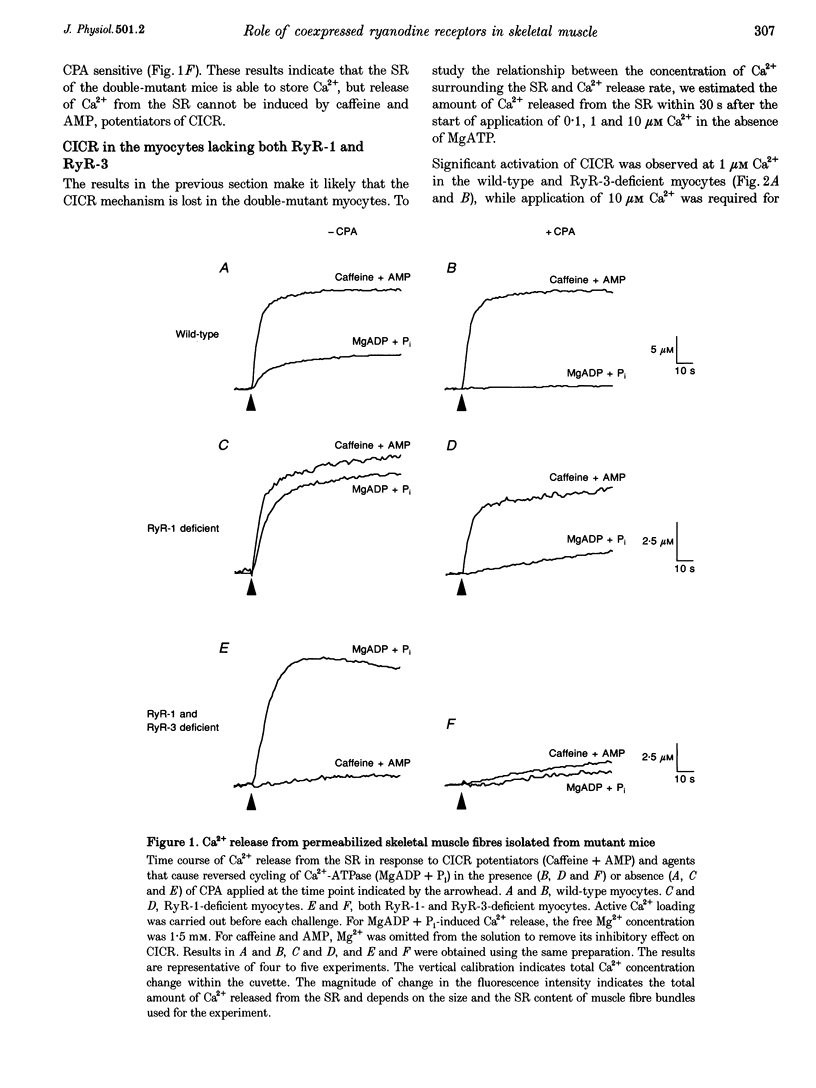
Abstract
1. We generated mice with targeted disruptions in the genes for both ryanodine receptor type 1 (RyR-1) and type 3 (RyR-3) to study the functional roles of RyR subtypes in skeletal muscle. 2. In permeabilized myocytes lacking both the RyRs, the Ca(2+)-induced Ca2+ release (CICR) mechanism was completely lost, and caffeine failed to induce Ca2+ release. 3. Replacement of potassium methanesulphonate in an experimental intracellular solution with choline chloride resulted in Ca2+ release in the wild-type muscle but not in the mutant muscle lacking RyR-1. 4. The double-mutant mice exhibited more severe muscular degeneration than RyR-1-deficient mice with formation of large vacuoles and swollen mitochondria while structural coupling between T-tubules and the sarcoplasmic reticulum was retained. 5. These results demonstrate that CICR is mediated solely by RyR-1 and RyR-3 in skeletal muscle cells, and suggest that RyR-1 is involved in Cl(-)-induced Ca2+ release. The results also suggest the presence of molecular components other than RyRs responsible for the triad formation. RyR-3 may have a role in the normal morphogenesis of skeletal muscle cells, although functionally it can be replaced by RyR-1.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barlogie B., Hasselbach W., Makinose M. Activation of calcium efflux by ADP and inorganic phosphate. FEBS Lett. 1971 Jan 30;12(5):267–268. doi: 10.1016/0014-5793(71)80194-1. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and calcium signalling. Nature. 1993 Jan 28;361(6410):315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- Block B. A., Imagawa T., Campbell K. P., Franzini-Armstrong C. Structural evidence for direct interaction between the molecular components of the transverse tubule/sarcoplasmic reticulum junction in skeletal muscle. J Cell Biol. 1988 Dec;107(6 Pt 2):2587–2600. doi: 10.1083/jcb.107.6.2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo M. Calcium release from the sarcoplasmic reticulum. Physiol Rev. 1977 Jan;57(1):71–108. doi: 10.1152/physrev.1977.57.1.71. [DOI] [PubMed] [Google Scholar]
- Endo M., Iino M. Specific perforation of muscle cell membranes with preserved SR functions by saponin treatment. J Muscle Res Cell Motil. 1980 Mar;1(1):89–100. doi: 10.1007/BF00711927. [DOI] [PubMed] [Google Scholar]
- Franzini-Armstrong C. Simultaneous maturation of transverse tubules and sarcoplasmic reticulum during muscle differentiation in the mouse. Dev Biol. 1991 Aug;146(2):353–363. doi: 10.1016/0012-1606(91)90237-w. [DOI] [PubMed] [Google Scholar]
