Abstract
The 19-kDa conserved C-terminal part of the Plasmodium falciparum merozoite surface protein 1 (PfMSP119) is a malaria vaccine candidate antigen, and human antibody responses to PfMSP119 have been associated with protection against clinical malaria. In this longitudinal study carried out in an area of stable but seasonal malaria transmission with an estimated parasite inoculation of about 20 infective bites/year, we monitored 266 3- to 15-year-old Ghanaian children clinically and parasitologically over a period of 18 months. Blood samples were collected at the beginning of the study before the major malaria season in April and after the season in November. Using enzyme-linked immunosorbent assay, we measured antibody responses to recombinant gluthathione S-transferase–PfMSP119 fusion proteins corresponding to the Wellcome and MAD20 allelic variants in these samples. Prevalence of antibodies recognizing the Wellcome 19 construct containing both epidermal growth factor (EGF)-like motifs in Wellcome type PfMSP119 was about 30%. Prevalence of antibodies to constructs containing only the first EGF domain from either Wellcome or MAD20 type PfMSP119 was about 15%, whereas antibodies recognizing a construct containing only the second EGF domain of MAD20 type PfMSP119 was found in only about 4% of the donors. Neither the prevalence nor the levels of any of the antibody specificities varied significantly with season, age, or sex. Significantly, and in contrast to previous reports from other parts of West Africa, we found no evidence of an association between antibody responses to PfMSP119 and clinical protection against malaria.
The asexual blood stages of the Plasmodium falciparum parasite are responsible for the clinical manifestations of malaria, and attempts have consequently been made to identify asexual stage antigens that may be of importance in the development of protective immunity to the disease (43). One such well-characterized antigen is the P. falciparum merozoite surface protein 1 (PfMSP1), which is located on the surface of blood stage merozoites. It is synthesized as a 200-kDa protein during schizogony but processed into fragments with diverse molecular weights, most of which are discarded before erythrocyte invasion (30). The final processing of the C-terminal 42-kDa fragment yields a 33-kDa protein, which is shed, and a relatively conserved 19-kDa part (PfMSP119), which remains attached to the merozoite during erythrocyte invasion and is expressed by the parasite during the early ring stages (29). Antibodies against this fragment may block merozoite invasion of erythrocytes and also inhibit parasite multiplication inside the erythrocytes (28, 29). The objective of this study was to verify the previous finding of association between antibody responses to PfMSP119 and protection from clinical malaria (23) and to characterize how donor age and season influence the levels of these antibodies.
MATERIALS AND METHODS
Study area.
The study was conducted in Dodowa, a semirural town approximately 50 km northeast of Accra, Ghana. It is predominantly a subsistence farming community with a population of about 6,500. There are two rainy seasons in this area: a major rainy season from May to August, and a minor one occurring between October and November. This is followed by a relatively dry season from December to April. Malaria transmission is perennial, but is highest during or immediately after the major and minor rainy seasons (high-transmission season) and lowest during the dry season (low-transmission season). It has been estimated that individuals in Dodowa are exposed to about 20 infective bites per year, and 98% of the infections are due to P. falciparum (1). Dodowa can thus be described as an area of hyperendemic and seasonal malaria transmission. The transmission is stable since it does not vary considerably from year to year.
Study population and clinical surveillance.
The study population consisted of a cohort of 300 schoolchildren, 3 to 15 years of age, of whom 54% were males and 46% were females. The cohort included between 13 and 37 children at each year of age. Informed parental consent was obtained after thorough explanation of all procedures involved in the study, which was approved by the Ghanaian Ministry of Health. The children selected were typed negative for sickle cell trait prior to the start of the study in April 1994. The study was completed in August 1995. During this period, the cohort was monitored clinically and parasitologically with the help of six field assistants who were resident in the town. Each child was visited once a week; during each visit, information regarding the health status in the previous week was recorded on a standard questionnaire form, and measurement of axillary temperatures was determined with a digital thermometer. Blood slide samples for detection of parasitemia were made from children with temperatures of ≥37.5°C and from children complaining of symptoms suggestive of malaria. Parents were also instructed to bring sick children to the field assistants outside the weekly scheduled visits, for recording of temperature and blood sampling by fingerprick. Any child with detectable parasitemia and fever was immediately treated with chloroquine, but for the analysis of data individuals were considered to have malaria only if (i) they reported fever and/or had a measured temperature higher of than 37.5°C and (ii) they had parasitemia of ≥5,000 parasites/μl. For the duration of the study, blood slide samples were obtained from all children once a month to determine the point prevalence of parasitemia.
The serological data included in this report are from samples obtained from the 266 children for whom clinical and parasitological data were available for the duration of the 18-month follow-up period and from whom two venous blood samples were obtained (see below). The composition of this group was essentially identical to that of the full cohort (data not shown).
Blood sampling.
Venous blood samples were obtained from the cohort on two occasions. The first samples were collected in April 1994, just before the onset of major rainy season (low-transmission-season samples), and the second samples were collected during November 1994 after the rainy seasons (high-transmission-season samples). Ten to 20 ml of venous blood from each donor was drawn aseptically into heparinized Vacutainer tubes (Becton Dickinson, Rutherford, N.J.). Plasma and cell samples were separated under sterile conditions by density centrifugation on Lymphoprep (Nyegaard, Oslo, Norway). Plasma samples were stored at −20°C. Negative control plasma samples were obtained from 31 healthy Danish adults who had never lived in a malaria-endemic area. A pool of plasma samples obtained from five adults living in Dodowa and selected for high antibody reactivity to the PfMSP119 constructs were used as positive controls.
