Abstract
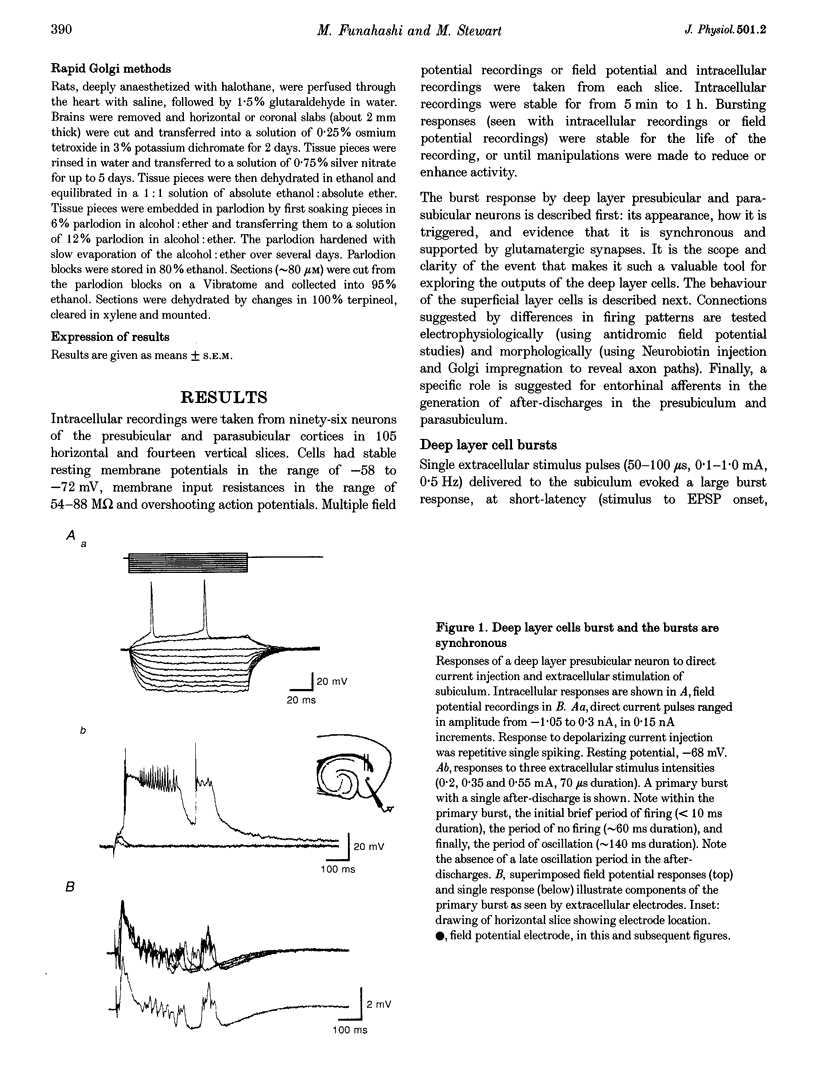
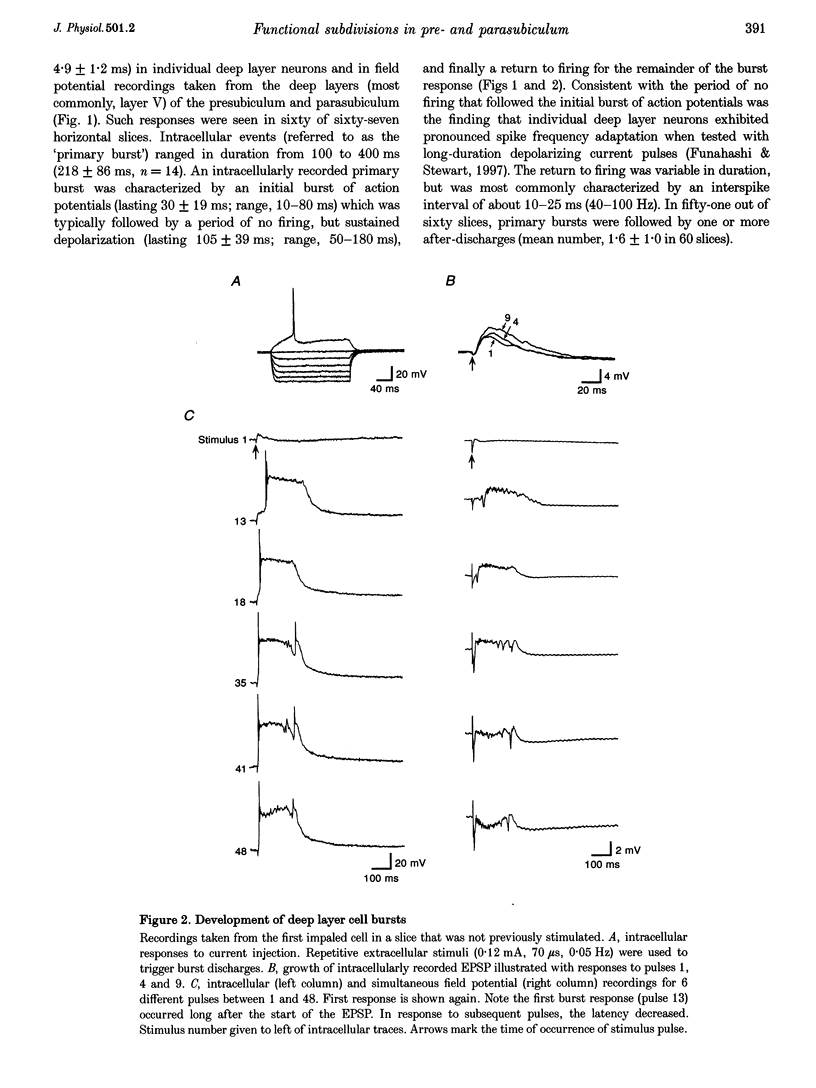
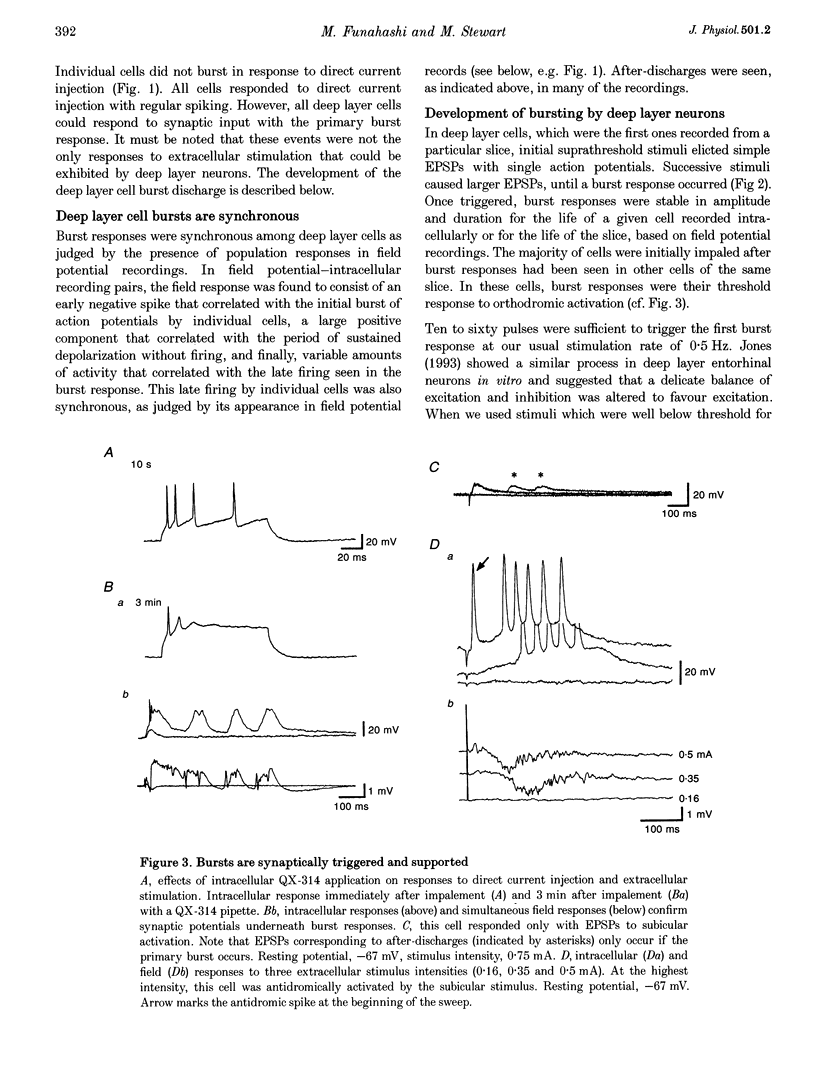
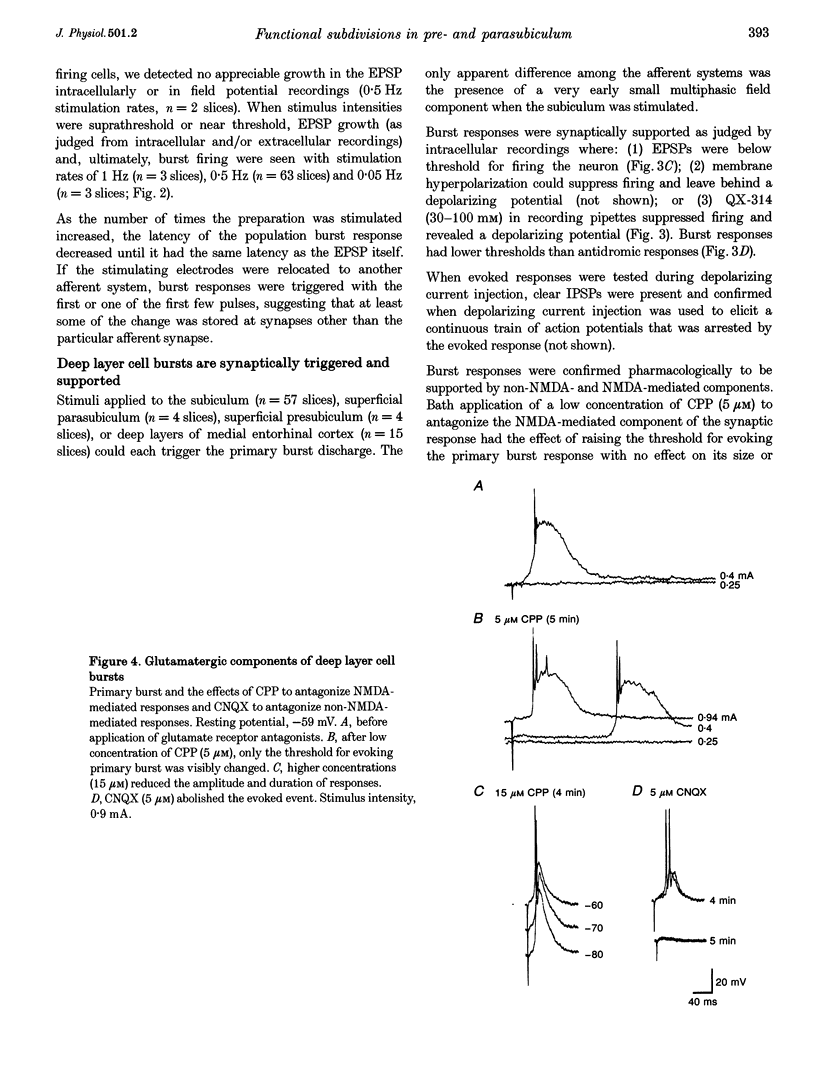
1. The presubiculum and parasubiculum are retrohippocampal structures bordered by the subiculum and medial entorhinal cortex. Deep layer (IV-VI) neurons from this region exhibit stable synaptically triggered burst behaviour which distinguishes them from superficial layer (I-III) cells. This functional separation was examined with intracellular and field potential recordings from horizontal slices of rat brain. Neurobiotin labelling and rapid Golgi techniques were used to obtain anatomical evidence of axonal trajectories. 2. Extracellular stimulation of the subiculum, deep medial entorhinal cortex or superficial pre- or parasubiculum caused, in deep layer cells only, a short latency burst discharge which could be followed by one or more after-discharges. Bursts appeared after repetitive stimulation and were stable for the life of the slice. Each event was supported by giant excitatory postsynaptic potentials (EPSPs). Events were similar whether they were evoked in horizontal slices or slices cut perpendicular to the horizontal plane. 