Abstract
Nontypeable Haemophilus influenzae (NTHI) is an opportunistic pathogen, and heterogeneity in the surface-exposed immunodominant domains of NTHI proteins is thought to be associated with the failure of an infection to stimulate an immune response that is cross-protective against heterologous NTHI strains. The aim of this study was to assess the vaccine potential of a surface-exposed component of the NTHI human transferrin receptor, TbpB, and to determine if the antibody response elicited was cross-reactive with heterologous strains of NTHI. The efficacy of immunization with a recombinant form of TbpB (rTbpB) was determined by assessing the pulmonary clearance of viable bacteria 4 h after a live challenge with NTHI. There was a significant reduction in the number of viable bacteria in both the bronchoalveolar lavage fluid (34% for the 20-μg dose and 58% for the 40-μg dose) and lung homogenates (26% for the 20-μg dose and 60% for the 40-μg dose) of rats immunized with rTbpB compared to the control animals. While rTbpB-specific antibodies from immunized rats were nonspecific in the recognition of TbpB from six heterologous NTHI strains on Western blots, these antibodies differed in their ability to block transferrin binding to heterologous strains and to cross-react in bactericidal assays. If bactericidal antibodies are key indicators of the efficacy of the immune response in eliminating NTHI, this data suggests that while immunization with rTbpB stimulates protective responses against the homologous isolate, variability in the recognition of TbpB from heterologous isolates may limit the potential of rTbpB as an NTHI vaccine component.
The unencapsulated or nontypeable form of Haemophilus influenzae (NTHI) is commonly found as a minor component of the oropharyngeal microbiota (13). However, NTHI is also associated with recurrent, opportunistic infections of mucosal sites, in particular otitis media in children (12) and exacerbations of chronic bronchitis in adults (20). Heterogeneity in the surface-exposed immunodominant domains of proteins, especially the outer membrane proteins P2 and P5, has been proposed as a mechanism of evasion of the host immune response (4, 8, 28). This heterogeneity is the major difficulty faced in the development of cross-protective immunity against NTHI, and considerable efforts have been invested in an attempt to identify conserved epitopes that could be used as candidates for vaccine development. These efforts have recently focused on the proteins that comprise the transferrin binding receptor.
The importance of iron withholding or “nutritional immunity” as an antibacterial defense mechanism is apparent from the prevalence of bacterial infections when this mechanism fails. The consequence of hemochromatosis and hyperferremia is often bacterial sepsis (22, 31). All pathogenic bacteria have a basic physiological requirement for iron; however, host mechanisms maintain free iron to levels below that required to sustain bacterial growth (21). Nevertheless, iron-starved inhibition of the growth of the majority of NTHI strains can be overcome by the addition of human transferrin (9, 10), and the observation that this required direct interaction of transferrin with the bacterial surface suggested the presence of a bacterial transferrin receptor rather than siderophore-mediated scavenging of iron from transferrin (19, 32).
The transferrin receptor is composed of two subunits. The interaction of the receptor with transferrin is probably initiated by transferrin binding protein B (TbpB), a peripheral lipoprotein that forms a complex with TbpA, a TonB-dependent integral outer membrane protein that is thought to form a gated pore to facilitate the transport of transferrin-derived iron across the outer membrane (7). Affinity chromatography has identified two transferrin receptor subunits in H. influenzae type b (Hib) (26), and recently the genes encoding TbpA and TbpB were cloned and characterized from both Hib and NTHI (7, 17). Although the potential of the NTHI Tbp proteins as vaccine components has not been characterized, passive transfer of hyperimmune anti-TbpB but not anti-TbpA serum protected against bacteremia in a rat pup model of Hib infection (17). In addition, antibodies specific for TbpB but not those specific for TbpA were found to be bactericidal against Neisseria meningitidis, and immunization of mice with TbpB was as protective as immunization with whole killed bacteria against a challenge with a lethal dose of the homologous meningococcal strain (16).
While sequence analysis of tbpB genes from six NTHI strains demonstrated regions of homology throughout the genes, the overall homology was as low as 66% in some isolates (17). Despite the relatively low level of conservation in some strains, studies have demonstrated that Tbp proteins from H. influenzae, Actinobacillus pleuropneumoniae, and N. meningitidis are antigenically related (11, 27), leading to the suggestion that TbpB may be used as a cross-protective antigen against these bacteria (11). Although the topology of these shared epitopes is unknown, it is conceivable that amino acid variation in the surface-exposed domains of TbpB that interact with human transferrin would be functionally conserved, providing potential targets for the induction of cross-protective immune responses.
Transferrin selectively accumulates in the lungs and is found primarily in the alveoli (3, 29), where it appears to act as an important antioxidant in the protection of cellular membranes against iron-dependent lipid peroxidation (23). Notably, NTHI stimulates the production of transferrin, which is increased in the sputum sol phase of patients with chronic bronchitis who are infected with this bacterium (29). Thus, the ability of NTHI to gain access to the human iron pool by sequestering iron from transferrin may provide an important mechanism that enhances bacterial survival within the respiratory tract. The physiological importance of Tbp proteins is also reflected by the large number of isolates of NTHI that have the capacity to bind and derive iron from human transferrin (18, 25). Therefore, stimulation of TbpB-specific antibodies may not only target protective immune responses but may also provide a mechanism that results in the attenuation of NTHI growth in the respiratory tract by limiting the access of this bacterium to a physiologically important source of iron.
The aim of the investigation described here was to determine the efficacy of mucosal-directed immunization with recombinant TbpB (rTbpB) in enhancing the clearance of NTHI from the lungs of rats with acute infection and to assess the cross-reactivity of the induced TbpB-specific antibodies.
MATERIALS AND METHODS
Chemicals.
Reagents were purchased from Sigma (St. Louis, Mo.) unless otherwise indicated.
NTHI strains.
The NTHI strains used in the cross-reactivity assays were derived either from middle ear fluid from patients with otitis media or from sputum from infected patients with chronic respiratory disease (Table 1). These strains were generously supplied by L. Tetlow, Medical Microbiologist, Capital Pathology, Australian Capital Territory, Australia. The strains were screened for the absence of agglutination with anti-b capsule antiserum (Murex Diagnostics, Dartford, England) and for the requirement for growth factors X (hemin) and V (NAD) with Microring XV impregnated discs (Microdiagnostics, Brisbane, Australia). The strains were biotyped with the api NH identification system (bioMérieux, Marcy-l’Etoile, France).
