Abstract
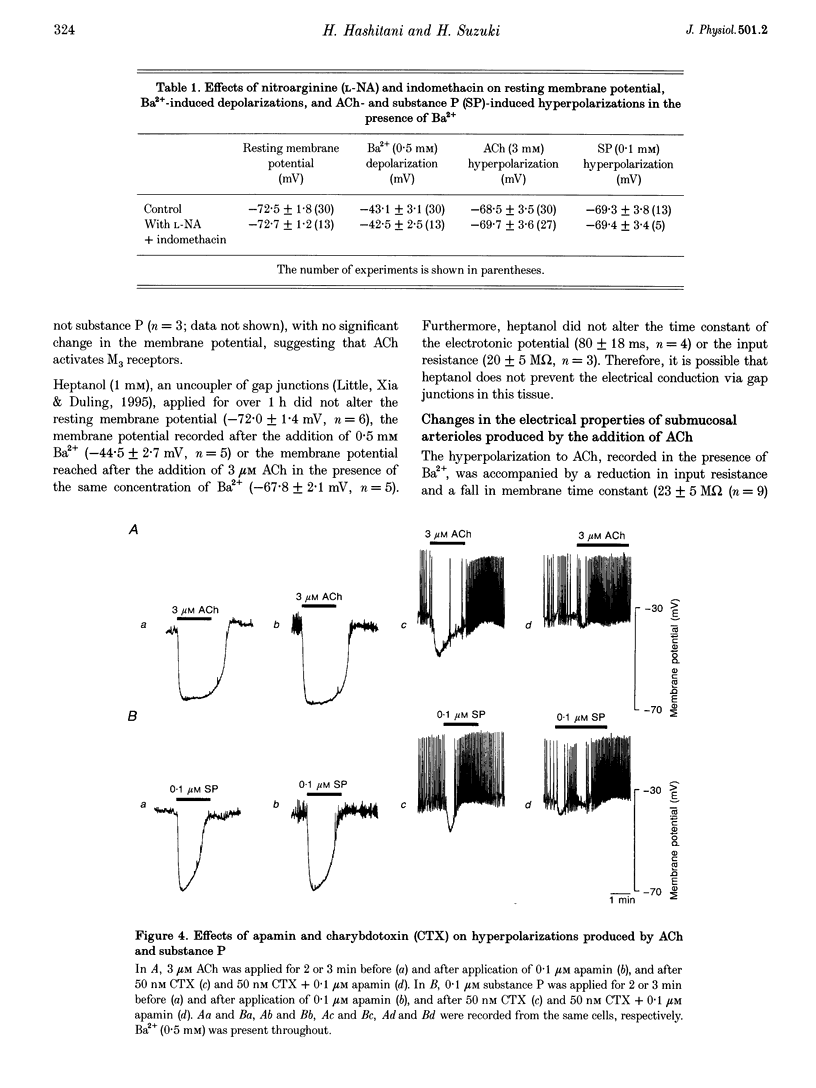
1. Membrane potentials were recorded from submucosal arterioles (diameter, 30-80 microns) of the guinea-pig small intestine, using conventional microelectrode techniques. In control solution the resting membrane potential was about -73 mV, and the addition of 0.5 mM Ba2+ depolarized the membrane to about -43 mV. 2. ACh (10 nM to 10 microM), or substance P (0.1 microM), caused a membrane hyperpolarization in preparations which had been depolarized by Ba2+ but not in control preparations. ACh produced a sustained hyperpolarization, whereas substance P produced a transient hyperpolarization, without being affected by either nitroarginine (0.1 mM) or indomethacin (10 microM). 3. In the presence of 50 microM BAPTA (acetoxymethyl ester form), the membrane potentials were not altered in the control solution or in the presence of Ba2+, but Ba2+ caused a smooth depolarization of the membrane. Following this procedure, both ACh and substance P caused membrane depolarization instead of hyperpolarization, suggesting that the ACh- and substance P-induced hyperpolarization in arteriolar smooth muscle are intracellular [Ca2+] dependent. 4. In short segments (200-500 microns) of arteriole, the time constant of electrotonic potentials produced by passing current pulses through the recording electrode was about 75 ms. The addition of Ba2+ increased both the input resistance and the time constant. 5. The hyperpolarizations produced by ACh or substance P were associated with a reduction in the amplitude and the time constant of electrotonic potential. 6. The reversal potential for the ACh-induced hyperpolarization, estimated from the current-voltage relationship, was about -86 mV, a value close to the equilibrium potential for K+. 7. In the presence of 50 nM charybdotoxin the hyperpolarization produced by ACh became transient and was reduced in amplitude: the residual response was further reduced by apamin (0.1 microM). The response produced by substance P was also reduced by 50 nM charybdotoxin: again the residual response was sensitive to 0.1 microM apamin. The hyperpolarizations produced by either ACh or substance P were insensitive to glibenclamide (10 microM) and 4-aminopyridine (1 mM). 8. It is suggested that in submucosal arterioles of the guinea-pig ileum, ACh- or substance P-induced hyperpolarizations of smooth muscle result from activation of both charybdotoxin-sensitive and apamin-sensitive K+ channels, with the former being predominant. The results are discussed in relation to the possible involvement of one or more endothelium-dependent hyperpolarizing factors in ACh- and substance P-induced hyperpolarization.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andriantsitohaina R., Surprenant A. Acetylcholine released from guinea-pig submucosal neurones dilates arterioles by releasing nitric oxide from endothelium. J Physiol. 