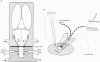
Abstract
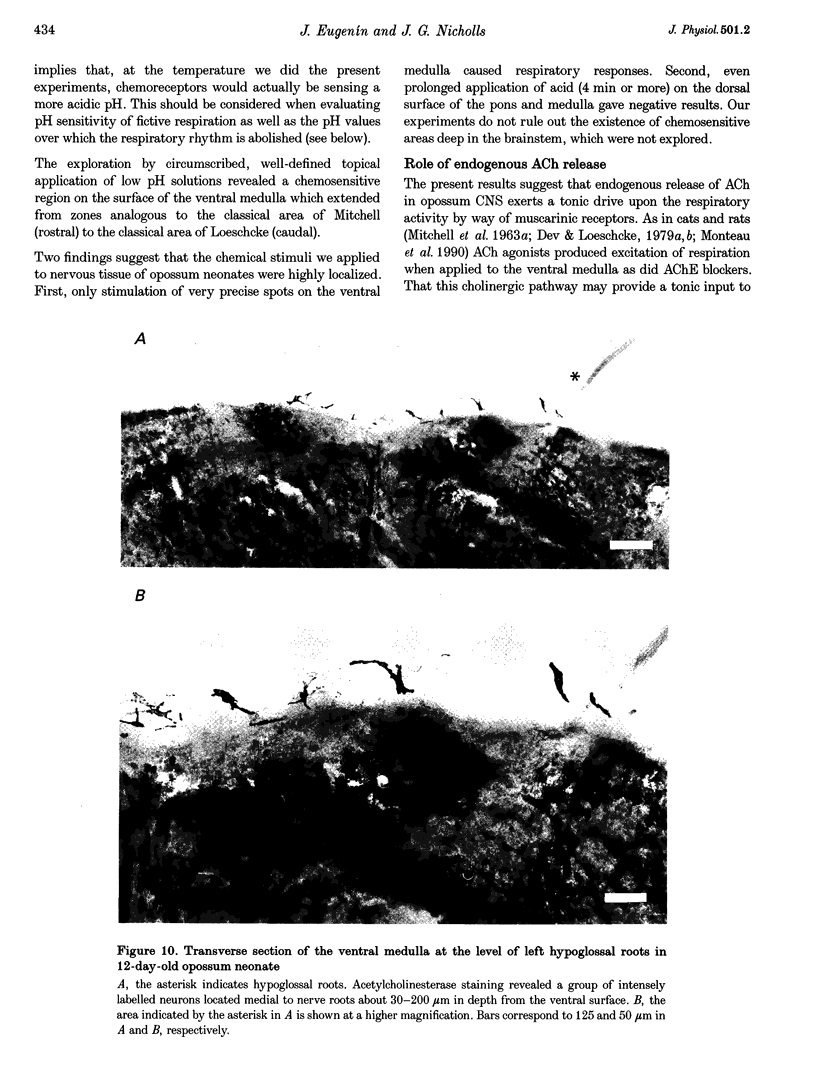
1. The aim of the present experiments was to characterize the central chemical drive of fictive respiration in the isolated CNS of the newborn opossum, Monodelphis domestica. This opossum preparation, in contrast to those of neonatal rats and mice, produces respiratory rhythm of high frequency in vitro. 2. Fictive respiration was recorded from C3-C5 ventral roots of the isolated CNS of 4- to 14-day-old opossums using suction electrodes. At room temperature (21-23 degrees C) the frequency of respiration was 43 +/- 5.3 min-1 (mean +/- S.E.M., n = 50) in basal medium Eagle's medium (BMEM) equilibrated with 5% CO2-95% O2, pH 7.37-7.40. Respiratory discharges remained regular throughout 8 h experiments and continued for more than 20 h in culture. 3. Superfusion of the brainstem confirmed that solutions of pH 6.3-7.2 increased both the amplitude and frequency of respiration. High pH solutions (7.5-7.7) had the opposite effect and abolished the rhythm at pH 7.7. Addition of ACh (50-100 microM) or carbachol (0.01-10 microM) to the brainstem superfusion also increased the amplitude and frequency of respiratory activity, as did physostigmine (50-100 microM) or neostigmine (20-50 microM). Conversely, scopolamine (50-100 microM) reduced the amplitude and frequency of the basal respiratory rhythm by about 30%. 4. H(+)- and cholinergic-sensitive areas on the surface of the isolated CNS were explored with a small micropipette (outer tip diameter, 100 microns) filled with BMEM (pH 6.5) or 1 microM carbachol. Carbachol applied to H(+)- and cholinergic-sensitive areas in the ventral medulla mimicked the changes of respiratory pattern produced by low pH application. Responses to altered pH and carbachol were abolished by scopolamine (50 microM). Histochemistry demonstrated several medullary groups of neurons stained for acetylcholinesterase. The superficial location of one of these groups coincided with a functional and anatomically well-defined pH- and carbachol-sensitive area placed medial to the hypoglossal roots. 5. Exploration of chemosensitive areas revealed that application of drugs or solutions of different pH to a single well-defined spot could have selective and distinctive effects upon amplitude and frequency of respiratory activity. 6. These results show that fictive respiration in the isolated CNS of the newborn opossum is tonically driven by chemical- and cholinergic-sensitive areas located on the ventral medulla, the activity of which regulates frequency and amplitude of respiration. They suggest that a cholinergic relay, although not essential for rhythm generation, is involved in the central pH chemosensory mechanism, or that cholinergic and chemical inputs converge upon the same input pathway to the respiratory pattern generator.