Recombinant antigens.
Recombinant PfMSP119 and gluthathione S-transferase (GST) fusion proteins from the Wellcome and MAD20 strains of P. falciparum were expressed in Escherichia coli transformed with pGEX3 plasmids and selected for ampicillin resistance as described previously (22). Briefly, overnight cultures from single colonies of PfMSP119-specific E. coli were expanded in Lewis broth containing 100 μg of ampicillin per ml at 37°C with shaking (180 rpm). The cultures were maintained for 3 h to attain the exponential growth phase and then stimulated with 0.2 mM isopropyl-β-d-thiogalactopyranoside to induce protein synthesis for a further 4 h. The E. coli cell pellet was lysed by freeze-thaw cycles, and the fusion protein was affinity purified with gluthathione-agarose beads. The purity of the extracted proteins was confirmed by the presence of single bands on Coomassie blue-stained sodium dodecyl sulfate-polyacrylamide gels. Fusion proteins containing the first epidermal growth factor (EGF)-like motif (Wellcome EGF1) of PfMSP119 or both the first and the second EGF-like motifs (Wellcome 19) of the Wellcome strain were prepared. In addition, we prepared two fusion proteins corresponding to the MAD20-type PfMSP119 antigens: MAD20 EGF1 and MAD20 EGF2, containing the first and the second EGF-like motifs, respectively. Control GST protein was prepared from E. coli with only GST gene constructs.
Antibody measurements.
Plasma antibodies to the recombinant PfMSP1-GST antigens were measured by enzyme-linked immunosorbent assay (ELISA) as described elsewhere (22). Briefly, microtiter test and control plates (Nunc, Roskilde, Denmark) were coated in parallel with recombinant PfMSP1-GST fusion protein (1 μg/ml) and GST control protein (5 μg/ml), respectively, and incubated at 4°C overnight. This was followed by blocking with 1% nonfat skimmed milk at 37°C for 1 h. Test samples diluted 1,000 times were then added in duplicate to both PfMSP1 fusion protein- and GST-coated plates and incubated overnight at 4°C. The plates were subsequently developed with peroxidase-conjugated rabbit anti-human immunoglobulin G (IgG) (Dako, Glostrup, Denmark), followed by H2O2 with o-phenylenediamine, and absorbance was read at 492 nm. Background optical density (OD) from GST-coated plates was subtracted from the OD of PfMSP-1 coated plates to obtain PfMSP-1-specific OD values as described previously (23). The two samples collected from each individual were assayed at the same time. To account for day-to-day variation, results were calculated as relative OD: (ODsample − ODbuffer control)/(ODpositive control − ODbuffer control). The lower limit of positivity was determined as the mean of ELISA readings of plasmas from 31 unexposed Danish donors, plus 2 standard deviations. All samples that were positive for total IgG were tested for IgG subclasses 1, 2, 3, and 4 in a similar ELISA, which was optimized to detect IgG subclasses by titration experiments. We used an antigen coating concentration of 5 μg/ml, plasma dilution of 1/200, and IgG subclass horseradish peroxidase conjugate (Zymed, San Francisco, Calif.) diluted 1/500.
Statistical analysis.
Statistical analysis was done with the SigmaStat software package (Jandel Scientific Corporation, San Rafael, Calif.). The χ2 test was used to compare proportions of antibody responders in protected and unprotected children, and in males and females, while the Mann-Whitney and Wilcoxon signed rank tests were used to compare the antibody levels between the groups for paired and unpaired data, respectively. Spearman’s rank correlation test was used to correlate antibody responses in low- and high-malaria-transmission samples and to assess associations between antibody levels and age. Differences were considered statistically significant if P was <0.05.
RESULTS
P. falciparum infections in the study cohort.
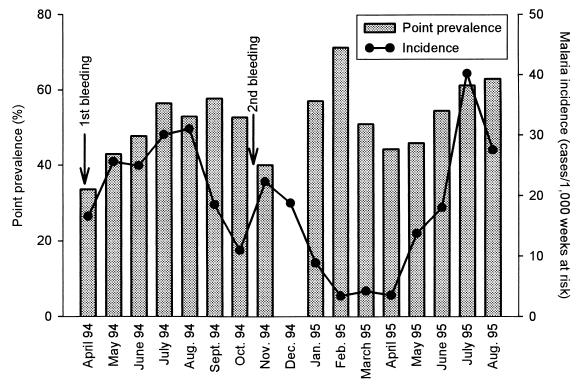
All except two of the children in the study cohort were parasitemic at one or more of the monthly parasite screenings done to determine point prevalence. As shown in Fig. 1, the point prevalence of patent P. falciparum parasitemia fluctuated around 50% during the 18-month study period. The incidence of clinical malaria was estimated as cases per 1,000 weeks at risk, taking account of the children who were absent for specified periods during the study period. It varied considerably over the year peaking in August 1994 and July 1995, reaching the lowest in April 1994 and February 1995 (Fig. 1). Children were not considered to be at risk for 1 month after a clinical episode of malaria. In the cases where several episodes of malaria occurred in the same individual, they were considered to constitute discrete episodes only if they were separated by at least 1 month.