TABLE 1.
Origin of NTHI isolates used in the cross-reactivity assays
| Strain | Biotype | Isolation sitea |
|---|---|---|
| UC19 | I | Sputum |
| UC27 | II | Sputum |
| UC28 | II | Middle ear |
| UC77 | I | Middle ear |
| UC84 | IV | Middle ear |
| UC103 | II | Sputum |
Sputum was obtained from infected patients with chronic respiratory disease, and middle ear fluid was obtained from patients with otitis media.
Cloning and purification of recombinant NTHI TbpB.
The NTHI strain UC19 (289-I) was originally derived from the sputum of a patient with chronic bronchitis and has been routinely used in this laboratory as the challenge strain when assessing the efficacy of immunization with NTHI antigens (14, 15). Chromosomal DNA was prepared from UC19 that had been cultured overnight at 37°C in 3 ml of brain heart infusion broth (Oxoid, Basingstoke, United Kingdom) supplemented with 10 μg (each) of hemin and NAD per ml. The bacteria were pelleted in a microcentrifuge; washed with phosphate-buffered saline (PBS); resuspended in 400 μl of buffer containing 50 mM glucose, 10 mM EDTA, and 25 mM Tris (pH 8.0); and then lysed in 0.1 M NaOH–0.5% sodium dodecyl sulfate (SDS). The lysate was digested at 37°C with a final concentration of 1.25 mg of heat-treated RNase per ml for 15 min and then with 2.5 mg of protease K per ml for 30 min. The digest was phenol extracted and ethanol precipitated, and the dried pellet was resuspended in 500 μl of H2O.
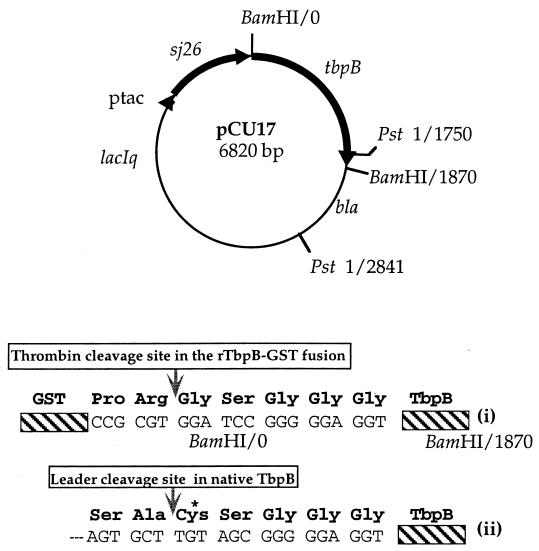
The gene encoding the mature form of UC19 TbpB was then amplified with the forward primer, 96-8 (5′-TTTATTAAGTGCTGGATCCGGGGGAGGTGGC-3′), and the reverse primer, 96-9 (5′-GTCATTTTTAGGATCCCATTACTT-3′), which contain BamHI restriction sites (underlined). The first triplet in the restriction site in 96-8 (bold) encodes a glycine residue that replaces the lipid-modified cysteine that normally occurs at the N terminus of the mature protein, and the first triplet in the restriction site in 96-9 (bold) occurs immediately after the tbpB stop codon. The PCR mixture contained 50 ng of chromosomal DNA, 0.1 mM deoxynucleoside triphosphates (Boehringer, Mannheim, Germany), and 20 pmol of each primer in reaction buffer [10 mM KCl, 6 mM (NH4)2SO4, 20 mM Tris-HCl (pH 8.0), 4 mM MgCl2, 0.1% Triton X-100, 10 mg of bovine serum albumin per ml] in a total volume of 50 μl. Native Pfu DNA polymerase (Stratagene, La Jolla, Calif.), which was used for high fidelity, was added after 5 min at 94°C. This was followed by 30 cycles of 94°C for 45 s, 50°C for 1 min, and 72°C for 3 min in a Perkin-Elmer thermal cycler (Roche, Branchburg, N.J.). Agarose gel analysis of the PCR product demonstrated a ∼1.8 kb fragment, which was purified with Bresaclean resin (Bresatec Ltd. Adelaide, Australia), digested with BamHI, and cloned into the BamHI restriction sites in plasmid pGEX2T (Pharmacia Biotech, Uppsala, Sweden) to produce plasmid pCU17. The orientation of the insert was determined by digestion with PstI. This plasmid is engineered to express recombinant TbpB as a glutathione S-transferase (GST) fusion protein with a thrombin cleavage recognition site between the two proteins.