1992;453:493–502. doi: 10.1113/jphysiol.1992.sp019241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton T. B., Lang R. J., Takewaki T. Mechanisms of action of noradrenaline and carbachol on smooth muscle of guinea-pig anterior mesenteric artery. J Physiol. 1984 Jun;351:549–572. doi: 10.1113/jphysiol.1984.sp015262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brayden J. E., Large W. A. Electrophysiological analysis of neurogenic vasodilatation in the isolated lingual artery of the rabbit. Br J Pharmacol. 1986 Sep;89(1):163–171. doi: 10.1111/j.1476-5381.1986.tb11132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busse R., Fichtner H., Lückhoff A., Kohlhardt M. Hyperpolarization and increased free calcium in acetylcholine-stimulated endothelial cells. Am J Physiol. 1988 Oct;255(4 Pt 2):H965–H969. doi: 10.1152/ajpheart.1988.255.4.H965. [DOI] [PubMed] [Google Scholar]
- Bény J. L., Brunet P. C. Electrophysiological and mechanical effects of substance P and acetylcholine on rabbit aorta. J Physiol. 1988 Apr;398:277–289. doi: 10.1113/jphysiol.1988.sp017042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell W. B., Gebremedhin D., Pratt P. F., Harder D. R. Identification of epoxyeicosatrienoic acids as endothelium-derived hyperpolarizing factors. Circ Res. 1996 Mar;78(3):415–423. doi: 10.1161/01.res.78.3.415. [DOI] [PubMed] [Google Scholar]
- Chen G. F., Suzuki H. Calcium dependency of the endothelium-dependent hyperpolarization in smooth muscle cells of the rabbit carotid artery. J Physiol. 1990 Feb;421:521–534. doi: 10.1113/jphysiol.1990.sp017959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G., Suzuki H. Some electrical properties of the endothelium-dependent hyperpolarization recorded from rat arterial smooth muscle cells. J Physiol. 1989 Mar;410:91–106. doi: 10.1113/jphysiol.1989.sp017522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G., Yamamoto Y., Miwa K., Suzuki H. Hyperpolarization of arterial smooth muscle induced by endothelial humoral substances. Am J Physiol. 1991 Jun;260(6 Pt 2):H1888–H1892. doi: 10.1152/ajpheart.1991.260.6.H1888. [DOI] [PubMed] [Google Scholar]
- Doods H. N., Mathy M. J., Davidesko D., van Charldorp K. J., de Jonge A., van Zwieten P. A. Selectivity of muscarinic antagonists in radioligand and in vivo experiments for the putative M1, M2 and M3 receptors. J Pharmacol Exp Ther. 1987 Jul;242(1):257–262. [PubMed] [Google Scholar]
- Edwards F. R., Hirst G. D. Inward rectification in submucosal arterioles of guinea-pig ileum. J Physiol. 1988 Oct;404:437–454. doi: 10.1113/jphysiol.1988.sp017298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards G., Niederste-Hollenberg A., Schneider J., Noack T., Weston A. H. Ion channel modulation by NS 1619, the putative BKCa channel opener, in vascular smooth muscle. Br J Pharmacol. 1994 Dec;113(4):1538–1547. doi: 10.1111/j.1476-5381.1994.tb17171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton D., McGiff J. C., Quilley J. Role of K+ channels in the vasodilator response to bradykinin in the rat heart. Br J Pharmacol. 1994 Nov;113(3):954–958. doi: 10.1111/j.1476-5381.1994.tb17085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland C. J., Plane F., Kemp B. K., Cocks T. M. Endothelium-dependent hyperpolarization: a role in the control of vascular tone. Trends Pharmacol Sci. 1995 Jan;16(1):23–30. doi: 10.1016/s0165-6147(00)88969-5. [DOI] [PubMed] [Google Scholar]