FIG. 1.
Malaria transmission in Dodowa, southern Ghana, during the period of surveillance. Point prevalence of P. falciparum parasitemia and incidence of malaria in the study cohort are shown. Point prevalence was not estimated in December 1994. The timing of collection of venous blood samples is indicated by arrows.
During the 18-month period of surveillance, 108 (41%) of the children had at least one clinical episode of malaria, and these children were considered to be susceptible to malaria (group 1). One hundred three (39%) of the children did not have complaints of fevers or measured febrile temperatures in the presence of asexual parasitemia ≥5,000 parasites/μl at any of the weekly screenings, and these children were considered to be clinically protected from malaria (group 2). Fifty-five of children had measured fevers or complaints of fevers associated with parasitemia <5,000 parasites/μl. In these children it is uncertain whether the symptoms were due to malaria (38), and these children were categorized as group 3. Children in group 1 tended to be younger (median age in years, 5; range, 3 to 15) than the children in either group 2 (median, 10; range, 3 to 15) or group 3 (median, 9; range, 3 to 15). Only groups 1 and 2 were used for comparing antibody responses in children considered susceptible or immune to malaria.
Antibody recognition of the PfMSP119 recombinant antigens.
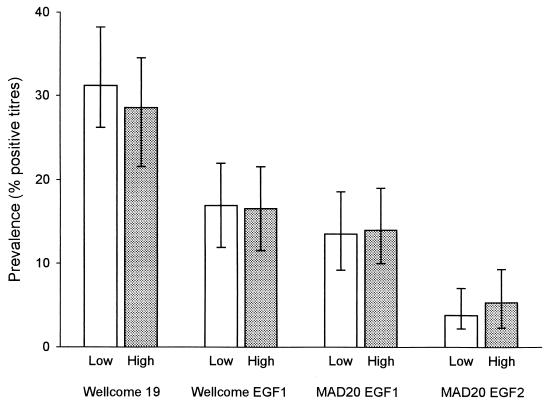
Antibody responses were measured to four recombinant antigens derived from the 19-kDa C-terminal part of the PfMSP1. Of these, the most commonly recognized (frequency of about 30%) was the Wellcome 19 construct, followed by the two EGF1 constructs (about 15%), while only about 4% of the plasma samples contained detectable levels of antibodies to the MAD20 EGF2 antigen (Fig. 2). The frequencies of positive antibody responses and the levels of antibodies to all recombinant antigens were similar in samples collected during the low- and high-transmission seasons (Fig. 2 and data not shown).
FIG. 2.
Point prevalence of positive antibody titers to recombinant PfMSP119 antigens during the low- and high-transmission seasons. Error bars indicate 95% confidence intervals for estimates.
Anti-PfMSP119 antibodies were detected in all age groups of the cohort in both low- and high-transmission seasons. Neither antibody levels nor the proportion of positive samples varied with age or sex for any of the four antigens studied (Table 1 and data not shown).
TABLE 1.
Correlation between levels of antibodies to PfMSP1 constructs and age in Ghanaian children
| Antigen construct | Low-transmission season
|
High-transmission season
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All samples
|
Positive responses only
|
All samples
|
Positive responses only
|
|||||||||
| rs | P(rs) | n | rs | P(rs) | n | rs | P(rs) | n | rs | P(rs) | n | |
| Wellcome 19 | 0.09 | 0.13 | 266 | 0.04 | 0.69 | 83 | 0.01 | 0.86 | 266 | −0.02 | 0.88 | 78 |
| Wellcome EGF1 | 0.04 | 0.56 | 266 | 0.16 | 0.30 | 45 | 0.09 | 0.16 | 266 | −0.02 | 0.90 | 44 |
| MAD20 EGF1 | 0.00 | 0.94 | 265 | 0.22 | 0.17 | 36 | −0.06 | 0.38 | 265 | 0.05 | 0.74 | 37 |
| MAD20 EGF2 | 0.04 | 0.52 | 265 | −0.01 | 0.77 | 10 | −0.03 | 0.63 | 265 | −0.15 | 0.59 | 14 |
rs, Spearman rank correlation coefficient; P(rs), associated level of significance; N, number of samples tested.
Correlation of responses at different measurements in the same individual.
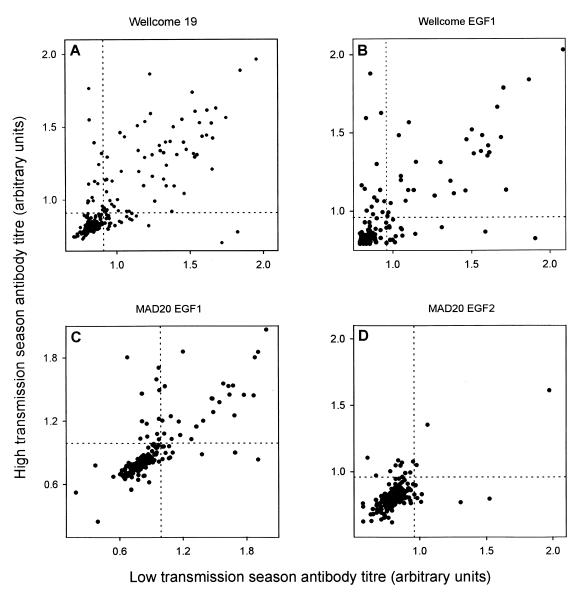
Although no overall seasonal variation in the antibody responses could be detected, we examined the correlation of antibody levels in matched pairs of plasma samples obtained during the low- and high-transmission seasons from the same individual. As shown in Fig. 3, the antibody levels measured in the samples collected in April and November were closely correlated [P(rs) < 0.001 for all correlations].