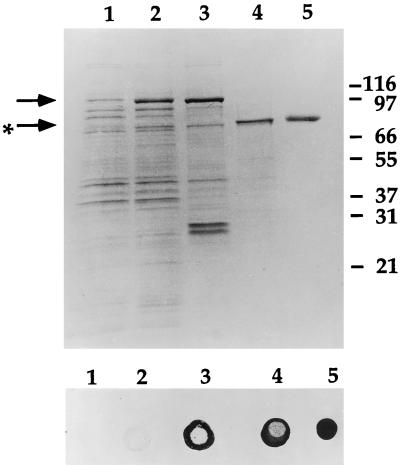
To purify rTbpB, cultures of BL21 [F− ompT hsd SB (rB− mB−) gal dcm] (Novagen, Madison, Wis.) containing pCU17 were grown in Luria-Bertani broth supplemented with 3.6 g of glucose per liter and 100 μg of ampicillin per ml. To reduce the degradation of the GST-rTbpB fusion, the cultures were grown at 25°C to 130 Klett units (∼8 × 108 cells/ml) before undergoing induction for 3 h with 10 μM isopropyl-β-d-thiogalactopyranoside (IPTG). The bacteria were harvested, washed, resuspended in 10 ml of PBS per g containing complete protease inhibitors (Boehringer Mannheim, Germany), and disrupted in an RF-1 Ribi cell fractionator (Sorvall, Dupont, Newtown, Conn.). Octyl-β-d-glucopyranoside (10 mg/ml; Boehringer) was added to the smashed cells to enhance the solubilization of the fusion protein; the mixture was then stirred on ice for 20 min and centrifuged at 12,000 × g to remove cellular debris. The cleared lysate was incubated at 4°C for 60 min with 1 ml of glutathione-Sepharose (Pharmacia Biotech) for each 50 ml of lysate. The Sepharose was then extensively washed with PBS to remove unbound proteins, and rTbpB was released from the Sepharose-bound rTbpB-GST by incubating the slurry at 4°C overnight with 50 U of human thrombin in a total of 5 ml of PBS. Following digestion, thrombin activity in the eluant was inhibited with 1 mM AEBSF [4-(2-aminoethyl)benzenesulfonyl fluoride; Calbiochem, San Diego, Calif.]. Recombinant TbpB was further purified on a UNO S1 cation-exchange column (Bio-Rad, Hercules, Calif.) with 10 mM MES [2-(N-morpholino)ethanesulfonic acid] buffer (pH 6.5) with a 0 to 0.5 M NaCl gradient. Fractions were screened for human transferrin binding activity by a dot enzyme assay essentially as described previously (25). Positive fractions, which were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), contained a single protein band of ∼75 kDa that was eluted from the cation-exchange column at approximately 0.3 M NaCl. Appropriate fractions were pooled, buffer exchanged on a PD10 column, (Pharmacia Biotech), and lyophilized. The protein concentration was estimated by the micro-BCA protein assay with a bovine serum albumin standard (Pierce, Rockford, Ill.). No endotoxin contamination could be detected in the purified rTbpB by using the E-TOXATE Limulus amebocyte assay (Sigma).
SDS-PAGE.
Samples for analysis were boiled for 3 min in sample buffer (62.5 mM Tris-HCl [pH 6.8], 10% [vol/vol] glycerol, 5% [vol/vol] β-mercaptoethanol, 0.001% bromophenol blue) and electrophoretically separated by denaturing Tris-glycine SDS-PAGE with prepoured 4 to 20% polyacrylamide gradient gels (Novex, San Diego, Calif.), with TBE buffer (89 mM Tris base [pH 8.3], 89 mM boric acid, 2 mM EDTA) and a constant voltage of 125 V. Proteins were detected with colloidal Coomassie G-250 (ICN, Costa Mesa, Calif.), and Mark 12 (Novex) was used as the molecular weight marker.
Immunization with rTbpB.
Lyophilized rTbpB was resuspended in PBS and emulsified in an equal volume of incomplete Freund’s adjuvant (IFA) to give a final protein concentration of either 400 or 800 μg/ml. Rats were immunized with rTbpB essentially as described previously (15, 30). Briefly, Peyer’s patches in male, 8-week-old Wistar rats were each injected with 2 to 5 μl of antigen, with each rat receiving a total of either 20 or 40 μg of rTbpB. A control group of animals was either sham immunized with PBS-IFA or left unimmunized. The animals were boosted intratracheally 14 days later with the same antigen dose as in the primary immunization in 50 μl of PBS.
Bacterial challenge.
The procedure for bacterial challenge was as described previously (15, 30). Briefly, 7 days after the booster dose, the rats were lightly sedated with halothane and 5 × 108 CFU of UC19 in 50 μl was instilled into the lungs via an intratracheal cannula. After 4 h, the animals were killed and bronchoaveolar lavage (BAL) fluid, serum, and homogenized lung samples were obtained. The numbers of viable bacteria in BAL fluid and lung homogenates were estimated by plating serial dilutions onto chocolate blood agar.
rTbpB-specific ELISAs.
Enzyme-linked immunosorbent assays (ELISAs) were performed essentially as described by Kyd et al. (15). Briefly, Polysorb microtiter wells (Nunc, Roskilde, Denmark) were coated overnight at 4°C with 0.4 μg of purified rTbpB in 100 μl of coating buffer (15 mM Na2CO3, 35 mM NaHCO3 [pH 9.6]). After the wells had been washed and blocked, 100 μl of diluted BAL fluid (1/2 to 1/16) or serum (1/200 to 1/500 for nonimmune samples; 1/500 to 1/4,000 for immune samples) in blocking buffer was added to each well, and the plates were incubated at room temperature for 90 min. The presence of rTbpB-specific antibodies was detected by incubation with Fc-specific horseradish peroxidase (HRP)-conjugated goat anti-rat immunoglobulin A (IgA) (diluted 1/1,000) or IgG (diluted 1/2,000) (Nordic Immunological Laboratories, Tilburg, The Netherlands) for 60 min. The plates were developed with tetramethylbenzidine in phosphate-citrate buffer (pH 5.0) containing 0.05% H2O2. The reaction was stopped with 0.5 M H2SO4, and absorbances were read at 450 nm on a 3550 microplate reader (Bio-Rad). The concentration of rTbpB-specific antibodies was calculated from an IgG or IgA standard curve.
Cross-reactivity of rTbpB-specific antiserum.
To assess the cross-reactivity of the UC19 rTbpB antibody on Western immunoblots, a panel of NTHI clinical isolates were iron starved as described previously (25), and a sample (10 μl) was boiled in sample buffer, electrophoretically separated on SDS-PAGE, and transferred to a polyvinylidene difluoride membrane by using a Multiphor Novablot semidry transfer system (Pharmacia) at a constant current of 0.8 mA/cm2 for 60 min. The membrane was blocked in 2% skim milk powder in TBST (Tris-buffered saline, 0.05% Tween 20) for 60 min, incubated with either nonimmune control serum or rTbpB-specific antiserum (diluted 1/200) for 60 min, and washed in TBST. Blots were then incubated with HRP-conjugated goat anti-rat IgG (Fc specific; diluted 1/1,000) and, after being washed, were developed with HRP-stabilized substrate (Promega Corp., Madison, Wis.).
To determine the ability of UC19 rTbpB-specific antibody to block the binding of human transferrin to heterologous NTHI strains, microtiter plates were coated with iron-starved bacteria as well as purified rTbpB as described previously (1). Essentially, bacteria and rTbpB (20 μg/ml) in PBS were allowed to dry onto the plates overnight at 37°C. The bacterial concentration used was optimized at the highest dilution to give saturation binding (data not shown). The plates were washed and blocked as for the ELISAs. After being washed, the wells were incubated with a 1/10 dilution of heat-inactivated nonimmune or UC19 rTbpB-immune sera in blocking buffer for 60 min, washed as above, and incubated with 1 μg of HRP-conjugated human transferrin (Pierce) per ml for 90 min at room temperature. After being washed, the wells were developed with tetramethylbenzidine substrate and the absorbances were read as above. Controls were treated in the same manner and consisted of bacteria or serum only.