Full text
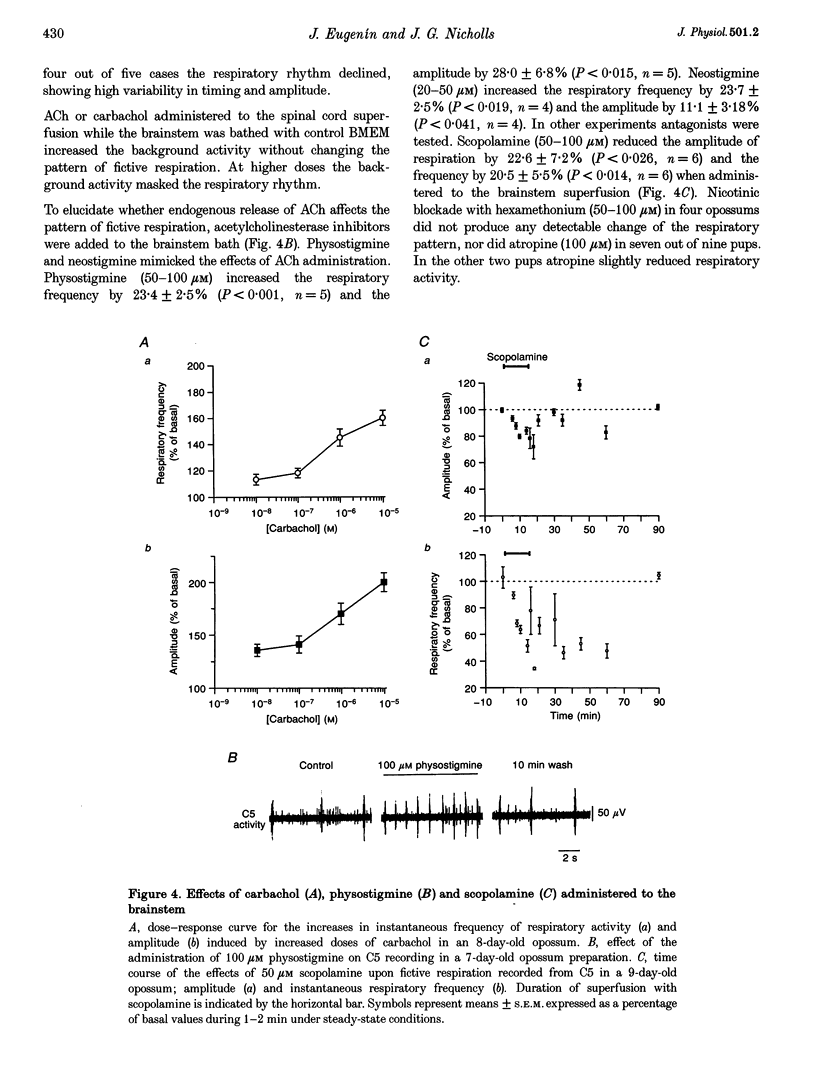
PDF

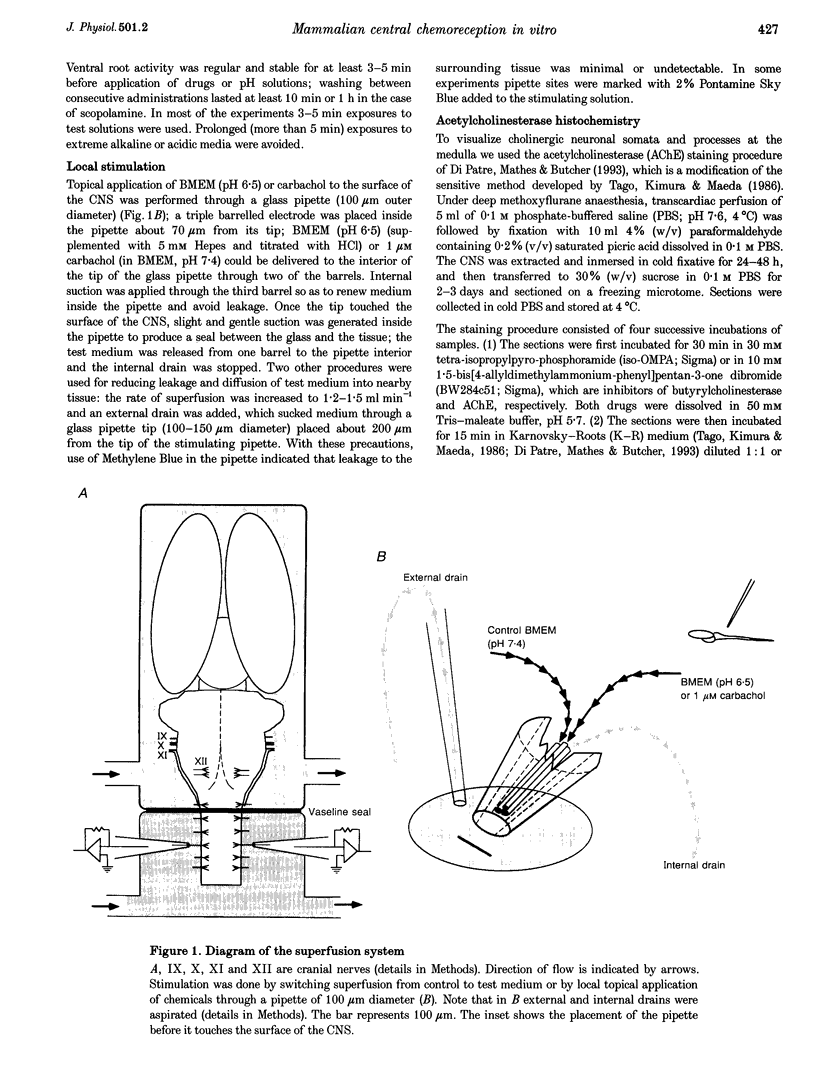
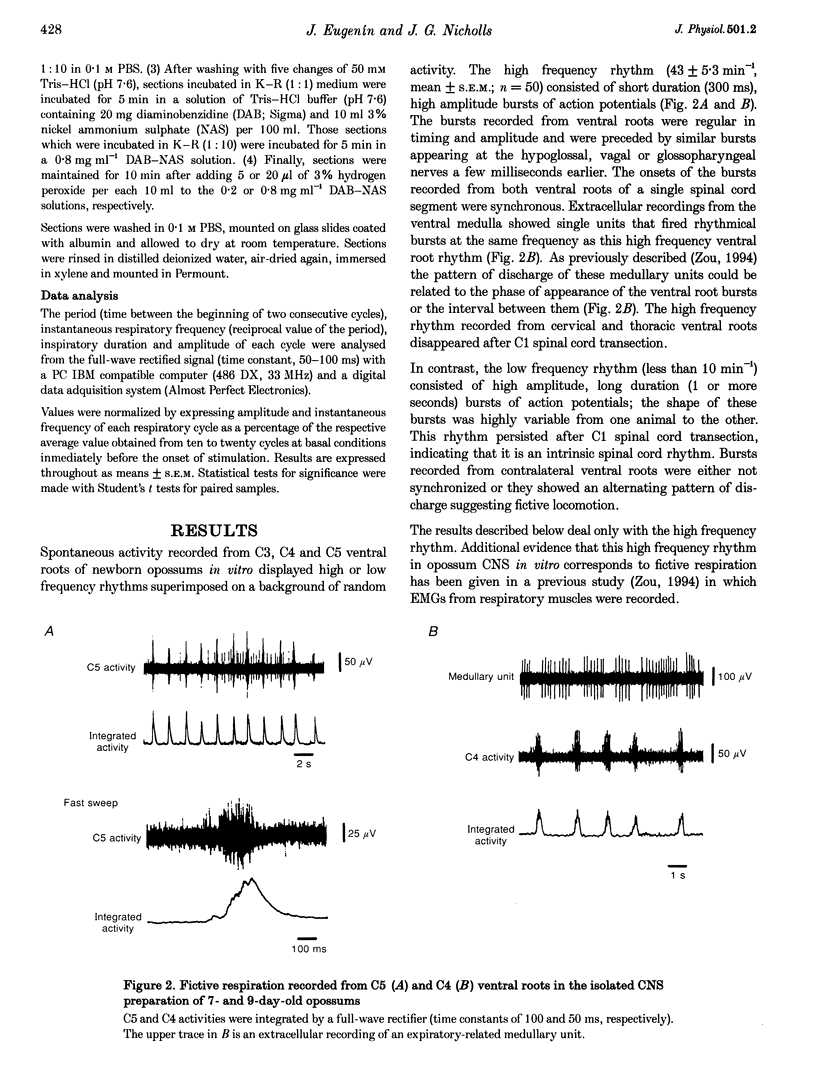
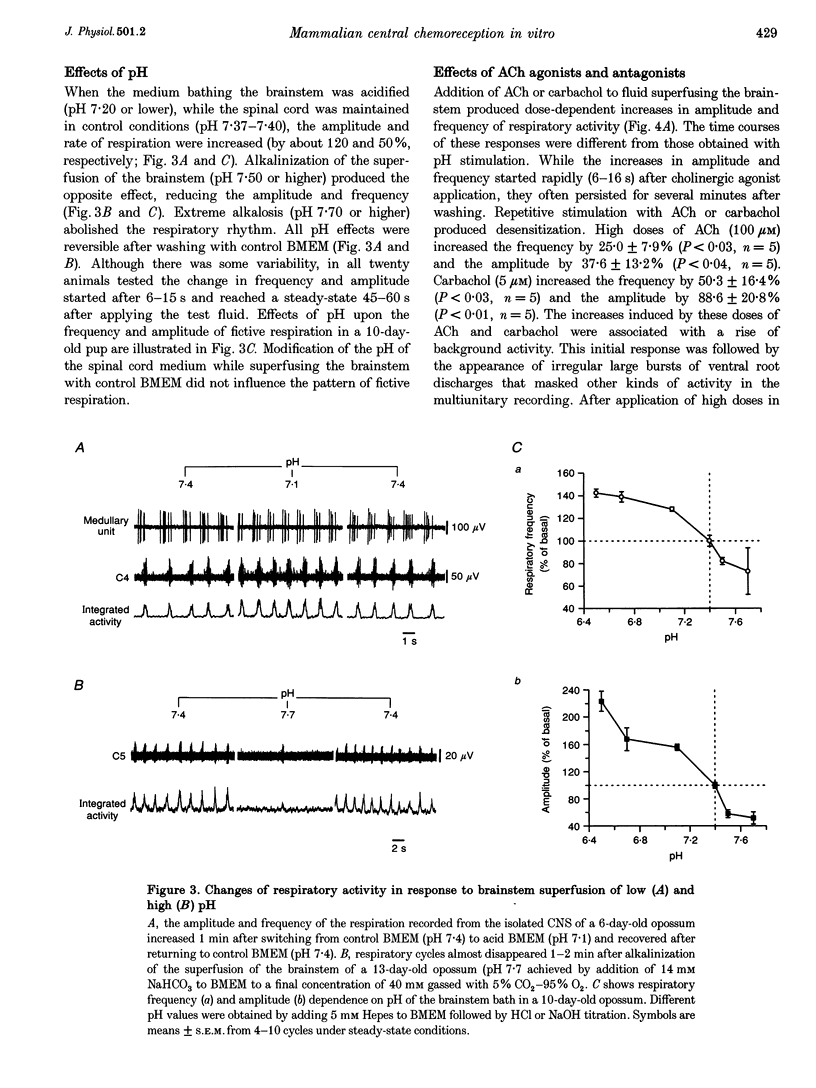
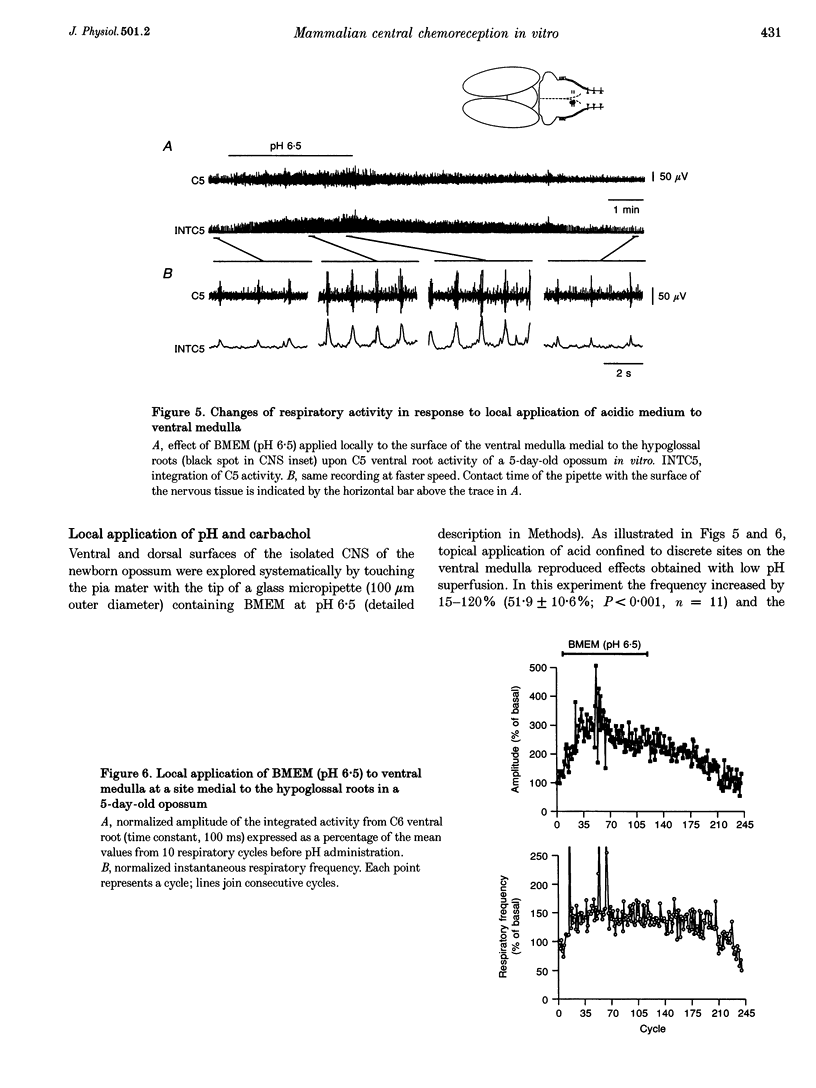
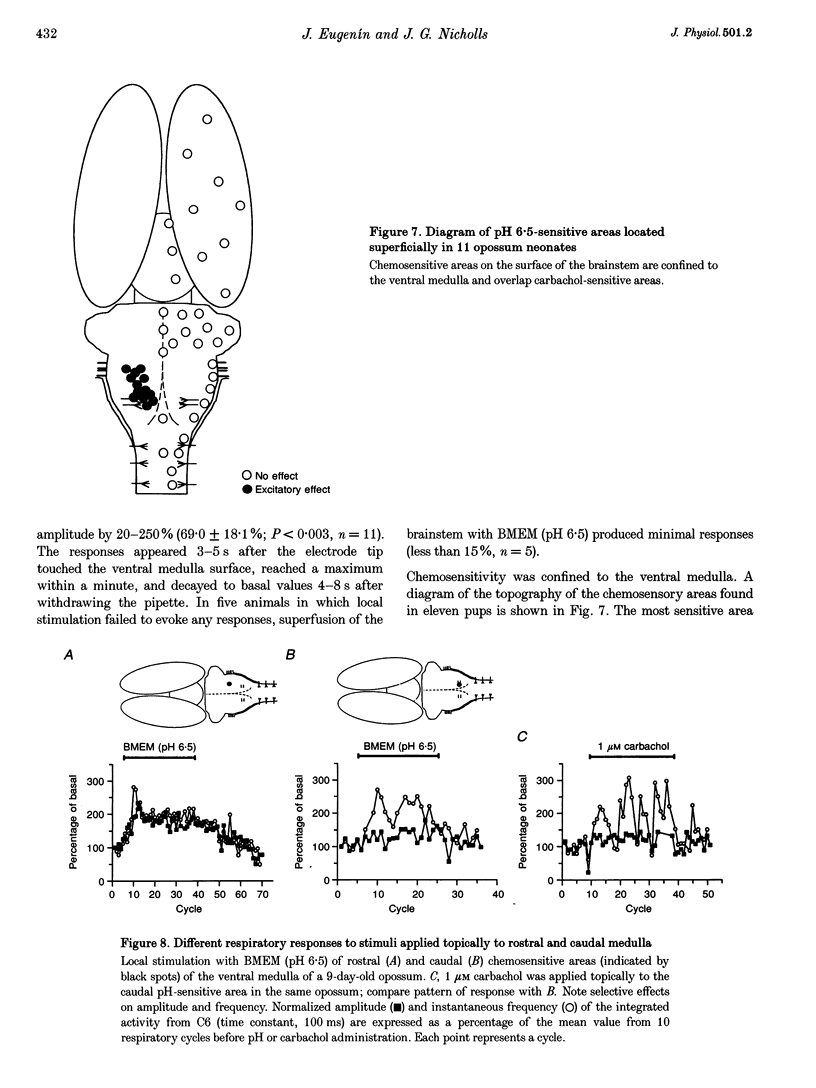



Images in this article
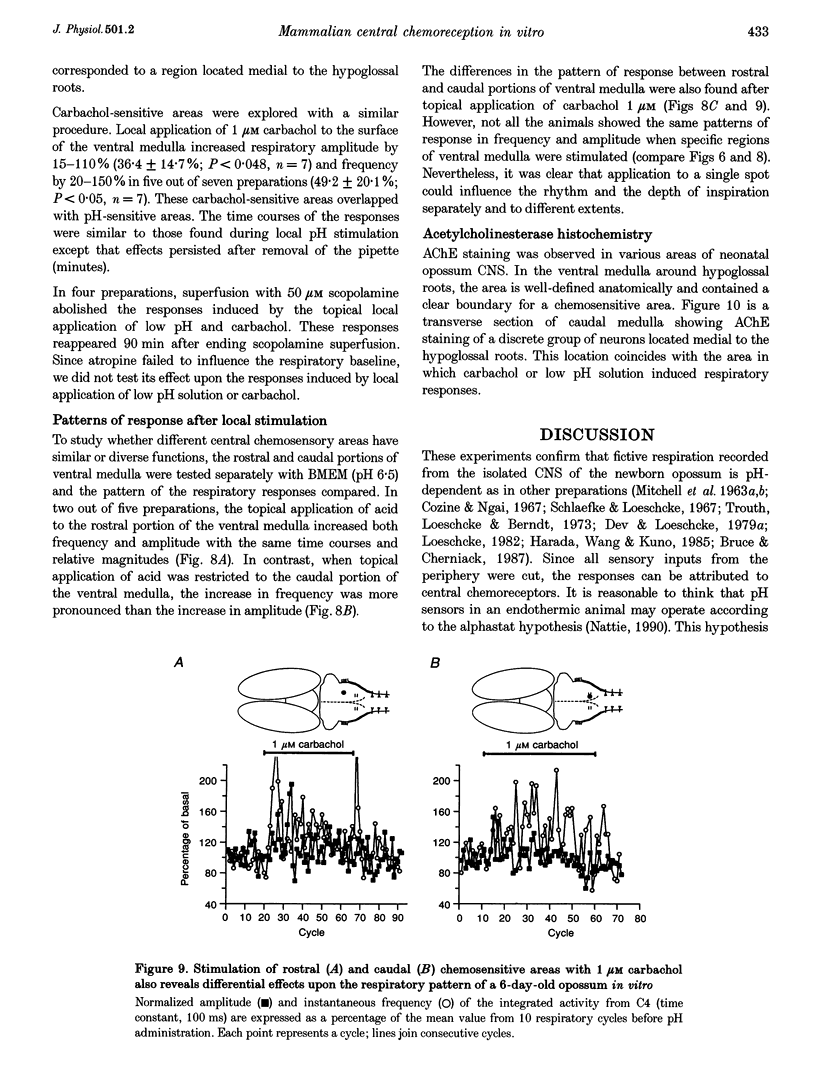
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bruce E. N., Cherniack N. S. Central chemoreceptors. J Appl Physiol (1985) 1987 Feb;62(2):389–402. doi: 10.1152/jappl.1987.62.2.389. [DOI] [PubMed] [Google Scholar]
- Campbell J. A. Changes in the tensions of CO(2) and O(2) in gases injected under the skin and into the abdominal cavity. J Physiol. 1924 Aug 12;59(1):1–16. doi: 10.1113/jphysiol.1924.sp002157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherniack N. S., von Euler C., Homma I., Kao F. F. Graded changes in central chemoceptor input by local temperature changes on the ventral surface of medulla. J Physiol. 1979 Feb;287:191–211. doi: 10.1113/jphysiol.1979.sp012654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark F. J., von Euler C. On the regulation of depth and rate of breathing. J Physiol. 1972 Apr;222(2):267–295. doi: 10.1113/jphysiol.1972.sp009797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coates E. L., Li A., Nattie E. E. Widespread sites of brain stem ventilatory chemoreceptors. J Appl Physiol (1985) 1993 Jul;75(1):5–14. doi: 10.1152/jappl.1993.75.1.5. [DOI] [PubMed] [Google Scholar]