3. Bath application of the NMDA receptor antagonist 3-[2-carboxypiperazin-4-yl]-propyl-1-phosphonic acid (CPP; 5 microM) elevated the threshold for evoking the giant EPSP. Higher concentrations (10-15 microM) reduced the amplitude and duration of the giant EPSP. Bath application of the AMPA receptor antagonist beta-cyano-7-nitroquinoxaline-2,3-dione (CNQX; 5 microM) eliminated the evoked EPSP. 4. In intact slices, superficial layer neurons of pre- and parasubiculum could exhibit EPSPs coincident with bursts recorded in the deep layers. However, in isolated subsections of horizontal slices or in 'vertical slices', both of which contained only pre- and/or parasubiculum, evoked or picrotoxin-induced bursts occurred only in deep layer cells. Superficial layer cells in these subsections showed no response to deep layer events. 5. Antidromic population spikes confirmed projections from superficial cell layers of pre- and parasubiculum down to their deep cell layers. Reciprocal antidromic responses were absent. 6. Axons of superficial layer stellate and pyramidal cells had horizontal collaterals and at least one ascending and one descending collateral. Branches of the descending collaterals were given off in layer V and some axons were found to reach the angular bundle. Axons of deep layer stellate and pyramidal cells also had horizontal collaterals and descending collaterals which could be traced to the angular bundle. One ascending axon collateral was found among the thirty-one deep layer cells examined morphologically. 7. We conclude that the deep layer cells of the presubiculum and parasubiculum are richly interconnected with excitatory synapses. These interconnections can generate giant excitatory synaptic potentials that support the bursting behaviour exhibited by these neurons. Any of the excitatory inputs to deep layer cells can trigger the population bursts and specific inputs from entorhinal cortex produce the after-discharges. Further, connections between superficial and deep layer cells appear to be almost exclusively in the direction of superficial to deep. The absence of significant ascending input can account for the functional separation of superficial and deep layer neurons of presubiculum and parasubiculum.