To determine the bactericidal cross-reactivity of UC19 rTbpB-specific antibody, iron-starved isolates of NTHI were diluted in Hanks balanced salt solution (1.4 mM CaCl2, 0.8 mM MgSO4, 5 mM KCl, 0.4 mM KH2PO4, 140 mM NaCl, 0.3 mM Na2PO4, 4 mM NaHCO3, 0.1% [wt/vol] glucose) to 5 × 106 CFU/ml. A source of complement was prepared from fresh rat serum that had been absorbed twice for 60 min with 1011 CFU of NTHI per ml of serum and then filtered. The NTHI strain used for absorbtion was the same strain used as the target strain in the bactericidal assay. Reaction mixtures, which were assembled in Nunclon microtiter plates (Nunc), were composed of 20 μl of serially diluted heat-inactivated UC19 rTbpB-specific serum, 20 μl of complement, 20 μl containing 105 CFU of NTHI, and 40 μl of Hanks balanced salt solution. The reaction mixtures were incubated at 37°C for 2 h and then serially diluted onto chocolate agar plates. The bactericidal titer was calculated as the dilution that reduced the viability of NTHI by more than 50% compared to the same dilution of nonimmune serum. Serum from animals immunized as above with formalin-killed UC19 (generously supplied by J. Kyd) was used a positive control.
Statistical analysis.
Data were expressed as the mean ± standard error of the mean. Analysis for statistical significance between immune and nonimmune groups was performed by an unpaired Student t test, and P < 0.05 was considered statistically significant. All calculations were performed with the Macintosh Instat program.
RESULTS
Cloning and purification of rTbpB.
Several unsuccessful attempts were made to purify TbpB from NTHI UC19 by standard transferrin affinity chromatography methods that had been used by other investigators to purify TbpB from Hib and Neisseria spp. (5, 26, 27). Attempts to purify rTbpB with an intact leader sequence and a C-terminal polyhistidine tail were also unsuccessful due to the toxicity of the recombinant protein. The plasmid encoding this protein was constructed from a PCR product that had been generated with primers based on the TbpB sequence from a Hib strain (7). Since the N terminus of the mature form of TbpB was found to be heterologous (reference 17, this study and data not shown), DNA sequence information was obtained from this plasmid to design PCR primers that enabled the mature form of UC19 TbpB to be cloned into the vector pGEX2T to produce plasmid pCU17 (Fig. 1). This plasmid encodes rTbpB with an N-terminal GST extension and a thrombin cleavage recognition site between rTbpB and GST. Although expression of the GST-rTbpB fusion was not toxic, most of the recombinant fusion protein was degraded when recombinant cultures were grown at 37°C and induced with 0.1 mM IPTG (data not shown). This degradation could be considerably reduced by using the OmpT-deficient strain BL21 as the host, growing the cultures at 25°C, inducing with a lower concentration of IPTG (10 μM), and using protease inhibitors during the initial stages of purification. The GST-rTbpB fusion protein from the soluble fraction of disrupted cells was immobilized on glutathione-Sepharose, and rTbpB was cleaved from the fusion with human thrombin. Recombinant TbpB was further purified by cation-exchange chromatography and was shown to be folded into a native conformation as assessed by transferrin binding activity. SDS-PAGE of samples from various stages of the purification and a dot enzyme assay of the corresponding transferrin binding activity are shown in Fig. 2. The yield of purified protein varied from 50 to 60 μg/liter of culture.
FIG. 1.
Plasmid construct of pCU17, which expresses a rTbpB as a GST (sj26) fusion protein. Details of the rTbpB N terminus are depicted below the construct and show that after thrombin cleavage of the fusion, the N-terminal lipid-modified cysteine (*) in the native protein (ii) is replaced by glycine in rTbpB (i).
FIG. 2.
SDS-PAGE of samples from the purification of rTbpB and the corresponding transferrin dot enzyme assay. The GST-rTbpB fusion protein is present in the cell lysate of the IPTG-induced culture of BL21/pCU17 (arrow) in lane 2 compared to the uninduced lysate in lane 1. The fusion protein binds to glutathione-Sepharose (lane 3), although some free GST (∼26 kDa), probably resulting from endogenous Escherichia coli protease activity, is also bound. rTbpB (starred arrow) is released from the Sepharose after thrombin cleavage (lane 4) and further purified by cation-exchange high-pressure liquid chromatography (lane 5). The lower panel demonstrates that samples in lanes 2 to 5 have transferrin binding activity, although the activity in the induced E. coli cell lysate (lane 2) was weak.
Clearance of NTHI after immunization with rTbpB.
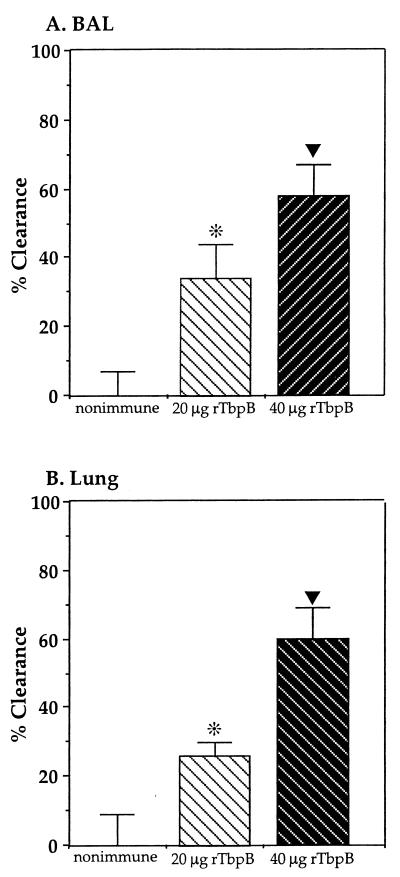
A mucosa-directed regimen was used to immunize rats with rTbpB. The animals were challenged with a bolus inoculum of UC19, and the rates of clearance of NTHI at 4 h from both BAL fluid and lung homogenates of rTbpB-immune animals were compared with those for the nonimmune animals (Fig. 3). Immunization with 20 μg of rTbpB per rat resulted in a significantly enhanced clearance of UC19 from both the BAL fluid (34%, P < 0.05) and lung homogenates (26%, P < 0.05), while immunization with the higher dose (40 μg of rTbpB per rat) increased the clearance in both BAL fluid (58%, P < 0.005) and lung homogenates (60%, P < 0.005).