- Dev N. B., Loeschcke H. H. A cholinergic mechanism involved in the respiratory chemosensitivity of the medulla oblongata in the cat. Pflugers Arch. 1979 Feb 14;379(1):29–36. doi: 10.1007/BF00622901. [DOI] [PubMed] [Google Scholar]
- Dev N. B., Loeschcke H. H. Topography of the respiratory and circulatory responses to acetylcholine and nicotine on the ventral surface of the medulla oblongata. Pflugers Arch. 1979 Feb 14;379(1):19–27. doi: 10.1007/BF00622900. [DOI] [PubMed] [Google Scholar]
- Di Patre P. L., Mathes C. W., Butcher L. L. Differential visualization of cholinesterasic neuronal somata and fibers by use of modifications of acetylcholinesterase pharmacohistochemistry. J Histochem Cytochem. 1993 Jan;41(1):129–135. doi: 10.1177/41.1.7678024. [DOI] [PubMed] [Google Scholar]
- Fedorko L., Kelly E. N., England S. J. Importance of vagal afferents in determining ventilation in newborn rats. J Appl Physiol (1985) 1988 Sep;65(3):1033–1039. doi: 10.1152/jappl.1988.65.3.1033. [DOI] [PubMed] [Google Scholar]
- Fukuda Y., Loeschcke H. H. A cholinergic mechanism involved in the neuronal excitation by H+ in the respiratory chemosensitive structures of the ventral medulla oblongata of rats in vitro. Pflugers Arch. 1979 Mar 16;379(2):125–135. doi: 10.1007/BF00586938. [DOI] [PubMed] [Google Scholar]
- Fung M. L., Wang W., St John W. M. Medullary loci critical for expression of gasping in adult rats. J Physiol. 1994 Nov 1;480(Pt 3):597–611. doi: 10.1113/jphysiol.1994.sp020387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer J. J., Carter J. E., Allan D. W. Respiratory rhythm generation in a precocial rodent in vitro preparation. Respir Physiol. 1996 Feb;103(2):105–112. doi: 10.1016/0034-5687(95)00073-9. [DOI] [PubMed] [Google Scholar]
- Harada Y., Kuno M., Wang Y. Z. Differential effects of carbon dioxide and pH on central chemoreceptors in the rat in vitro. J Physiol. 1985 Nov;368:679–693. doi: 10.1113/jphysiol.1985.sp015883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada Y., Wang Y. Z., Kuno M. Central chemosensitivity to H+ and CO2 in the rat respiratory center in vitro. Brain Res. 1985 May 6;333(2):336–339. doi: 10.1016/0006-8993(85)91588-4. [DOI] [PubMed] [Google Scholar]
- Issa F. G., Remmers J. E. Identification of a subsurface area in the ventral medulla sensitive to local changes in PCO2. J Appl Physiol (1985) 1992 Feb;72(2):439–446. doi: 10.1152/jappl.1992.72.2.439. [DOI] [PubMed] [Google Scholar]