Full text
PDF


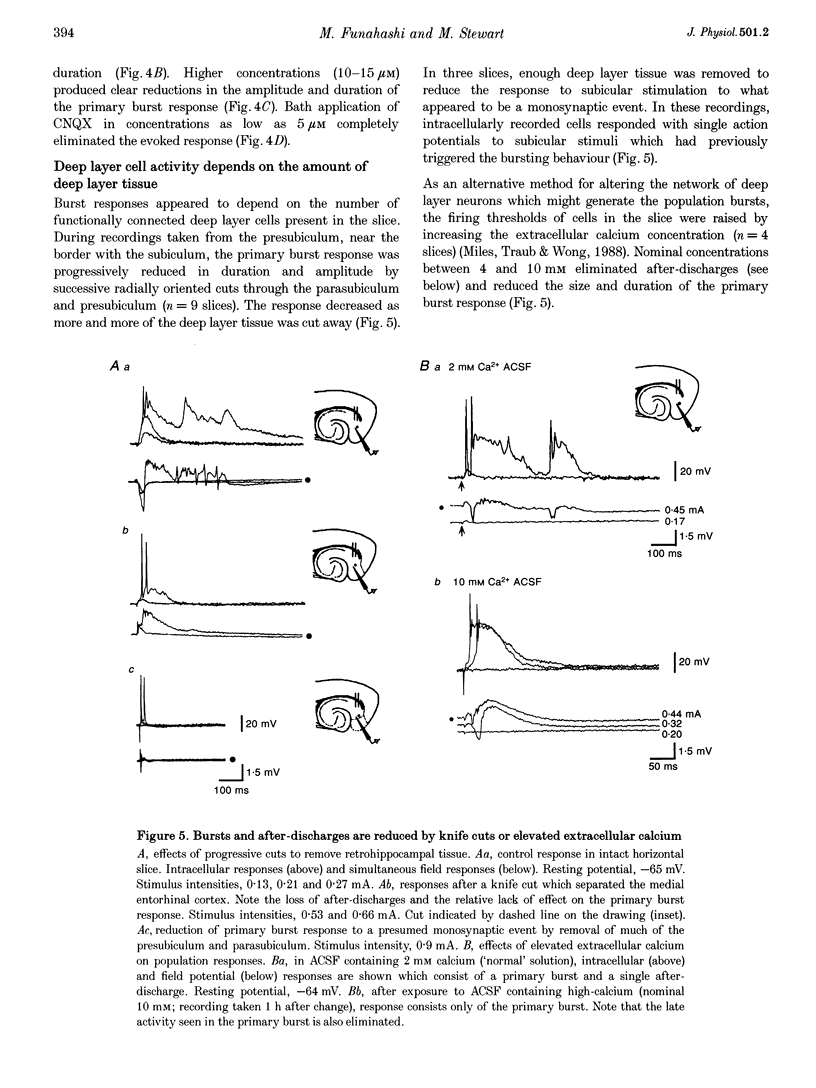
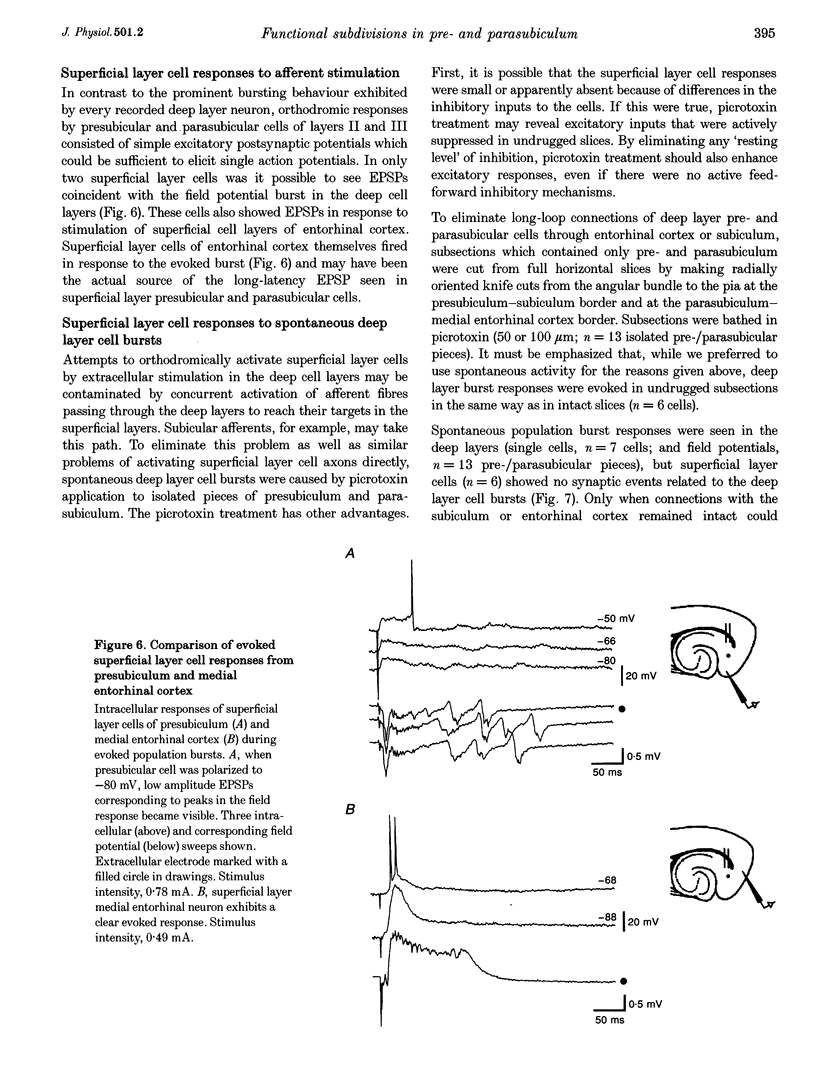
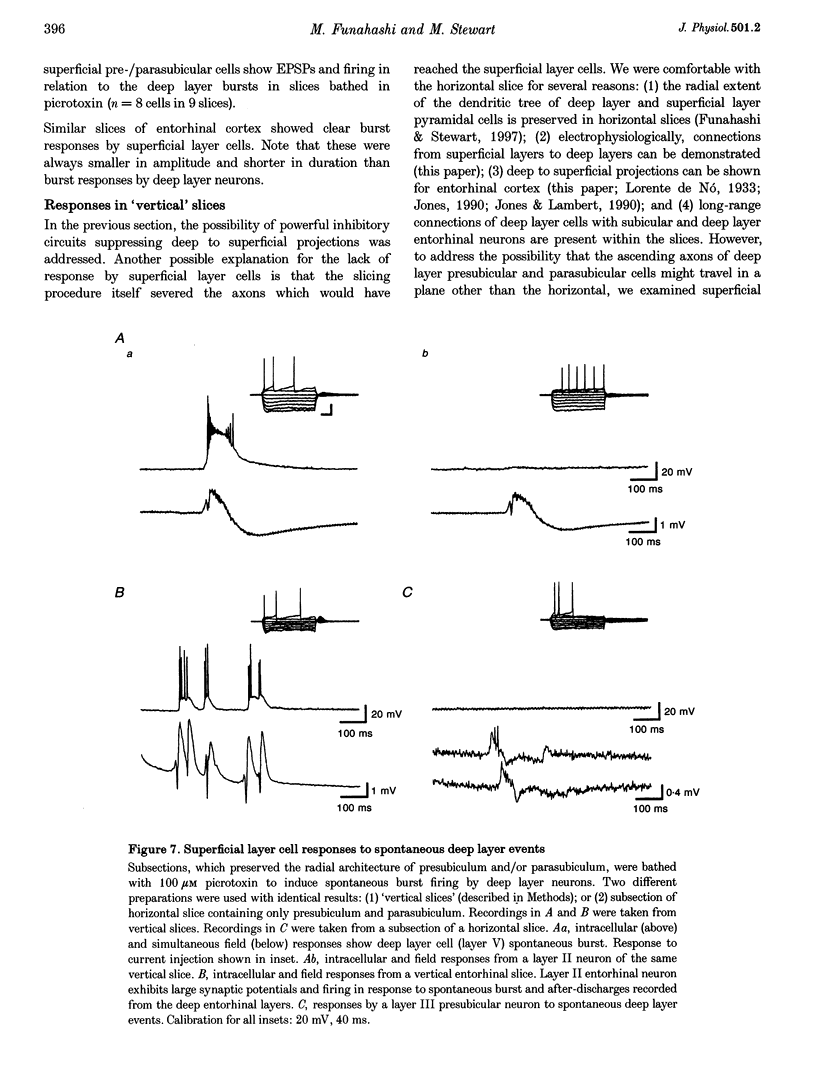
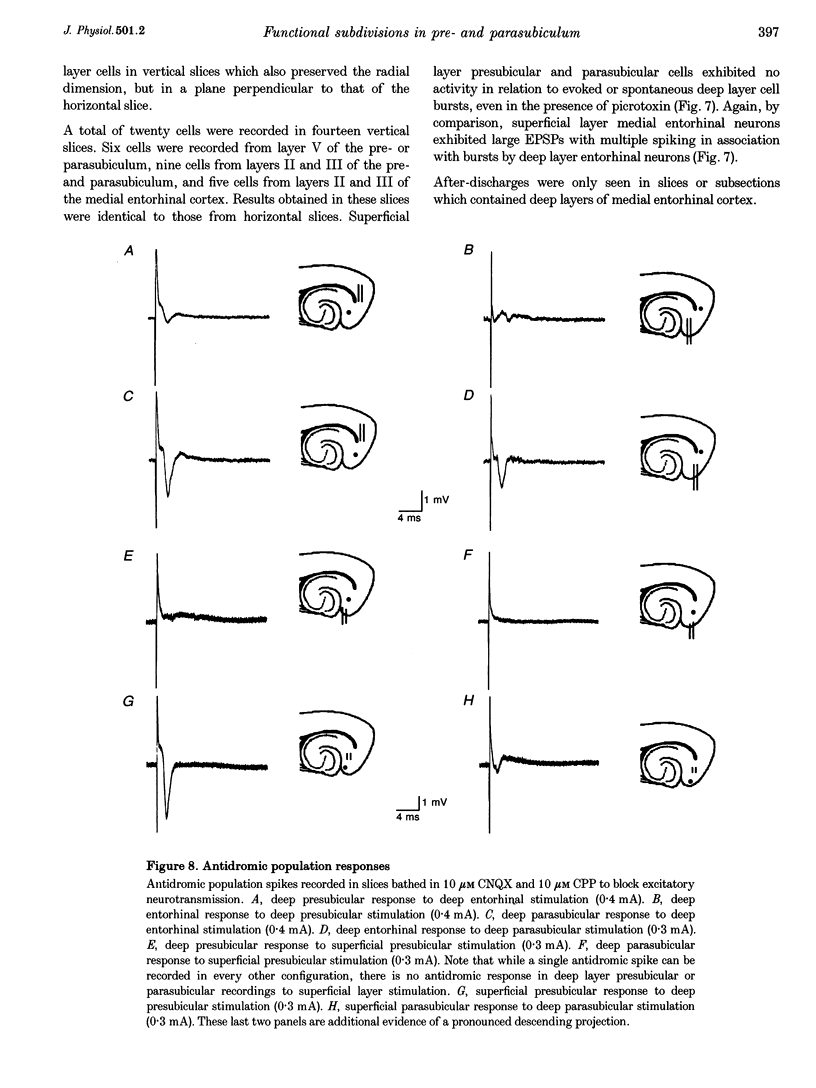
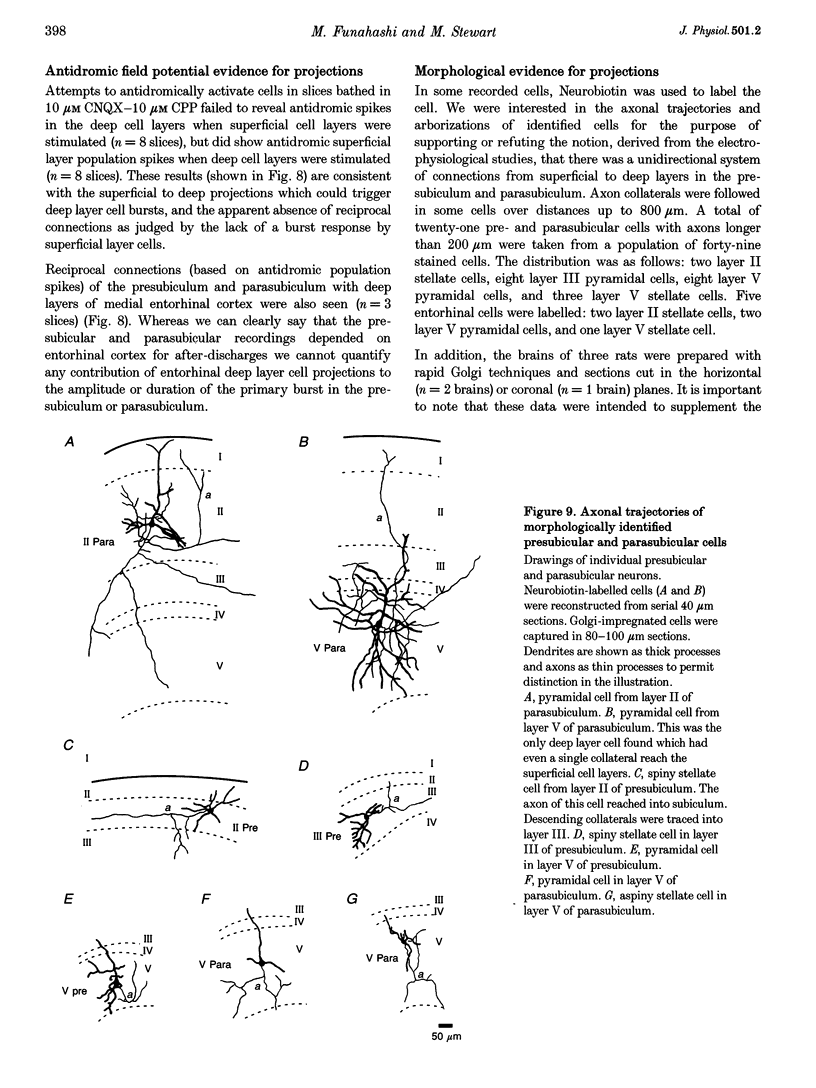
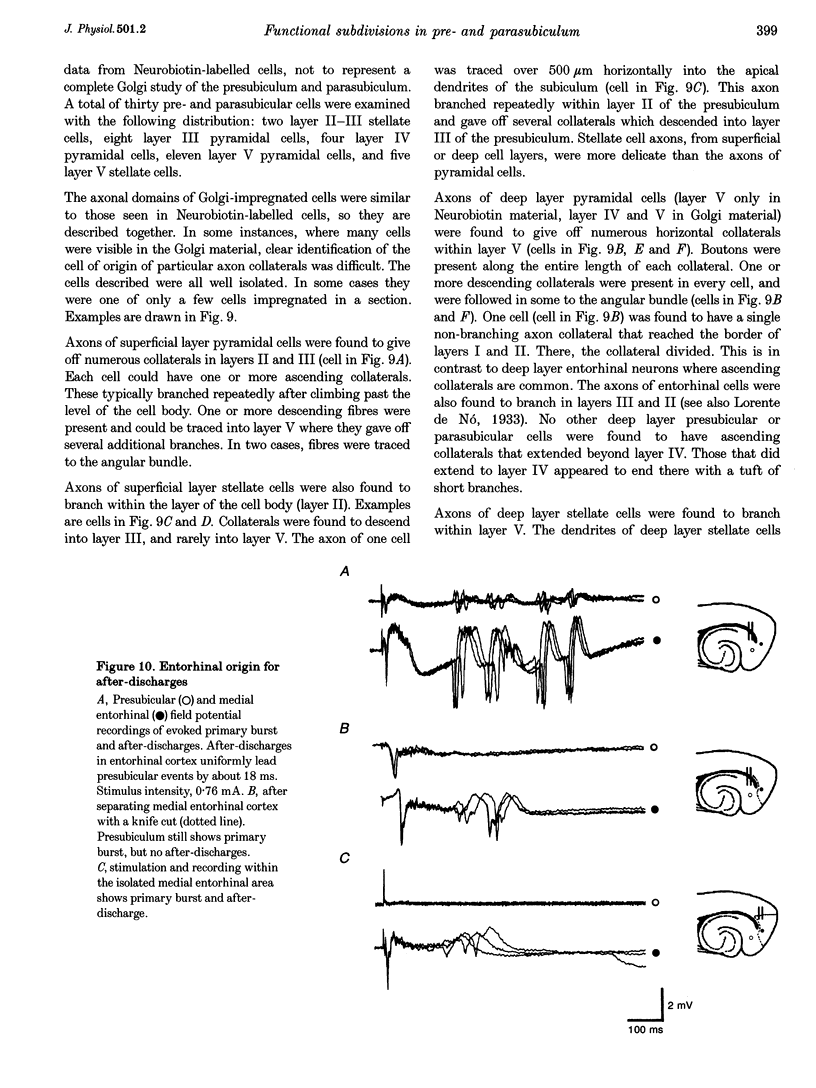




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alonso A., García-Austt E. Neuronal sources of theta rhythm in the entorhinal cortex of the rat. II. Phase relations between unit discharges and theta field potentials. Exp Brain Res. 1987;67(3):502–509. doi: 10.1007/BF00247283. [DOI] [PubMed] [Google Scholar]
- Barkai E., Friedman A., Grossman Y., Gutnick M. J. Laminar pattern of synaptic inhibition during convulsive activity induced by 4-aminopyridine in neocortical slices. J Neurophysiol. 1995 Apr;73(4):1462–1467. doi: 10.1152/jn.1995.73.4.1462. [DOI] [PubMed] [Google Scholar]
- Buzsáki G. Two-stage model of memory trace formation: a role for "noisy" brain states. Neuroscience. 1989;31(3):551–570. doi: 10.1016/0306-4522(89)90423-5. [DOI] [PubMed] [Google Scholar]
- Chrobak J. J., Buzsáki G. High-frequency oscillations in the output networks of the hippocampal-entorhinal axis of the freely behaving rat. J Neurosci. 1996 May 1;16(9):3056–3066. doi: 10.1523/JNEUROSCI.16-09-03056.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrobak J. J., Buzsáki G. Selective activation of deep layer (V-VI) retrohippocampal cortical neurons during hippocampal sharp waves in the behaving rat. J Neurosci. 