FIG. 3.
Correlation between antibody titers and recombinant PfMSP119 antigens measured in matched plasma samples collected during the low- and high-transmission seasons. Dotted lines indicate cutoff levels for titer positivity.
PfMSP119 responses in relation to clinical protection against malaria.
An association between anti-PfMSP1 antibody levels and clinical protection against malaria has previously been reported found in several studies (2, 12, 23, 27, 35). To verify this association, we compared antibody responses in the children in our study who were susceptible to clinical malaria (group 1) with those seen in children who appeared clinically protected (group 2). As shown in Table 2, the proportions of positive antibody responses to the recombinant PfMSP119 antigens were not significantly higher in the protected than in the susceptible children [P(χ2) > 0.28 in all cases]. This was the case for samples collected during both low- and high-transmission seasons. Indeed, the proportion of responders tended to be higher among the susceptible than the protected children during the low-transmission season, suggesting that a positive antibody response reflects recent high parasitemia. A similar picture emerged when we examined the levels of anti-PfMSP119 antibodies rather than proportions of positive responses. Thus, antibody levels were not significantly different between protected and susceptible children, whether analysis was restricted to samples having a positive antibody titer only (Table 3) or whether all data points were included (data not shown). Recategorization of the children as “protected” and “susceptible” based on shorter periods of surveillance (between 1 and 10 months following the first blood sampling) did not change the lack of association between anti-PfMSP1 antibody levels and clinical protection (data not shown). Taken together, these data do not support the hypothesis of an important role of anti-PfMSP119 antibodies in acquired immunological protection against malaria.
TABLE 2.
Prevalence of antibody responses to PfMSP1 constructs in Ghanaian children susceptible to, and protected against, P. falciparum malariaa
| Antigen construct | Low-transmission season
|
High-transmission season
|
||||||
|---|---|---|---|---|---|---|---|---|
| Susceptible (n = 108) | Protected (n = 103) | χ2 | P(χ2) | Susceptible (n = 108) | Protected (n = 103b) | χ2 | P(χ2) | |
| Wellcome 19 | 0.34 | 0.30 | 0.25 | 0.62 | 0.27 | 0.28 | 0.00 | 0.95 |
| Wellcome EGF1 | 0.20 | 0.16 | 0.30 | 0.59 | 0.14 | 0.20 | 1.15 | 0.28 |
| MAD20 EGF1 | 0.18 | 0.13 | 0.62 | 0.43 | 0.15 | 0.14 | 0.00 | 0.98 |
| MAD20 EGF2 | 0.04 | 0.02 | 0.12 | 0.73 | 0.06 | 0.06 | 0.04 | 0.85 |
n, number of samples tested; χ2, test statistic (1 degree of freedom) for comparison of antibody prevalence in susceptible and protected children; P(χ2); associated level of significance.
n = 102 for MAD20 constructs.
TABLE 3.
Levels of antibodies of PfMSP 1 constructs in Ghanaian children susceptible to, and protected against, P. falciparum malariaa
| Antigen construct | Low-transmission season
|
High-transmission season
|
||||
|---|---|---|---|---|---|---|
| Susceptible levelb | Protected levelb | P(z) | Susceptible level | Protected level | P(z) | |
| Wellcome 19 | 1.22 ± 1.07 (37) | 1.17 ± 1.07 (31) | 0.38 | 1.24 ± 1.09 (29) | 1.26 ± 1.07 (29) | 0.85 |
| Wellcome EGF1 | 1.31 ± 1.11 (22) | 1.20 ± 1.09 (17) | 0.23 | 1.42 ± 1.11 (15) | 1.21 ± 1.08 (21) | 0.20 |
| MAD20 EGF1 | 1.36 ± 1.12 (19) | 1.24 ± 1.11 (13) | 0.27 | 1.41 ± 1.11 (16) | 1.34 ± 1.10 (14) | 0.44 |
| MAD20 EGF2 | 1.00 (4) | 1.20 (2) | 0.17 | 1.07 ± 1.10 (6) | 1.05 ± 1.04 (6) | 0.73 |
Only data for children with a positive antibody titer are presented (see also Fig. 4).
Geometric mean relative OD value ± 95% confidence interval, determined as described in Materials and Methods; number of samples tested given in parentheses.
IgG subclass specificities of anti-PfMSP119 antibody responses.
It has previously been suggested that the isotypes or isotype balance of antibodies, rather than the levels of antibodies per se, are important in antibody-mediated protection against malaria (10). However, in our study population, IgG1-specific responses dominated in all anti-PfMSP119 IgG-positive samples, and no relationship between specific isotypes or isotype balance could be discerned (data not shown).