- Fruen B. R., Kane P. K., Mickelson J. R., Louis C. F. Chloride-dependent sarcoplasmic reticulum Ca2+ release correlates with increased Ca2+ activation of ryanodine receptors. Biophys J. 1996 Nov;71(5):2522–2530. doi: 10.1016/S0006-3495(96)79445-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goeger D. E., Riley R. T., Dorner J. W., Cole R. J. Cyclopiazonic acid inhibition of the Ca2+-transport ATPase in rat skeletal muscle sarcoplasmic reticulum vesicles. Biochem Pharmacol. 1988 Mar 1;37(5):978–981. doi: 10.1016/0006-2952(88)90195-5. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Iino M. Calcium-induced calcium release mechanism in guinea pig taenia caeci. J Gen Physiol. 1989 Aug;94(2):363–383. doi: 10.1085/jgp.94.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inui M., Saito A., Fleischer S. Purification of the ryanodine receptor and identity with feet structures of junctional terminal cisternae of sarcoplasmic reticulum from fast skeletal muscle. J Biol Chem. 1987 Feb 5;262(4):1740–1747. [PubMed] [Google Scholar]
- Lai F. A., Erickson H. P., Rousseau E., Liu Q. Y., Meissner G. Purification and reconstitution of the calcium release channel from skeletal muscle. Nature. 1988 Jan 28;331(6154):315–319. doi: 10.1038/331315a0. [DOI] [PubMed] [Google Scholar]
- Makinose M., Hasselbach W. ATP synthesis by the reverse of the sarcoplasmic calcium pump. FEBS Lett. 1971 Jan 30;12(5):271–272. doi: 10.1016/0014-5793(71)80196-5. [DOI] [PubMed] [Google Scholar]
- Nakajima Y., Endo M. Release of calcium induced by 'depolarisation' of the sarcoplasmic reticulum membrane. Nat New Biol. 1973 Dec 19;246(155):216–218. doi: 10.1038/newbio246216a0. [DOI] [PubMed] [Google Scholar]
- Sutko J. L., Airey J. A. Ryanodine receptor Ca2+ release channels: does diversity in form equal diversity in function? Physiol Rev. 1996 Oct;76(4):1027–1071. doi: 10.1152/physrev.1996.76.4.1027. [DOI] [PubMed] [Google Scholar]
- Takekura H., Nishi M., Noda T., Takeshima H., Franzini-Armstrong C. Abnormal junctions between surface membrane and sarcoplasmic reticulum in skeletal muscle with a mutation targeted to the ryanodine receptor. Proc Natl Acad Sci U S A. 1995 Apr 11;92(8):3381–3385. doi: 10.1073/pnas.92.8.3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeshima H., Iino M., Takekura H., Nishi M., Kuno J., Minowa O., Takano H., Noda T. Excitation-contraction uncoupling and muscular degeneration in mice lacking functional skeletal muscle ryanodine-receptor gene. Nature. 1994 Jun 16;369(6481):556–559. doi: 10.1038/369556a0. [DOI] [PubMed] [Google Scholar]
- Takeshima H., Ikemoto T., Nishi M., Nishiyama N., Shimuta M., Sugitani Y., Kuno J., Saito I., Saito H., Endo M. Generation and characterization of mutant mice lacking ryanodine receptor type 3. J Biol Chem. 1996 Aug 16;271(33):19649–19652. doi: 10.1074/jbc.271.33.19649. [DOI] [PubMed] [Google Scholar]
- Takeshima H., Yamazawa T., Ikemoto T., Takekura H., Nishi M., Noda T., Iino M. Ca(2+)-induced Ca2+ release in myocytes from dyspedic mice lacking the type-1 ryanodine receptor. EMBO J. 1995 Jul 3;14(13):2999–3006. doi: 10.1002/j.1460-2075.1995.tb07302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]