1994 Oct;14(10):6160–6170. doi: 10.1523/JNEUROSCI.14-10-06160.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funahashi M., Stewart M. Presubicular and parasubicular cortical neurons of the rat: electrophysiological and morphological properties. Hippocampus. 1997;7(2):117–129. doi: 10.1002/(SICI)1098-1063(1997)7:2<117::AID-HIPO1>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Jones R. S. Complex synaptic responses of entorhinal cortical cells in the rat to subicular stimulation in vitro: demonstration of an NMDA receptor-mediated component. Neurosci Lett. 1987 Oct 16;81(1-2):209–214. doi: 10.1016/0304-3940(87)90000-0. [DOI] [PubMed] [Google Scholar]
- Jones R. S. Entorhinal-hippocampal connections: a speculative view of their function. Trends Neurosci. 1993 Feb;16(2):58–64. doi: 10.1016/0166-2236(93)90018-h. [DOI] [PubMed] [Google Scholar]
- Jones R. S., Heinemann U. Synaptic and intrinsic responses of medical entorhinal cortical cells in normal and magnesium-free medium in vitro. J Neurophysiol. 1988 May;59(5):1476–1496. doi: 10.1152/jn.1988.59.5.1476. [DOI] [PubMed] [Google Scholar]
- Jones R. S., Lambert J. D. Synchronous discharges in the rat entorhinal cortex in vitro: site of initiation and the role of excitatory amino acid receptors. Neuroscience. 1990;34(3):657–670. doi: 10.1016/0306-4522(90)90172-z. [DOI] [PubMed] [Google Scholar]