DISCUSSION
Clinical malaria is caused by the multiplication of the erythrocytic stages of the malaria parasites (43). Antibodies against blood-stage merozoite antigens may block parasite invasion of erythrocytes, leading to a reduction in parasitemia and thus protect against the disease (9, 28). The merozoite surface protein 1 (MSP1) is processed during schizogony giving rise to a 19-kDa C-terminal fragment (MSP19), which contains two cysteine-rich EGF-like domains and which remains attached to the merozoite during erythrocyte invasion (7, 8). In vaccination experiments in monkeys and mice, MSP1 has been shown to be protective against homologous challenge (15, 19, 32, 33, 40), although MSP1 vaccination did not protect Aotus nacymai monkeys against homologous or heterologous challenge in another study (13).
MSP119-specific monoclonal antibodies can inhibit the invasion of erythrocytes by P. falciparum merozoites in vitro (17). In addition, the two EGF-like motifs of P. falciparum MSP119 (PfMSP119) are recognized by human antibodies (37), and the presence of such antibodies has been associated with a lowered risk of clinical malaria in immunoepidemiological studies (2, 12, 23, 27, 35). The present study was specifically designed to verify the hypothesis that antibodies to the C-terminal part of PfMSP119 confer protection against malaria and to further characterize the natural acquisition of these antibodies in an area of stable but seasonal malaria transmission.
The prevalence of antibodies to the recombinant PfMSP119 antigens included in the study varied between 31% (Wellcome 19) and 4% (MAD20 EGF2). The prevalence of antibody responders did not increase with age, and the lack of antibody response to the PfMSP1 antigens in many of the children is unlikely to be due to lack of exposure to the antigen, since all children must have been exposed to the parasite repeatedly. Although the prevalence of antibody responses to PfMSP119 antigens was quite low in the present study, similar levels have previously been found in the Gambia and Sierra Leone (23). The tendency for responding or nonresponding individuals to remain as such at the two times of sampling regardless of the level of transmission indicates that host factors play an important role for the capacity to respond to the C-terminal part of PfMSP1. In recent studies with several antigens from both blood and gametocyte stages of the parasites, it was suggested that bias toward a particular responder type may reflect the type of exposure encountered during childhood, akin to the phenomenon of clonal imprinting or “original antigenic sin” (26, 39). Another possibility is that host genetic factors are important for responder status as is the case for the response to Pf155/RESA (41).
Additional putative reasons for the low antibody prevalence include the short half-life of anti-PfMSP1 antibodies, which are mainly found shortly after clinical episodes (12, 14, 25), and low immunogenicity or lack of adequate T-cell help for antibody production (21, 31, 42). Although the prevalence of antibody responses to PfMSP119 in this study compares with the prevalence reported for other West African countries (23), we did not detect associations between the responses to any of the constructs and protection against malaria. The categorization of individuals protected from, or susceptible to, malaria was based on 18 months of parasitological and clinical survey. It may be argued that we did not detect any association of anti-PfMSP119 antibodies and protection from malaria because of the long survey periods between the blood sampling times, and that antibodies to PfMSP119 may decay and thus decrease to levels that may not be functionally protective. This is unlikely to be the case since the nonassociation with protection from clinical malaria of anti-PfMSP119 antibody remained when the follow-up period used in defining protected and susceptible individuals was restricted to between 1 and 10 months after the first blood sampling. Thus, the simplest explanation for the lack of association between clinical protection and PfMSP119 antibodies here is that no such association exists under the epidemiological circumstances prevalent in our study area. This notwithstanding, it remains a possibility that antibodies against the C-terminal part of PfMSP1 do play a role in the protection against malaria as shown by other studies (2, 12, 23, 27, 35, 36). In one of these studies, it was concluded that maternal antibodies appeared to have a greater protective capacity than infant antibodies (12). There are data available to support the notion that adults and children have intrinsically different capacities for mounting protective immune responses (5, 6). It is thus conceivable that adults tend to produce more protective anti-PfMSP119 antibodies than children do. It could also be that the fine specificity and the balance between antibodies of different subclasses, which may be dependent on the intensity of transmission, influence the protective function of anti-PfMSP1 antibodies (10, 11, 16, 18). The fine specificity of anti-PfMSP1 antibodies relating to the particular amino acid composition and conformation might be of importance to the protective efficacy, since only some anti-PfMSP119 monoclonal antibodies inhibit the in vitro merozoite invasion of erythrocytes (16, 17). Naturally acquired antibodies to PfMSP119 may thus be a mixture of protective and nonprotective types. The inhibition of merozoite invasion of erythrocytes is dependent on the inhibition of the final processing of the C-terminal PfMSP1 protein, and the natural population of anti-PfMSP119 antibody species may be nonprotective if protective species are inhibited by nonprotective ones (7, 9). Alternatively, cross-reacting antibodies and antibodies of IgM class may inhibit protective ones (3, 34).
The balance between IgG subclass-specific responses, and especially that of cytophilic IgG1 and IgG3, has been associated with protection against malaria (4, 20, 24). However, this did not appear to be of importance in the present study, as the antibody responses to the various PfMSP119 constructs were predominantly IgG1 in all children.
In conclusion, we found no significant association between the prevalence or levels of anti-PfMSP119 antibody with protection from clinical malaria, nor did we find any correlation with age. This study emphasizes the need to characterize immune responses to PfMSP119 in subjects from various epidemiological settings to establish the role of such responses in acquired protective immunity to malaria, and they also suggests that new reagents are required to discriminate fine differences in epitope specificity, which may predict the protective efficacy of the immune response.