- Loeschcke H. H. Central chemosensitivity and the reaction theory. J Physiol. 1982 Nov;332:1–24. doi: 10.1113/jphysiol.1982.sp014397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteau R., Morin D., Hilaire G. Acetylcholine and central chemosensitivity: in vitro study in the newborn rat. Respir Physiol. 1990 Aug;81(2):241–253. doi: 10.1016/0034-5687(90)90049-5. [DOI] [PubMed] [Google Scholar]
- Morin-Surun M. P., Boudinot E., Schäfer T., Denavit-Saubié M. Localization of chemosensitive structures in the isolated brainstem of adult guinea-pig. J Physiol. 1995 May 15;485(Pt 1):203–212. doi: 10.1113/jphysiol.1995.sp020724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Møllgård K., Balslev Y., Janas M. S., Treherne J. M., Saunders N. R., Nichols J. G. Development of spinal cord in the isolated CNS of a neonatal mammal (the opossum Monodelphis domestica) maintained in longterm culture. J Neurocytol. 1994 Mar;23(3):151–165. doi: 10.1007/BF01181557. [DOI] [PubMed] [Google Scholar]
- Nicholls J. G., Stewart R. R., Erulkar S. D., Saunders N. R. Reflexes, fictive respiration and cell division in the brain and spinal cord of the newborn opossum, Monodelphis domestica, isolated and maintained in vitro. J Exp Biol. 1990 Sep;152:1–15. doi: 10.1242/jeb.152.1.1. [DOI] [PubMed] [Google Scholar]