FIG. 3.
Clearance of NTHI UC19 from BAL fluid (A) and homogenized lung (B) following challenge of rats immunized with either 20 or 40 μg of rTbpB compared to nonimmune animals. The percent clearance in the rTbpB-immunized groups (five rats per group) was calculated as 100 minus the percent ratio of the mean CFU recovered from the immunized group divided by the mean of the nonimmune group. The mean of the CFU recovered from nonimmune animals (10 rats) was given the value of 0% clearance. The error bar represents the standard error of the mean expressed as a percentage. The clearance of immune compared to nonimmune groups was dose dependent, and immunization with both doses of rTbpB resulted in significantly enhanced clearance at 4 h (*, P < 0.05; ▾, P < 0.005).
rTbpB-specific antibodies in BAL fluid and serum.
Antibody responses in both BAL fluid (IgA and IgG) and serum (IgG) were measured by ELISA (Table 2). Significant increases in rTbpB-specific IgG levels in serum for animals immunized with both the 20-μg dose (P < 0.005) and the 40-μg dose (P < 0.005) were observed. There were also significant increases in TbpB-specific IgG levels in BAL fluid (20-μg dose, P < 0.05; 40-μg dose, P < 0.005); however, while there was a trend toward increased levels of rTbpB-specific IgA in BAL fluid, only levels induced in response to the higher rTbpB dose were significantly different from those for nonimmune animals (P < 0.05).
TABLE 2.
rTbpB-specific antibodies in serum and BAL fluid after immunization with either 20 or 40 μg of rTbpB
| Sample | rTbpB dose (μg/rat) used for immunization | Levela of:
|
|
|---|---|---|---|
| rTbpB-specific IgG | rTbpB-specific IgA | ||
| Serum | 0b | 28.60 ± 4.14 | NAc |
| BAL | 0b | 93.79 ± 10.24 | 4.03 ± 0.77 |
| Serum | 20 | 155.20 ± 55.40 | NA |
| BAL | 20 | 463.54 ± 160.50 | 11.64 ± 7.06 |
| Serum | 40 | 218.96 ± 69.40 | NA |
| BAL | 40 | 785.12 ± 351.12 | 24.46 ± 15.73 |
IgG levels in serum are given in micrograms per milliliter; IgG and IgA levels in BAL fluid are given in nanograms per milliliter.
Nonimmune.
NA, not assayed.
Cross-reactivity of rTbpB antibody.
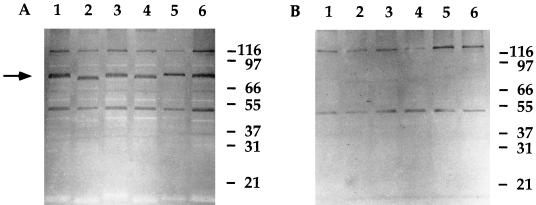
The cross-reactivity of UC19 rTbpB-specific antibodies for TbpB expressed by other NTHI clinical isolates was assessed. A panel of NTHI clinical isolates (Table 1) was iron starved, and the cross-reactivity of the rTbpB antiserum for the TbpB expressed by these strains (including the parent strain, UC19) was determined by Western immunoblot analysis (Fig. 4). While some nonspecific bands were detected with both rTbpB-immune and nonimmune serum, only the immune serum detected a band in all strains that corresponded to a band with a similar molecular weight to that for the purified rTbpB (Fig. 2). There was relatively no difference in the specificity of the immune serum for these strains.
FIG. 4.
Western immunoblot analysis of the specificity of rTbpB-specific immune serum (A) and nonimmune serum (B) for heterologous strains of NTHI. Whole-cell lysates of the parent strain, UC19 (lane 1), and the heterologous strains, UC27 (lane 2), UC28 (lane 3), UC77 (lane 4), UC84 (lane 5), and UC103 (lane 6), were probed with either immune or nonimmune sera. The position of TbpB is indicated by an arrow and the molecular mass markers are indicated on the right in kilodaltons.
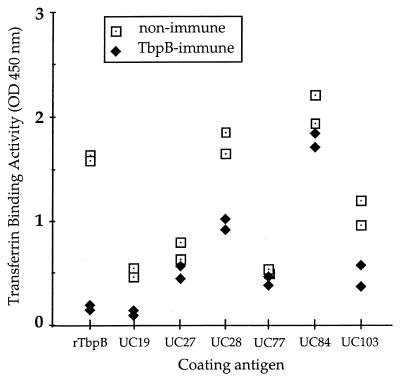
Since Western immunoblotting is unable to discriminate between surface-exposed or membrane-buried domains of proteins, an assay was developed to determine the cross-reactivity of rTbpB-specific antibodies for surface-exposed domains of the transferrin receptor and the ability of these antibodies to block transferrin binding to NTHI clinical isolates as well as to purified rTbpB. While UC19 rTbpB-specific immune sera blocked 89 and 80% of transferrin binding to purified rTbpB and the parent strain UC19, respectively, the inhibition of transferrin binding to other strains was less efficient, ranging from 2% for UC77 to 50% for UC103 (Fig. 5). There was no correlation in the percentage of inhibition with either the site of isolation of the NTHI strain, the size of the corresponding TbpB as seen on the immunoblot, or the transferrin binding efficiency of individual strains.
FIG. 5.