ACKNOWLEDGMENTS
Ben Abuakwa and Anne Corfitz are thanked for excellent field and technical assistance, respectively. Gillian Wagner is thanked for assistance with antigen preparation and serological assays.
This study received financial support from the ENRECA program of the Danish International Development Agency and the Danish Medical Research Council.
REFERENCES
- 1.Afari E A, Appawu M, Dunyo S, Baffoe-Wilmot A, Nkrumah F K. Malaria infection, morbidity and transmission in two ecological zones in southern Ghana. Afr J Health Sci. 1995;2:312–316. [PubMed] [Google Scholar]
- 2.Al-Yaman F, Genton B, Kramer K J, Chang S P, Hui G S, Baisor M, Alpers M P. Assessment of the role of naturally acquired antibody levels to Plasmodium falciparum merozoite surface protein-1 in protecting Papua New Guinean children from malaria morbidity. Am J Trop Med Hyg. 1996;54:443–448. doi: 10.4269/ajtmh.1996.54.443. [DOI] [PubMed] [Google Scholar]
- 3.Anders R F. Multiple cross-reactivities amongst antigens of Plasmodium falciparum impair the development of protective immunity against malaria. Parasite Immunol. 1986;8:529–539. doi: 10.1111/j.1365-3024.1986.tb00867.x. [DOI] [PubMed] [Google Scholar]
- 4.Aribot G, Rogier C, Sarthou J L, Trape J F, Balde A T, Druilhe P, Roussilhon C. Pattern of immunoglobulin isotype response to Plasmodium falciparum blood-stage antigens in individuals living in a holoendemic area of Senegal (Dielmo, West Africa) Am J Trop Med Hyg. 1996;54:449–457. doi: 10.4269/ajtmh.1996.54.449. [DOI] [PubMed] [Google Scholar]
- 5.Baird J K, Jones T R, Danudirgo E W, Annis B A, Bangs M J, Basri H, Purnomo, Masbar S. Age-dependent acquired protection against Plasmodium falciparum in people having two years exposure to hyperendemic malaria. Am J Trop Med Hyg. 1991;45:65–76. doi: 10.4269/ajtmh.1991.45.65. [DOI] [PubMed] [Google Scholar]
- 6.Baird J K, Purnomo, Basri H, Bangs M J, Andersen E M, Jones T R, Masbar S, Harjosuwarno S, Subianto B, Arbani P R. Age-specific prevalence of Plasmodium falciparum among six populations with limited histories of exposure to endemic malaria. Am J Trop Med Hyg. 1993;49:707–719. doi: 10.4269/ajtmh.1993.49.707. [DOI] [PubMed] [Google Scholar]
- 7.Blackman M J, Chappel J A, Shai S, Holder A A. A conserved parasite serine protease processes the Plasmodium falciparum merozoite surface protein-1. Mol Biochem Parasitol. 1993;62:103–114. doi: 10.1016/0166-6851(93)90182-w. [DOI] [PubMed] [Google Scholar]
- 8.Blackman M J, Holder A A. Secondary processing of the Plasmodium falciparum merozoite surface protein-1 (MSP1) by a calcium-dependent membrane-bound serine protease: shedding of MSP133 as a noncovalently associated complex with other fragments of the MSP1. Mol Biochem Parasitol. 1992;50:307–315. doi: 10.1016/0166-6851(92)90228-c. [DOI] [PubMed] [Google Scholar]
- 9.Blackman M J, Scott F T, Shai S, Holder A A. Antibodies inhibit the protease-mediated processing of a malaria merozoite surface protein. J Exp Med. 1994;180:389–393. doi: 10.1084/jem.180.1.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bouharoun-Tayoun H, Druilhe P. Plasmodium falciparum malaria: evidence for an isotype imbalance which may be responsible for delayed acquisition of protective immunity. Infect Immun. 1992;60:1473–1481. doi: 10.1128/iai.60.4.1473-1481.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bouharoun-Tayoun H, Druilhe P. Antibodies in falciparum malaria: what matters most, quantity or quality? Mem Inst Oswaldo Cruz. 1992;87(Suppl. 3):229–234. doi: 10.1590/s0074-02761992000700038. [DOI] [PubMed] [Google Scholar]
- 12.Branch O H, Udhayakumar V, Hightower A W, Oloo A J, Hawley W A, Nahlen B L, Bloland P B, Kaslow D C, Lal A A. A longitudinal investigation of IgG and IgM antibody responses to the merozoite surface protein-1 19-kilodalton domain of Plasmodium falciparum in pregnant women and infants: associations with febrile illness, parasitemia, and anemia. Am J Trop Med Hyg. 1998;58:211–219. doi: 10.4269/ajtmh.1998.58.211. [DOI] [PubMed] [Google Scholar]
- 13.Burghaus P A, Wellde B T, Hall T, Richards R L, Egan A F, Riley E M, Ballou W P, Holder A A. Immunization of Aotus nancymai with recombinant C terminus of Plasmodium falciparum merozoite surface protein 1 in liposomes and alum adjuvant does not induce protection against a challenge infection. Infect Immun. 1996;64:3614–3619. doi: 10.1128/iai.64.9.3614-3619.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cavanagh D, Elhassan I M, Roper C, Robinson V J, Giha H, Holder A A, Hviid L, Theander T G, Arnot D E, McBride J S. A longitudinal study of type-specific antibody responses to Plasmodium falciparum merozoite surface protein1 in an area of unstable malaria in Sudan. J Immunol. 1998;161:347–359. [PubMed] [Google Scholar]
