeke H, Resch K, Uciechowski P. Lymphocyte-derived cytokines induce sequential expression of monocyte- and T cell-specific chemokines in human mesangial cells. Kidney Int. 1997;52:1521–1531. doi: 10.1038/ki.1997.482. [DOI] [PubMed] [Google Scholar]
- 19.Soto A, McWhinney P, Kibbler C, Cohen J. Cytokine release and mitogenic activity in the viridans streptococcal shock syndrome. Cytokine. 1998;10:370–376. doi: 10.1006/cyto.1997.0300. [DOI] [PubMed] [Google Scholar]
- 20.Standiford T, Arenberg D, Danforth J, Kunkel S, VanOtteren G, Strieter R. Lipoteichoic acid induces secretion of interleukin-8 from human blood monocytes: a cellular and molecular analysis. Infect Immun. 1994;62:119–125. doi: 10.1128/iai.62.1.119-125.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stashenko P, Dewhirst F E, Peros W J, Kent R L, Ago J M. Synergistic interactions between interleukin 1, tumor necrosis factor, and lymphotoxin in bone resorption. J Immunol. 1987;138:1464–1468. [PubMed] [Google Scholar]
- 22.Stein J, editor. Internal medicine. 4th ed. St. Louis, Mo: C. V. Mosby; 1994. pp. 189–193. [Google Scholar]
- 23.Street N, Mosmann T. Functional diversity of T lymphocytes due to secretion of different cytokine patterns. FASEB J. 1991;5:171–177. doi: 10.1096/fasebj.5.2.1825981. [DOI] [PubMed] [Google Scholar]
- 24.Suttles J, Miller R, Tao X, Stout R. T cells which do not express membrane tumor necrosis factor-alpha activate macrophage effector function by cell contact-dependent signaling of macrophage tumor necrosis factor-alpha production. Eur J Immunol. 1994;24:1736–1742. doi: 10.1002/eji.1830240803. [DOI] [PubMed] [Google Scholar]
- 25.Sylvester I, Rankin J, Yoshimura T, Tanaka S, Leonard E. Secretion of neutrophil attractant/activation protein by lipopolysaccharide-stimulated lung macrophages determined by both enzyme-linked immunosorbent assay and N-terminal sequence analysis. Am Rev Respir Dis. 1990;141:683–688. doi: 10.1164/ajrccm/141.3.683. [DOI] [PubMed] [Google Scholar]
- 26.Tani K, Su S, Utsunomiya I, Oppenheim J, Wang J. Interferon-gamma maintains the binding and functional capacity of receptors for IL-8 on cultured human T cells. Eur J Immunol. 1998;28:502–507. doi: 10.1002/(SICI)1521-4141(199802)28:02<502::AID-IMMU502>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 27.Tani-Ishii N, Wang C, Tanner A, Stashenko P. Changes in root canal microbiota during the development of rat periapical lesions. Oral Microbiol Immunol. 1994;9:129–135. doi: 10.1111/j.1399-302x.1994.tb00048.x. [DOI] [PubMed] [Google Scholar]
- 28.Thompson B, Mundy G, Chambers T. Tumor necrosis factors α and β induce osteoblastic cell to stimulate osteoclastic bone resorption. J Immunol. 1987;138:775–779. [PubMed] [Google Scholar]
- 29.Trinchieri G. Cytokines acting on or secreted by macrophages during intracellular infection (IL-10, IL-12, IFN-gamma) Curr Opin Immunol. 1997;9:17–23. doi: 10.1016/s0952-7915(97)80154-9. [DOI] [PubMed] [Google Scholar]
- 30.Ullman R, Miller S, Strampfer M, Cunha B. Streptococcus mutans endocarditis: report of three cases and review of the literature. Heart Lung. 1988;17:209–212. [PubMed] [Google Scholar]
- 31.van de Rijn I, George M, Bouvet A, Roberts R B. Enzyme-linked immunosorbent assay for the detection of antibodies to nutritionally variant streptococci in patients with endocarditis. J Infect Dis. 1986;153:116–121. doi: 10.1093/infdis/153.1.116. [DOI] [PubMed] [Google Scholar]
- 32.Van Winkelhoff A, Carlee A, de Graaff J. Bacteroides endodontalis and other black-pigmented Bacteroides species in odontogenic abcesses. Infect Immun. 1985;49:494–497. doi: 10.1128/iai.49.3.494-497.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Van Winkelhoff A, van Steenbergen M, de Graaff J. Porphyromonas (Bacteroides) endodontalis: its role in endodontal infections. J Endodont. 1992;18:431–434. doi: 10.1016/s0099-2399(06)80843-5. [DOI] [PubMed] [Google Scholar]
- 34.Wayman B, Murata S, Almeida R, Fowler C. A bacteriological and histological evaluation of 58 periapical lesions. J Endodont. 1992;18:152–155. doi: 10.1016/S0099-2399(06)81409-3. [DOI] [PubMed] [Google Scholar]
- 35.Zubery Y, Dunstan C, Story B, Kesavalu L, Ebersole J, Holt S, Boyce B. Bone resorption caused by three periodontal pathogens in vivo in mice is mediated in part by prostaglandin. Infect Immun. 1998;66:4158–4162. doi: 10.1128/iai.66.9.4158-4162.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]