- Gebremedhin D., Kaldunski M., Jacobs E. R., Harder D. R., Roman R. J. Coexistence of two types of Ca(2+)-activated K+ channels in rat renal arterioles. Am J Physiol. 1996 Jan;270(1 Pt 2):F69–F81. doi: 10.1152/ajprenal.1996.270.1.F69. [DOI] [PubMed] [Google Scholar]
- Hecker M., Bara A. T., Bauersachs J., Busse R. Characterization of endothelium-derived hyperpolarizing factor as a cytochrome P450-derived arachidonic acid metabolite in mammals. J Physiol. 1994 Dec 1;481(Pt 2):407–414. doi: 10.1113/jphysiol.1994.sp020449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst G. D., Neild T. O. Some properties of spontaneous excitatory junction potentials recorded from arterioles of guinea-pigs. J Physiol. 1980 Jun;303:43–60. doi: 10.1113/jphysiol.1980.sp013269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst G. D. Neuromuscular transmission in arterioles of guinea-pig submucosa. J Physiol. 1977 Dec;273(1):263–275. doi: 10.1113/jphysiol.1977.sp012093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst G. D., van Helden D. F. Ionic basis of the resting potential of submucosal arterioles in the ileum of the guinea-pig. J Physiol. 1982 Dec;333:53–67. doi: 10.1113/jphysiol.1982.sp014438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaizumi Y., Henmi S., Uyama Y., Atsuki K., Torii Y., Ohizumi Y., Watanabe M. Characteristics of Ca2+ release for activation of K+ current and contractile system in some smooth muscles. Am J Physiol. 1996 Sep;271(3 Pt 1):C772–C782. doi: 10.1152/ajpcell.1996.271.3.C772. [DOI] [PubMed] [Google Scholar]
- Kotecha N., Neild T. O. Vasodilatation and smooth muscle membrane potential changes in arterioles from the guinea-pig small intestine. J Physiol. 1995 Feb 1;482(Pt 3):661–667. doi: 10.1113/jphysiol.1995.sp020548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little T. L., Xia J., Duling B. R. Dye tracers define differential endothelial and smooth muscle coupling patterns within the arteriolar wall. Circ Res. 1995 Mar;76(3):498–504. doi: 10.1161/01.res.76.3.498. [DOI] [PubMed] [Google Scholar]
- Marchenko S. M., Sage S. O. Calcium-activated potassium channels in the endothelium of intact rat aorta. J Physiol. 1996 Apr 1;492(Pt 1):53–60. doi: 10.1113/jphysiol.1996.sp021288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchenko S. M., Sage S. O. Electrical properties of resting and acetylcholine-stimulated endothelium in intact rat aorta. J Physiol. 1993 Mar;462:735–751. doi: 10.1113/jphysiol.1993.sp019579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missiaen L., Taylor C. W., Berridge M. J. Spontaneous calcium release from inositol trisphosphate-sensitive calcium stores. Nature. 