- Okada Y., Mückenhoff K., Scheid P. Hypercapnia and medullary neurons in the isolated brain stem-spinal cord of the rat. Respir Physiol. 1993 Sep;93(3):327–336. doi: 10.1016/0034-5687(93)90078-o. [DOI] [PubMed] [Google Scholar]
- Onimaru H., Homma I. Respiratory rhythm generator neurons in medulla of brainstem-spinal cord preparation from newborn rat. Brain Res. 1987 Feb 17;403(2):380–384. doi: 10.1016/0006-8993(87)90080-1. [DOI] [PubMed] [Google Scholar]
- Saunders N. R., Balkwill P., Knott G., Habgood M. D., Møllgård K., Treherne J. M., Nicholls J. G. Growth of axons through a lesion in the intact CNS of fetal rat maintained in long-term culture. Proc Biol Sci. 1992 Dec 22;250(1329):171–180. doi: 10.1098/rspb.1992.0146. [DOI] [PubMed] [Google Scholar]
- St John W. M. Neurogenesis, control, and functional significance of gasping. J Appl Physiol (1985) 1990 Apr;68(4):1305–1315. doi: 10.1152/jappl.1990.68.4.1305. [DOI] [PubMed] [Google Scholar]
- Stewart R. R., Zou D. J., Treherne J. M., Møllgård K., Saunders N. R., Nicholls J. G. The intact central nervous system of the newborn opossum in long-term culture: fine structure and GABA-mediated inhibition of electrical activity. J Exp Biol. 1991 Nov;161:25–41. doi: 10.1242/jeb.161.1.25. [DOI] [PubMed] [Google Scholar]