- Kang Y., Kaneko T., Ohishi H., Endo K., Araki T. Spatiotemporally differential inhibition of pyramidal cells in the cat motor cortex. J Neurophysiol. 1994 Jan;71(1):280–293. doi: 10.1152/jn.1994.71.1.280. [DOI] [PubMed] [Google Scholar]
- Kita H., Armstrong W. A biotin-containing compound N-(2-aminoethyl)biotinamide for intracellular labeling and neuronal tracing studies: comparison with biocytin. J Neurosci Methods. 1991 Apr;37(2):141–150. doi: 10.1016/0165-0270(91)90124-i. [DOI] [PubMed] [Google Scholar]
- Köhler C. Intrinsic connections of the retrohippocampal region in the rat brain. II. The medial entorhinal area. J Comp Neurol. 1986 Apr 8;246(2):149–169. doi: 10.1002/cne.902460202. [DOI] [PubMed] [Google Scholar]
- Köhler C. Intrinsic projections of the retrohippocampal region in the rat brain. I. The subicular complex. J Comp Neurol. 1985 Jun 22;236(4):504–522. doi: 10.1002/cne.902360407. [DOI] [PubMed] [Google Scholar]
- Miles R., Traub R. D., Wong R. K. Spread of synchronous firing in longitudinal slices from the CA3 region of the hippocampus. J Neurophysiol. 1988 Oct;60(4):1481–1496. doi: 10.1152/jn.1988.60.4.1481. [DOI] [PubMed] [Google Scholar]
- Salin P. A., Prince D. A. Electrophysiological mapping of GABAA receptor-mediated inhibition in adult rat somatosensory cortex. J Neurophysiol. 1996 Apr;75(4):1589–1600. doi: 10.1152/jn.1996.75.4.1589. [DOI] [PubMed] [Google Scholar]
