Abstract
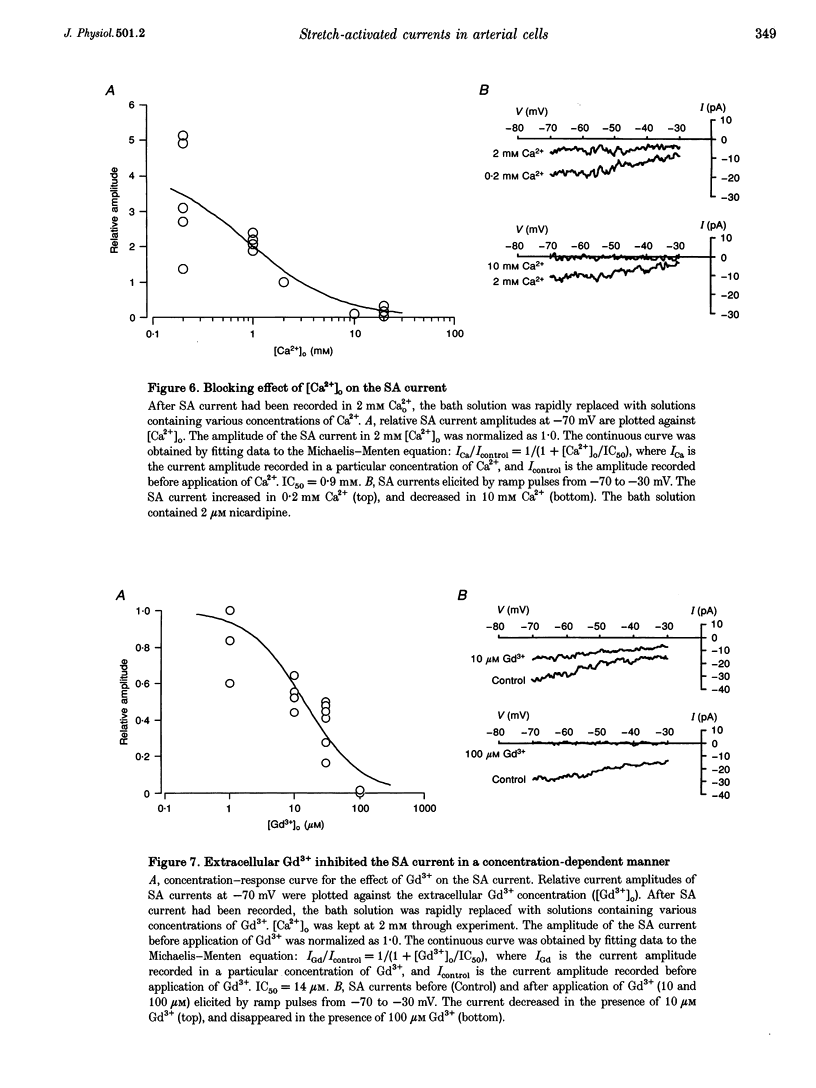
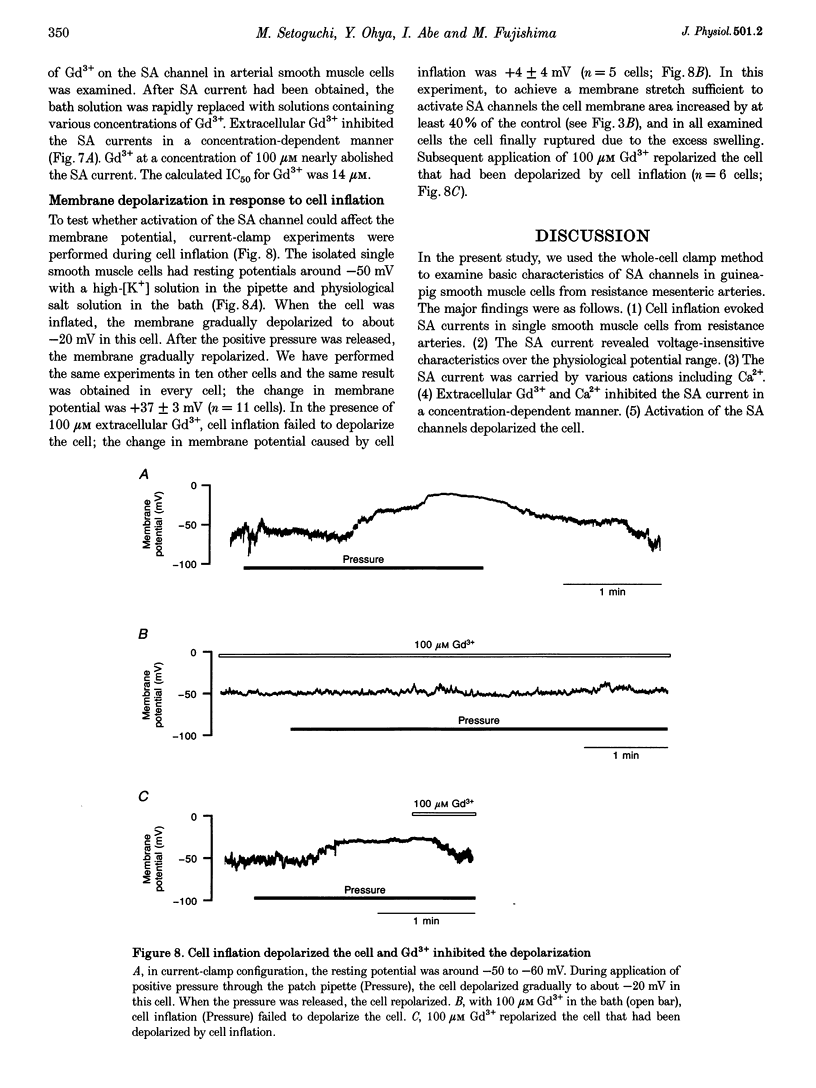
1. Stretch-activated (SA) channels were studied in smooth muscle cells isolated from mesenteric resistance arteries using the whole-cell patch-clamp method. Membrane stretch was achieved by cell inflation after application of positive pressure through a patch electrode. 2. In the voltage-clamp configuration, cell inflation increased and cell deflation decreased the membrane conductance. Conductance of the evoked current depended on the increase in cross-sectional area of the cell. The current-voltage relationship was linear between -80 and 0 mV, while further hyperpolarization showed a slight inward rectification. 3. The reversal potential of the SA current depended on the extracellular Na+ concentration, suggesting that the inward SA current was carried predominantly by Na+. The SA current was also carried by other cations, suggesting that the channel responsible for this current is a non-selective cation channel. The permeability sequence of cations as assessed by reversal potential was as follows: K+ > or = CS+ > or = Na+ > Li+. The channel was also permeable to Ca2+. 4. Extracellular Ca2+ and Gd3+ inhibited the SA current carried by monovalent cations in a concentration-dependent manner with IC50 (concentration giving 50% of maximal inhibition) values of 0.9 mM and 14 microM, respectively. 5. In the current-clamp configuration, membrane stretch depolarized the cell, and 100 microM Gd3+ inhibited the stretch-induced depolarization. 6. The results suggest that SA cation channels exist in arterial smooth muscle cells. Activation of the channels may modify membrane potential and intracellular ionic environment, and promote stretch-mediated cell responses.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bryant H. J., Harder D. R., Pamnani M. B., Haddy F. J. In vivo membrane potentials of smooth muscle cells in the caudal artery of the rat. Am J Physiol. 1985 Jul;249(1 Pt 1):C78–C83. doi: 10.1152/ajpcell.1985.249.1.C78. [DOI] [PubMed] [Google Scholar]
- Bülow A., Johansson B. Membrane stretch evoked by cell swelling increases contractile activity in vascular smooth muscle through dihydropyridine-sensitive pathways. Acta Physiol Scand. 1994 Dec;152(4):419–427. doi: 10.1111/j.1748-1716.1994.tb09824.x. [DOI] [PubMed] [Google Scholar]