- 15.Chang S P, Case S E, Gosnell W L, Kramer K J, Tam L Q, Hashiro C Q, Nikaido C M, Gibson H L, Lee-Ng C T, Barr P J, Yokota B T, Hui G S N. A recombinant baculovirus 42-kilodalton C-terminal fragment of Plasmodium falciparum merozoite surface protein 1 protects Aotus monkeys against malaria. Infect Immun. 1996;64:253–261. doi: 10.1128/iai.64.1.253-261.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chappel J A, Egan A F, Riley E M, Druilhe P, Holder A A. Naturally acquired human antibodies which recognize the first epidermal growth factor-like module in the Plasmodium falciparum merozoite surface protein 1 do not inhibit parasite growth in vitro. Infect Immun. 1994;62:4488–4494. doi: 10.1128/iai.62.10.4488-4494.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chappel J A, Holder A A. Monoclonal antibodies that inhibit Plasmodium falciparum invasion in vitro recognize the first growth factor-like domain of merozoite surface protein-1. Mol Biochem Parasitol. 1993;60:303–312. doi: 10.1016/0166-6851(93)90141-j. [DOI] [PubMed] [Google Scholar]
- 18.Cooper J A, Cooper L T, Saul A J. Mapping of the region predominantly recognized by antibodies to the Plasmodium falciparum merozoite surface antigen MSA 1. Mol Biochem Parasitol. 1992;51:301–312. doi: 10.1016/0166-6851(92)90080-4. [DOI] [PubMed] [Google Scholar]
- 19.Daly T M, Long C A. A recombinant 15-kilodalton carboxyl-terminal fragment of Plasmodium yoelii yoelii 17XL merozoite surface protein 1 induces a protective immune response in mice. Infect Immun. 1993;61:2462–2467. doi: 10.1128/iai.61.6.2462-2467.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Druilhe P, Perignon J-L. A hypothesis about the chronicity of malaria infection. Parasitol Today. 1997;13:353–357. doi: 10.1016/s0169-4758(97)01095-8. [DOI] [PubMed] [Google Scholar]
- 21.Egan A, Waterfall M, Pinder M, Holder A, Riley E. Characterization of human T- and B-cell epitopes in the C terminus of Plasmodium falciparum merozoite surface protein 1: evidence for poor T-cell recognition of polypeptides with numerous disulfide bonds. Infect Immun. 1997;65:3024–3031. doi: 10.1128/iai.65.8.3024-3031.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Egan A F, Chappel J A, Burghaus P A, Morris J S, McBride J S, Holder A A, Kaslow D C, Riley E M. Serum antibodies from malaria-exposed people recognize conserved epitopes formed by the two epidermal growth factor motifs of MSP119, the carboxy-terminal fragment of the major merozoite surface protein of Plasmodium falciparum. Infect Immun. 1995;63:456–466. doi: 10.1128/iai.63.2.456-466.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Egan A F, Morris J, Barnish G, Allen S, Greenwood B M, Kaslow D C, Holder A A, Riley E M. Clinical immunity to Plasmodium falciparum malaria is associated with serum antibodies to the 19-kDa C-terminal fragment of the merozoite surface antigen, PfMSP-1. J Infect Dis. 1996;173:765–769. doi: 10.1093/infdis/173.3.765. [DOI] [PubMed] [Google Scholar]
- 24.Ferrante A, Rzepczyk C M. Atypical IgG subclass antibody responses to Plasmodium falciparum asexual stage antigens. Parasitol Today. 1997;13:145–148. doi: 10.1016/s0169-4758(97)89812-2. [DOI] [PubMed] [Google Scholar]
- 25.Früh K, Doumbo O, Müller H-M, Koita O, McBride J, Crisanti A, Touré Y, Bujard H. Human antibody response to the major merozoite surface antigen of Plasmodium falciparum is strain specific and short-lived. Infect Immun. 1991;59:1319–1324. doi: 10.1128/iai.59.4.1319-1324.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Good M F, Zevering Y, Currier J, Bilsborough J. ‘Original antigenic sin’, T cell memory, and malaria sporozoite immunity: an hypothesis for immune evasion. Parasite Immunol. 1993;15:187–193. doi: 10.1111/j.1365-3024.1993.tb00599.x. [DOI] [PubMed] [Google Scholar]
- 27.Høgh B, Marbiah N T, Burghaus P A, Andersen P K. Relationship between maternally derived anti-Plasmodium falciparum antibodies and risk of infection and disease in infants living in an area of Liberia, west Africa, in which malaria is highly endemic. Infect Immun. 1995;63:4034–4038. doi: 10.1128/iai.63.10.4034-4038.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holder A A. The precursor to major merozoite surface antigens: structure and role in immunity. Prog Allergy. 1988;41:72–97. [PubMed] [Google Scholar]