- Suzue T. Respiratory rhythm generation in the in vitro brain stem-spinal cord preparation of the neonatal rat. J Physiol. 1984 Sep;354:173–183. doi: 10.1113/jphysiol.1984.sp015370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tago H., Kimura H., Maeda T. Visualization of detailed acetylcholinesterase fiber and neuron staining in rat brain by a sensitive histochemical procedure. J Histochem Cytochem. 1986 Nov;34(11):1431–1438. doi: 10.1177/34.11.2430009. [DOI] [PubMed] [Google Scholar]
- Treherne J. M., Woodward S. K., Varga Z. M., Ritchie J. M., Nicholls J. G. Restoration of conduction and growth of axons through injured spinal cord of neonatal opossum in culture. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):431–434. doi: 10.1073/pnas.89.1.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trouth C. O., Loeschcke H. H., Berndt J. A superficial substrate on the ventral surface of the medulla oblongata influencing respiration. Pflugers Arch. 1973 Mar 21;339(2):135–152. doi: 10.1007/BF00587180. [DOI] [PubMed] [Google Scholar]
- Varga Z. M., Fernandez J., Blackshaw S., Martin A. R., Muller K. J., Adams W. B., Nicholls J. G. Neurite outgrowth through lesions of neonatal opossum spinal cord in culture. J Comp Neurol. 