- Davis M. J., Donovitz J. A., Hood J. D. Stretch-activated single-channel and whole cell currents in vascular smooth muscle cells. Am J Physiol. 1992 Apr;262(4 Pt 1):C1083–C1088. doi: 10.1152/ajpcell.1992.262.4.C1083. [DOI] [PubMed] [Google Scholar]
- Davis M. J., Meininger G. A., Zawieja D. C. Stretch-induced increases in intracellular calcium of isolated vascular smooth muscle cells. Am J Physiol. 1992 Oct;263(4 Pt 2):H1292–H1299. doi: 10.1152/ajpheart.1992.263.4.H1292. [DOI] [PubMed] [Google Scholar]
- Davis M. J. Myogenic response gradient in an arteriolar network. Am J Physiol. 1993 Jun;264(6 Pt 2):H2168–H2179. doi: 10.1152/ajpheart.1993.264.6.H2168. [DOI] [PubMed] [Google Scholar]
- Dopico A. M., Kirber M. T., Singer J. J., Walsh J. V., Jr Membrane stretch directly activates large conductance Ca(2+)-activated K+ channels in mesenteric artery smooth muscle cells. Am J Hypertens. 1994 Jan;7(1):82–89. doi: 10.1093/ajh/7.1.82. [DOI] [PubMed] [Google Scholar]
- Guharay F., Sachs F. Stretch-activated single ion channel currents in tissue-cultured embryonic chick skeletal muscle. J Physiol. 1984 Jul;352:685–701. doi: 10.1113/jphysiol.1984.sp015317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara N., Masuda H., Shoda M., Irisawa H. Stretch-activated anion currents of rabbit cardiac myocytes. J Physiol. 1992 Oct;456:285–302. doi: 10.1113/jphysiol.1992.sp019337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Harder D. R., Gilbert R., Lombard J. H. Vascular muscle cell depolarization and activation in renal arteries on elevation of transmural pressure. Am J Physiol. 1987 Oct;253(4 Pt 2):F778–F781. doi: 10.1152/ajprenal.1987.253.4.F778. [DOI] [PubMed] [Google Scholar]
- Harder D. R. Pressure-dependent membrane depolarization in cat middle cerebral artery. Circ Res. 1984 Aug;55(2):197–202. doi: 10.1161/01.res.55.2.197. [DOI] [PubMed] [Google Scholar]
- Hisada T., Ordway R. W., Kirber M. T., Singer J. J., Walsh J. V., Jr Hyperpolarization-activated cationic channels in smooth muscle cells are stretch sensitive. Pflugers Arch. 1991 Jan;417(5):493–499. doi: 10.1007/BF00370945. [DOI] [PubMed] [Google Scholar]
- Hwa J. J., Bevan J. A. Stretch-dependent (myogenic) tone in rabbit ear resistance arteries. Am J Physiol. 1986 Jan;250(1 Pt 2):H87–H95. doi: 10.1152/ajpheart.1986.250.1.H87. [DOI] [PubMed] [Google Scholar]