Inhibition of the binding of human transferrin to NTHI isolates by rTbpB-specific immune serum. The NTHI parent strain, UC19, and heterologous NTHI isolates were iron starved and coated onto microtiter plates together with purified UC19 rTbpB. The ability of rTbpB-specific immune serum to block the binding of HRP-conjugated human transferrin to NTHI was assayed and compared to transferrin binding in the presence of nonimmune serum. The samples were assayed twice independently. The percent inhibition was calculated as 100 minus the percent ratio of the absorbance of the HRP end product (indicating the amount of HRP-transferrin bound) after incubation of NTHI with immune serum divided by the absorbance after incubation with nonimmune serum. The average percent inhibition of immune serum relative to the binding in the presence of nonimmune serum for each individual strain was as follows: purified rTbpB, 89%; UC19, 80%; UC27, 15%; UC28, 44%; UC77, 2%; UC84, 9%; and UC103, 50%.
In addition to the Western blot and the binding assays, bactericidal assays were performed to assess the cross-reactivity of UC19 rTbpB-specific antibodies. Serum from animals immunized with formalin-killed UC19 and a pool of UC19 rTbpB-specific immune serum both demonstrated bactericidal activity against UC19 (Table 3). However, the bactericidal titer of the rTbpB-specific immune serum ranged from only 4 to 8, in contrast to a titer of 32 to 64 for serum from UC19-immunized rats. Recombinant TbpB immune serum was cross-reactive with UC28 (titer of 2 to 4); however, no bactericidal activity could be detected against the isolates, UC27, UC77, UC84, or UC103.
TABLE 3.
Bactericidal activity of whole-killed UC19-specific and UC19 rTbpB-specific immune serum against heterologous isolates of NTHI
| NTHI isolate | Antigen-specific serum | Bactericidal titera |
|---|---|---|
| UC19 | Whole killed UC19 | 32–64 |
| UC19 | UC19 rTbpB | 4–8 |
| UC27 | UC19 rTbpB | NDb |
| UC28 | UC19 rTbpB | 2–4 |
| UC77 | UC19 rTbpB | ND |
| UC84 | UC19 rTbpB | ND |
| UC103 | UC19 rTbpB | ND |
The bactericidal titer is the reciprocal of the dilution of serum that resulted in greater than 50% killing compared to the same dilution of nonimmune serum. The range is the result of two independent experiments.
ND, no bactericidal activity detected.
DISCUSSION
There is currently considerable interest in TbpB as a vaccine candidate against a number of pathogenic bacteria including A. pleuropneumoniae, N. meningitidis, and Hib (2, 17, 24). Some reports have proposed that H. influenzae TbpB is “a likely candidate for a universal vaccine” (17) and that “shared epitopes…clearly strengthen the case for using Tbps as protective antigens” (11). However, these studies have relied strongly on data from Western immunoblots to determine the cross-reactivity of H. influenzae Tbp-specific antibodies, and no studies have reported the protection afforded by immunization with TbpB against a challenge with NTHI. In this study, two key issues were addressed: first, whether immunization with a recombinant form of TbpB enhanced the clearance of an NTHI challenge to the rat lung, and second, the degree of cross-reactivity of TbpB-specific antibodies to surface-exposed conformational epitopes.
A method was developed for purification of rTbpB from NTHI, and rats immunized with this protein demonstrated significantly enhanced clearance of NTHI, in a dose-dependent manner, compared to the nonimmune animals. This data supports previous observations that a TbpB-specific immune response is highly efficacious at reducing bacterial survival in vivo (16, 17). However, while significantly enhanced clearance was seen following rTbpB immunization, it was somewhat lower than that seen after immunization with the NTHI P6 outer membrane protein or the OMP26 protein (14, 15). However, the P6 and OMP26 studies, which were performed with different strains of rat from that used in the present study, may not be directly comparable.
Immunization with rTbpB elicited specific antibodies in a dose-dependent manner in both serum and BAL fluid, but these levels were also lower than those seen after immunization with either P6 or OMP26 (14, 15). Notably, previous studies have shown that removal of the N-terminal palmitoyl moiety from P6 decreased its immunogenicity, although its protective efficacy in the rat pup model of bacteremia was only slightly reduced (33). While the absence of an N-terminal lipid moiety in the rTbpB used in the present study may also result in a molecule that is less immunostimulatory than lipidated native TbpB, it is unknown what effect lipidation would have on protective efficacy.
A significant difficulty associated with the development of an effective vaccine against NTHI is that the potential vaccine candidate must elicit protective immune responses not only against the homologous strain but also against heterologous strains. Antigenic cross-reactivity has been demonstrated by Western blot analysis of transferrin binding proteins of H. influenzae, A. pleuropneumoniae, and N. meningitidis (11). In addition, since TbpB variants commonly interact with human transferrin, it would seem that certain TbpB surface-exposed domains would have to remain conserved for this interaction to be maintained. To determine if the antibody elicited by immunization with UC19 rTbpB recognized epitopes on TbpB from heterologous NTHI strains, Western immunoblot analysis was performed. A protein of variable electrophoretic mobility, although corresponding to a similar size to rTbpB from UC19, was detected with approximately the same specificity in the whole-cell lysates of the six NTHI isolates tested.
Nevertheless, despite the presence of shared TbpB epitopes, the important issue is whether these conserved epitopes are surface exposed when TbpB is assembled as a constituent of the transferrin receptor in the bacterial outer membrane and thus are accessible by bactericidal or opsonophagocytic antibodies. To begin to address this issue, the ability of rTbpB-specific antibodies to inhibit transferrin binding to heterologous strains was assessed. Binding to the purified rTbpB was inhibited and most of the binding to the parent strain, UC19, was also inhibited by UC19 rTbpB-specific antibody. These results suggested that rTbpB-specific antibodies recognized surface-exposed domains of TbpB when assembled in the outer membrane as a constituent of the transferrin receptor. While individual strains varied in the efficiency with which they bound transferrin, in contrast to the cross-reactivity of rTbpB-specific antibody detected by Western immunoblots, the inhibition binding assay gave variable cross-reactivity with heterologous strains. Since rTbpB-specific antibodies inhibit the interaction of transferrin with the parent strain, UC19, some of the epitopes recognized by these antibodies must be at or close to the transferrin binding domain. Alternatively, they may interact with TbpB to alter the conformation of the protein so that it no longer interacts with transferrin. However, regardless of the mechanism whereby rTbpB-specific antibodies inhibit the binding of transferrin, the corresponding epitopes in heterologous strains were variably recognized by these antibodies. These inhibition studies suggested that rTbpB-specific “blocking” antibodies may vary in their capacity to interact with heterologous isolates.