- Silva L. R., Amitai Y., Connors B. W. Intrinsic oscillations of neocortex generated by layer 5 pyramidal neurons. Science. 1991 Jan 25;251(4992):432–435. doi: 10.1126/science.1824881. [DOI] [PubMed] [Google Scholar]
- Stewart M., Quirk G. J., Barry M., Fox S. E. Firing relations of medial entorhinal neurons to the hippocampal theta rhythm in urethane anesthetized and walking rats. Exp Brain Res. 1992;90(1):21–28. doi: 10.1007/BF00229252. [DOI] [PubMed] [Google Scholar]
- Stewart M., Wong R. K. Intrinsic properties and evoked responses of guinea pig subicular neurons in vitro. J Neurophysiol. 1993 Jul;70(1):232–245. doi: 10.1152/jn.1993.70.1.232. [DOI] [PubMed] [Google Scholar]
- Taube J. S., Muller R. U., Ranck J. B., Jr Head-direction cells recorded from the postsubiculum in freely moving rats. II. Effects of environmental manipulations. J Neurosci. 1990 Feb;10(2):436–447. doi: 10.1523/JNEUROSCI.10-02-00436.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taube J. S. Place cells recorded in the parasubiculum of freely moving rats. Hippocampus. 1995;5(6):569–583. doi: 10.1002/hipo.450050608. [DOI] [PubMed] [Google Scholar]