- 29.Holder A A, Blackman M J, Burghaus P A, Chappel J A, Ling I T, McCallum D N, Shai S. A malaria merozoite surface protein (MSP1)-structure, processing and function. Mem Inst Oswaldo Cruz. 1992;87(Suppl. 3):37–42. doi: 10.1590/s0074-02761992000700004. [DOI] [PubMed] [Google Scholar]
- 30.Holder A A, Sandhu J S, Hillman Y, Davey L S, Nicholls S C, Cooper H, Lockyer M J. Processing of the precursor to the major merozoite surface antigens of Plasmodium falciparum. Parasitology. 1987;94:199–208. doi: 10.1017/s0031182000053889. [DOI] [PubMed] [Google Scholar]
- 31.Hui G S, Nikaido C, Hashiro C, Kaslow D C, Collins W E. Dominance of conserved B-cell epitopes of the Plasmodium falciparum merozoite surface protein, MSP1, in blood-stage infections of naive Aotus monkeys. Infect Immun. 1996;64:1502–1509. doi: 10.1128/iai.64.5.1502-1509.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kumar S, Yadava A, Keister D B, Tian J H, Ohl M, Perdue Greenfield K A, Miller L H, Kaslow D C. Immunogenicity and in vivo efficacy of recombinant Plasmodium falciparum merozoite surface protein-1 in Aotus monkeys. Mol Med. 1995;1:325–332. [PMC free article] [PubMed] [Google Scholar]
- 33.Ling I T, Ogun S A, Holder A A. Immunization against malaria with a recombinant protein. Parasite Immunol. 1994;16:63–67. doi: 10.1111/j.1365-3024.1994.tb00324.x. [DOI] [PubMed] [Google Scholar]
- 34.Nguer C M, Diallo T O, Diouf A, Tall A, Dieye A, Perraut R, Garraud O. Plasmodium falciparum- and merozoite surface protein 1-specific antibody isotype balance in immune Senegalese adults. Infect Immun. 1997;65:4873–4876. doi: 10.1128/iai.65.11.4873-4876.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Riley E M, Allen S J, Wheeler J G, Blackman M J, Bennett S, Takacs B, Schönfeld H-J, Holder A A, Greenwood B M. Naturally acquired cellular and humoral immune responses to the major merozoite surface antigen (PfMSP1) of Plasmodium falciparum are associated with reduced malaria morbidity. Parasite Immunol. 1992;14:321–337. doi: 10.1111/j.1365-3024.1992.tb00471.x. [DOI] [PubMed] [Google Scholar]
- 36.Riley E M, Morris-Jones S, Blackman M J, Greenwood B M, Holder A A. A longitudinal study of naturally acquired cellular and humoral immune responses to a merozoite surface protein (MSP1) of Plasmodium falciparum in an area of seasonal malaria transmission. Parasite Immunol. 1993;15:513–524. doi: 10.1111/j.1365-3024.1993.tb00639.x. [DOI] [PubMed] [Google Scholar]
- 37.Shai S, Blackman M J, Holder A A. Epitopes in the 19kDa fragment of the Plasmodium falciparum major merozoite surface protein-1 (PfMSP-119) recognized by human antibodies. Parasite Immunol. 1995;17:269–275. doi: 10.1111/j.1365-3024.1995.tb01025.x. [DOI] [PubMed] [Google Scholar]
- 38.Smith T, Schellenberg J A, Hayes R. Attributable fraction estimates and case definitions for malaria in endemic areas. Stat Med. 1994;13:2345–2358. doi: 10.1002/sim.4780132206. [DOI] [PubMed] [Google Scholar]
- 39.Taylor R R, Egan A, McGuinness D, Jepson A, Adair R, Drakely C, Riley E. Selective recognition of malaria antigens by human serum antibodies is not genetically determined but demonstrates some features of clonal imprinting. Int Immunol. 1996;8:905–915. doi: 10.1093/intimm/8.6.905. [DOI] [PubMed] [Google Scholar]
- 40.Tian J H, Kumar S, Kaslow D C, Miller L H. Comparison of protection induced by immunization with recombinant proteins from different regions of merozoite surface protein 1 of Plasmodium yoelii. Infect Immun. 1997;65:3032–3036. doi: 10.1128/iai.65.8.3032-3036.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Troye-Blomberg M, Sjoberg K, Olerup O, Riley E M, Kabilan L, Perlmann H, Marbiah N T, Perlmann P. Characterization of regulatory T cell responses to defined immunodominant T cell epitopes of the Plasmodium falciparum antigen Pf155/RESA. Immunol Lett. 1990;25:129–134. doi: 10.1016/0165-2478(90)90103-w. [DOI] [PubMed] [Google Scholar]
- 42.Udhayakumar V, Anyona D, Kariuki S, Shi Y P, Bloland P B, Branch O H, Weiss W, Nahlen B L, Kaslow D C, Lal A A. Identification of T and B cell epitopes recognized by humans in the C-terminal 42-kDa domain of the Plasmodium falciparum merozoite surface protein (MSP)-1. J Immunol. 1995;154:6022–6030. [PubMed] [Google Scholar]
- 43.Warrell D A. Clinical features of malaria. In: Gillis H M, Warrell D A, editors. Bruce-Chwatt’s essential malariology. London, England: Edward Arnold; 1993. pp. 35–49. [Google Scholar]