Bactericidal antibodies have been proposed in many studies as important effectors in the elimination of bacteria. Recombinant TbpB-specific antibodies were found to be bactericidal against the homologous strain, UC19, as well as the heterologous strain, UC28. However, this bactericidal titer was much lower than that observed with the serum from animals immunized with formalin-killed UC19. No cross-reactive rTbpB-specific bactericidal activity could be demonstrated against the heterologous strains, UC27, UC77, UC84, and UC103. Notably, the bactericidal cross-reactivity of UC19 rTbpB serum with UC28 is of interest, considering that good cross-reactivity was also observed in the transferrin binding inhibition assay. A recent study of the bactericidal antibodies elicited in response to immunization with meningococcal Tbps demonstrated that the choice of adjuvant played a significant role in both the bactericidal titer and cross-reactivity (6). It is therefore difficult to draw conclusions about the cross-reactivity of NTHI rTbpB-specific bactericidal antibodies that would be elicited in response to vaccination regimens with adjuvants other than IFA, which was used in the present study. However, based solely on the data reported here, the cross-reactivity of both blocking and bactericidal antibodies elicited in response to mucosa-directed immunization with UC19 rTbpB emulsified in IFA seems to be limited.
In conclusion, immunization with rTbpB significantly enhanced the clearance of NTHI from the rat lung during acute pulmonary infection compared to the control group of animals. However, despite cross-reactivity on Western immunoblots, rTbpB antibodies were variable in their ability to block the binding of transferrin to heterologous isolates as well as in their cross-reactive bactericidal activity. It is possible that the human immune responses to TbpB epitopes are different from those in the rat and that different adjuvants or immunization regimens will elicit a different profile of cross-reactive antibodies. However, if bactericidal antibodies to surface-exposed epitopes are key mediators in the elimination of NTHI, the data presented here suggest that the value of TbpB as a vaccine component may be limited due to variable cognate recognition of both blocking and bactericidal antibodies to surface-exposed TbpB epitopes in heterologous strains of NTHI.
ACKNOWLEDGMENTS
We are extremely grateful to Paul Foster for critical reading of the manuscript, to Graeme Cox for generous provision of experimental resources, and to Russell Taylor for large-scale bacterial culture.
D.W. was supported by a University of Canberra Postgraduate Research Award and a Collaborative Research Scholarship provided by The John Curtin School of Medical Research, ANU.
REFERENCES
- 1.Abdillahi H, Poolman J T. Whole-cell ELISA for typing Neisseria meningitidis with monoclonal antibodies. FEMS Microbiol Lett. 1987;48:367–371. [PubMed] [Google Scholar]
- 2.Danve B, Lissolo L, Mignon M, Dumas P, Colombani S, Schryvers A B, Quentin-Millet M-J. Transferrin-binding proteins isolated from Neisseria meningitidis elicit protective and bactericidal antibodies in laboratory animals. Vaccine. 1993;11:1214–1220. doi: 10.1016/0264-410x(93)90045-y. [DOI] [PubMed] [Google Scholar]
- 3.Davis W B, Pacht E R. Extracellular antioxidant defenses. In: Crystal R G, West J B, editors. The lung: scientific foundations. New York, N.Y: Raven Press; 1991. pp. 1821–1827. [Google Scholar]
- 4.Duim B, Bowler L D, Eijk P P, Jansen H M, Dankert J, van Alphen L. Molecular variation in the major outer membrane protein P5 gene of nonencapsulated Haemophilus influenzae during chronic infections. Infect Immun. 1997;65:1351–1356. doi: 10.1128/iai.65.4.1351-1356.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferrón L, Ferreirós C M, Criado M T, Andrade M P. Purification of the Neisseria meningitidis transferrin binding protein-2 (TBP2) to homogeneity using column chromatography. FEMS Microbiol Lett. 1993;109:159–166. doi: 10.1111/j.1574-6968.1993.tb06161.x. [DOI] [PubMed] [Google Scholar]
- 6.Gómez J A, Hernández E, Criada M T, Ferreirós C M. Effect of adjuvants in the isotypes and bactericidal activity of antibodies against the transferrin-binding proteins of Neisseria meningitidis. Vaccine. 1998;16:1633–1639. doi: 10.1016/s0264-410x(98)00062-0. [DOI] [PubMed] [Google Scholar]
- 7.Gray-Owen S D, Loosmore S, Schryvers A B. Identification and characterization of genes encoding the human transferrin-binding proteins from Haemophilus influenzae. Infect Immun. 1995;63:1201–1210. doi: 10.1128/iai.63.4.1201-1210.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Groeneveld K, van Alphen L, Eijk P P, Jansen H M, Zanen H C. Changes in outer membrane proteins of nontypable Haemophilus influenzae in patients with chronic obstructive pulmonary disease. J Infect Dis. 1988;158:360–365. doi: 10.1093/infdis/158.2.360. [DOI] [PubMed] [Google Scholar]
- 9.Hardie K R, Adams R A, Towner K J. Transferrin-binding ability of invasive and commensal isolates of Haemophilus spp. J Med Microbiol. 1993;39:218–224. doi: 10.1099/00222615-39-3-218. [DOI] [PubMed] [Google Scholar]
- 10.Herrington D A, Sparling P F. Haemophilus influenzae can use human transferrin as a sole source for required iron. Infect Immun. 1985;48:248–251. doi: 10.1128/iai.48.1.248-251.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holland J, Parsons T R, Hasan A A, Cook S M, Stevenson P, Griffiths E, Williams P. Conservation and antigenic cross-reactivity of the transferrin-binding proteins of Haemophilus influenzae, Actinobacillus pleuropneumoniae and Neisseria meningitidis. Microbiology. 1996;142:3505–3513. doi: 10.1099/13500872-142-12-3505. [DOI] [PubMed] [Google Scholar]