1991 Jul 18;352(6332):241–244. doi: 10.1038/352241a0. [DOI] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Murphy M. E., Brayden J. E. Apamin-sensitive K+ channels mediate an endothelium-dependent hyperpolarization in rabbit mesenteric arteries. J Physiol. 1995 Dec 15;489(Pt 3):723–734. doi: 10.1113/jphysiol.1995.sp021086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy M. E., Brayden J. E. Nitric oxide hyperpolarizes rabbit mesenteric arteries via ATP-sensitive potassium channels. J Physiol. 1995 Jul 1;486(Pt 1):47–58. doi: 10.1113/jphysiol.1995.sp020789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neild T. O., Shen K. Z., Surprenant A. Vasodilatation of arterioles by acetylcholine released from single neurones in the guinea-pig submucosal plexus. J Physiol. 1990 Jan;420:247–265. doi: 10.1113/jphysiol.1990.sp017910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkington H. C., Tare M., Tonta M. A., Coleman H. A. Stretch revealed three components in the hyperpolarization of guinea-pig coronary artery in response to acetylcholine. J Physiol. 1993 Jun;465:459–476. doi: 10.1113/jphysiol.1993.sp019687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilling W. P. Effect of membrane potential on cytosolic calcium of bovine aortic endothelial cells. Am J Physiol. 1989 Sep;257(3 Pt 2):H778–H784. doi: 10.1152/ajpheart.1989.257.3.H778. [DOI] [PubMed] [Google Scholar]
- Standen N. B., Quayle J. M., Davies N. W., Brayden J. E., Huang Y., Nelson M. T. Hyperpolarizing vasodilators activate ATP-sensitive K+ channels in arterial smooth muscle. Science. 1989 Jul 14;245(4914):177–180. doi: 10.1126/science.2501869. [DOI] [PubMed] [Google Scholar]
- Tare M., Parkington H. C., Coleman H. A., Neild T. O., Dusting G. J. Hyperpolarization and relaxation of arterial smooth muscle caused by nitric oxide derived from the endothelium. Nature. 1990 Jul 5;346(6279):69–71. doi: 10.1038/346069a0. [DOI] [PubMed] [Google Scholar]
- Vanhoutte P. M., Rubanyi G. M., Miller V. M., Houston D. S. Modulation of vascular smooth muscle contraction by the endothelium. Annu Rev Physiol. 1986;48:307–320. doi: 10.1146/annurev.ph.48.030186.001515. [DOI] [PubMed] [Google Scholar]
- Vanner S., Surprenant A. Neural reflexes controlling intestinal microcirculation. Am J Physiol. 1996 Aug;271(2 Pt 1):G223–G230. doi: 10.1152/ajpgi.1996.271.2.G223. [DOI] [PubMed] [Google Scholar]
- Zhang G., Yamamoto Y., Miwa K., Suzuki H. Vasodilation induced by substance P in guinea pig carotid arteries. Am J Physiol. 1994 Mar;266(3 Pt 2):H1132–H1137. doi: 10.1152/ajpheart.1994.266.3.H1132. [DOI] [PubMed] [Google Scholar]
- von der Weid P. Y., Bény J. L. Simultaneous oscillations in the membrane potential of pig coronary artery endothelial and smooth muscle cells. J Physiol. 1993 Nov;471:13–24. doi: 10.1113/jphysiol.1993.sp019888. [DOI] [PMC free article] [PubMed] [Google Scholar]