- Traub R. D., Whittington M. A., Colling S. B., Buzsáki G., Jefferys J. G. Analysis of gamma rhythms in the rat hippocampus in vitro and in vivo. J Physiol. 1996 Jun 1;493(Pt 2):471–484. doi: 10.1113/jphysiol.1996.sp021397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witter M. P., Groenewegen H. J., Lopes da Silva F. H., Lohman A. H. Functional organization of the extrinsic and intrinsic circuitry of the parahippocampal region. Prog Neurobiol. 1989;33(3):161–253. doi: 10.1016/0301-0082(89)90009-9. [DOI] [PubMed] [Google Scholar]
- Wouterlood F. G., Härtig W., Brückner G., Witter M. P. Parvalbumin-immunoreactive neurons in the entorhinal cortex of the rat: localization, morphology, connectivity and ultrastructure. J Neurocytol. 1995 Feb;24(2):135–153. doi: 10.1007/BF01181556. [DOI] [PubMed] [Google Scholar]
- Ylinen A., Bragin A., Nádasdy Z., Jandó G., Szabó I., Sik A., Buzsáki G. Sharp wave-associated high-frequency oscillation (200 Hz) in the intact hippocampus: network and intracellular mechanisms. J Neurosci. 1995 Jan;15(1 Pt 1):30–46. doi: 10.1523/JNEUROSCI.15-01-00030.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Brederode J. F., Spain W. J. Differences in inhibitory synaptic input between layer II-III and layer V neurons of the cat neocortex. J Neurophysiol. 1995 Sep;74(3):1149–1166. doi: 10.1152/jn.1995.74.3.1149. [DOI] [PubMed] [Google Scholar]
- van Groen T., Wyss J. M. The connections of presubiculum and parasubiculum in the rat. Brain Res. 1990 Jun 4;518(1-2):227–243. doi: 10.1016/0006-8993(90)90976-i. [DOI] [PubMed] [Google Scholar]