- 12.Klein J O. Role of nontypeable Haemophilus influenzae in pediatric respiratory tract infections. Pediatr Infect Dis J. 1997;16:S5–S8. doi: 10.1097/00006454-199702001-00002. [DOI] [PubMed] [Google Scholar]
- 13.Kuklinska D, Kilian M. Relative proportions of Haemophilus species in the throat of healthy children and adults. Eur J Clin Microbiol. 1984;3:249–252. doi: 10.1007/BF02014895. [DOI] [PubMed] [Google Scholar]
- 14.Kyd J M, Cripps A W. Potential of a novel protein, OMP26, from nontypeable Haemophilus influenzae to enhance clearance in a rat model. Infect Immun. 1998;66:2272–2278. doi: 10.1128/iai.66.5.2272-2278.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kyd J M, Dunkley M L, Cripps A W. Enhanced respiratory clearance of nontypeable Haemophilus influenzae following mucosal immunization with P6 in a rat model. Infect Immun. 1995;63:2931–2940. doi: 10.1128/iai.63.8.2931-2940.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lissolo L, Maitre-Wilmotte G, Dumas P, Mignon M, Danve B, Quentin-Millet M-J. Evaluation of transferrin-binding protein 2 within the transferrin-binding protein complex as a potential antigen for future meningococcal vaccines. Infect Immun. 1995;63:884–890. doi: 10.1128/iai.63.3.884-890.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loosmore S M, Yang Y, Coleman D C, Shortreed J M, England D M, Harkness R E, Chong P S-C, Klein M H. Cloning and expression of the Haemophilus influenzae transferrin receptor genes. Mol Microbiol. 1996;19:575–586. doi: 10.1046/j.1365-2958.1996.406943.x. [DOI] [PubMed] [Google Scholar]
- 18.Morton D J, Musser J M, Stull T L. Expression of the Haemophilus influenzae transferrin receptor is repressible by hemin but not elemental iron alone. Infect Immun. 1993;61:4033–4037. doi: 10.1128/iai.61.10.4033-4037.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morton D J, Williams P. Siderophore-independent acquisition of transferrin-bound iron by Haemophilus influenzae type b. J Gen Microbiol. 1990;136:927–933. doi: 10.1099/00221287-136-5-927. [DOI] [PubMed] [Google Scholar]
- 20.Murphy T F, Apicella M A. Nontypable Haemophilus influenzae: a review of clinical aspects, surface antigens, and the human immune response to infection. Rev Infect Dis. 1987;9:1–15. doi: 10.1093/clinids/9.1.1. [DOI] [PubMed] [Google Scholar]
- 21.Neilands J B. Iron and its role in microbial physiology. In: Neilands J B, editor. Microbial iron metabolism. New York, N.Y: Academic Press, Inc.; 1974. pp. 3–34. [Google Scholar]
- 22.Otto B R, Verweij-van Vught A M J J, MacLaren D M. Transferrins and heme-compounds as iron sources for pathogenic bacteria. Crit Rev Microbiol. 1992;18:217–233. doi: 10.3109/10408419209114559. [DOI] [PubMed] [Google Scholar]
- 23.Pacht E R, Davis W B. Role of transferrin and ceruloplasmin in antioxidant activity of lung epithelium. J Appl Physiol. 1988;64:2092–2099. doi: 10.1152/jappl.1988.64.5.2092. [DOI] [PubMed] [Google Scholar]
- 24.Rossi-Campos A, Anderson C, Gerlach G-F, Klashinsky S, Potter A A, Willson P J. Immunization of pigs against Actinobacillus pleuropneumoniae with two recombinant protein preparations. Vaccine. 1992;10:512–518. doi: 10.1016/0264-410x(92)90349-o. [DOI] [PubMed] [Google Scholar]
- 25.Schryvers A B. Characterization of the human transferrin and lactoferrin receptors in Haemophilus influenzae. Mol Microbiol. 1988;2:467–472. doi: 10.1111/j.1365-2958.1988.tb00052.x. [DOI] [PubMed] [Google Scholar]
- 26.Schryvers A B. Identification of the transferrin and lactoferrin-binding proteins in Haemophilus influenzae. J Med Microbiol. 1989;29:121–130. doi: 10.1099/00222615-29-2-121. [DOI] [PubMed] [Google Scholar]
- 27.Stevenson P, Williams P, Griffiths E. Common antigenic domains in transferrin-binding protein 2 of Neisseria meningitidis, Neisseria gonorrhoeae, and Haemophilus influenzae type b. Infect Immun. 1992;60:2391–2396. doi: 10.1128/iai.60.6.2391-2396.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Alphen L, Eijk P, Geelen-van den Broek L, Dankert J. Immunochemical characterization of variable epitopes of outer membrane protein P2 of nontypeable Haemophilus influenzae. Infect Immun. 1991;59:247–252. doi: 10.1128/iai.59.1.247-252.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vogel L, Schoonbrood D, Geluk F, Hoek F, Bresser P, Out T, Jansen H, Dankert J, van Alphen L. Iron-binding proteins in sputum of chronic bronchitis patients with Haemophilus influenzae infections. Eur Respir J. 1997;10:2327–2333. doi: 10.1183/09031936.97.10102327. [DOI] [PubMed] [Google Scholar]
- 30.Wallace F J, Clancy R L, Cripps A W. An animal model demonstration of enhanced clearance of nontypable Haemophilus influenzae from the respiratory tract after antigen stimulation of gut-associated lymphoid tissue. Am Rev Respir Dis. 1989;140:311–316. doi: 10.1164/ajrccm/140.2.311. [DOI] [PubMed] [Google Scholar]
- 31.Weinberg E D. The iron-withholding defense system. ASM News. 1993;59:559–562. [Google Scholar]
- 32.Williams P, Morton D J, Towner K J, Stevenson P, Griffiths E. Utilization of enterobactin and other exogenous iron sources by Haemophilus influenzae, H. parainfluenzae and H. paraphrophilus. J Gen Microbiol. 1990;136:2343–2350. doi: 10.1099/00221287-136-12-2343. [DOI] [PubMed] [Google Scholar]
- 33.Yang Y-P, Munson R S, Grass S, Chong P, Harkness R E, Gisonni L, James O, Kwok Y, Klein M H. Effect of lipid modification on the physicochemical, structural, antigenic and immunoprotective properties of Haemophilus influenzae outer membrane protein P6. Vaccine. 1997;115:976–987. doi: 10.1016/s0264-410x(96)00296-4. [DOI] [PubMed] [Google Scholar]