- Kirber M. T., Walsh J. V., Jr, Singer J. J. Stretch-activated ion channels in smooth muscle: a mechanism for the initiation of stretch-induced contraction. Pflugers Arch. 1988 Sep;412(4):339–345. doi: 10.1007/BF01907549. [DOI] [PubMed] [Google Scholar]
- Langton P. D. Calcium channel currents recorded from isolated myocytes of rat basilar artery are stretch sensitive. J Physiol. 1993 Nov;471:1–11. doi: 10.1113/jphysiol.1993.sp019887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris C. E., Horn R. Failure to elicit neuronal macroscopic mechanosensitive currents anticipated by single-channel studies. Science. 1991 Mar 8;251(4998):1246–1249. doi: 10.1126/science.1706535. [DOI] [PubMed] [Google Scholar]
- Ohya Y., Abe I., Fujii K., Takata Y., Fujishima M. Voltage-dependent Ca2+ channels in resistance arteries from spontaneously hypertensive rats. Circ Res. 1993 Dec;73(6):1090–1099. doi: 10.1161/01.res.73.6.1090. [DOI] [PubMed] [Google Scholar]
- Ohya Y., Sperelakis N. ATP regulation of the slow calcium channels in vascular smooth muscle cells of guinea pig mesenteric artery. Circ Res. 1989 Jan;64(1):145–154. doi: 10.1161/01.res.64.1.145. [DOI] [PubMed] [Google Scholar]
- Sun D., Messina E. J., Kaley G., Koller A. Characteristics and origin of myogenic response in isolated mesenteric arterioles. Am J Physiol. 1992 Nov;263(5 Pt 2):H1486–H1491. doi: 10.1152/ajpheart.1992.263.5.H1486. [DOI] [PubMed] [Google Scholar]
- Wellner M. C., Isenberg G. Properties of stretch-activated channels in myocytes from the guinea-pig urinary bladder. J Physiol. 1993 Jul;466:213–227. [PMC free article] [PubMed] [Google Scholar]
- Wellner M. C., Isenberg G. Stretch effects on whole-cell currents of guinea-pig urinary bladder myocytes. J Physiol. 1994 Nov 1;480(Pt 3):439–448. doi: 10.1113/jphysiol.1994.sp020373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X. C., Sachs F. Block of stretch-activated ion channels in Xenopus oocytes by gadolinium and calcium ions. Science. 1989 Feb 24;243(4894 Pt 1):1068–1071. doi: 10.1126/science.2466333. [DOI] [PubMed] [Google Scholar]
- Yang X. C., Sachs F. Mechanically sensitive, nonselective cation channels. EXS. 1993;66:79–92. doi: 10.1007/978-3-0348-7327-7_5. [DOI] [PubMed] [Google Scholar]
- Yang Z., Noll G., Lüscher T. F. Calcium antagonists differently inhibit proliferation of human coronary smooth muscle cells in response to pulsatile stretch and platelet-derived growth factor. Circulation. 1993 Sep;88(3):832–836. doi: 10.1161/01.cir.88.3.832. [DOI] [PubMed] [Google Scholar]