1996 Mar 18;366(4):600–612. doi: 10.1002/(SICI)1096-9861(19960318)366:4<600::AID-CNE4>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Wang W., Fung M. L., Darnall R. A., St John W. M. Characterizations and comparisons of eupnoea and gasping in neonatal rats. J Physiol. 1996 Jan 1;490(Pt 1):277–292. doi: 10.1113/jphysiol.1996.sp021143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward S. K., Treherne J. M., Knott G. W., Fernandez J., Varga Z. M., Nicholls J. G. Development of connections by axons growing through injured spinal cord of neonatal opossum in culture. J Exp Biol. 1993 Mar;176:77–88. doi: 10.1242/jeb.176.1.77. [DOI] [PubMed] [Google Scholar]
- Zhou D., Wasicko M. J., Hu J. M., St John W. M. Differing activities of medullary respiratory neurons in eupnea and gasping. J Appl Physiol (1985) 1991 Mar;70(3):1265–1270. doi: 10.1152/jappl.1991.70.3.1265. [DOI] [PubMed] [Google Scholar]
- Zou D. J. Respiratory rhythm in the isolated central nervous system of newborn opossum. J Exp Biol. 1994 Dec;197:201–213. doi: 10.1242/jeb.197.1.201. [DOI] [PubMed] [Google Scholar]
- Zou D. J., Treherne J. M., Stewart R. R., Saunders N. R., Nicholls J. G. Regulation of GABAB receptors by histamine and neuronal activity in the isolated spinal cord of neonatal opossum in culture. Proc Biol Sci. 1991 Oct 22;246(1315):77–82. doi: 10.1098/rspb.1991.0127. [DOI] [PubMed] [Google Scholar]