Abstract
Peripheral ameloblastoma (PA) is a rare benign tumor that can occur mostly in the mandibular gingiva of the premolar area, originating from the residual odontogenic epithelium. The patient is usually asymptomatic and the lesion can be an occasional finding during routine intraoral examination. Due to the lack of clinical and radiographic pathognomonic features, the diagnosis is based on histopathological analysis, associated with 3D computed tomography (CT) imaging. Here, we report the case of a middle-aged man showing an asymptomatic, sessile, normochromic papillomatous mass of the lingual alveolar mucosa, in correspondence of tooth 4.4, which was histologically diagnosed as peripheral ameloblastoma. After the complete excision of the lesion, there were no recurrence and no symptoms during the 3-year follow-up. The treatment of choice for PA is a conservative surgical excision, which usually results in a good prognosis, together with a long-term follow-up, necessary to intercept possible recurrence or, more rarely, malignant transformation.
Keywords: peripheral ameloblastoma (PA), extraosseous lesion, benign tumor
1. Introduction
In the oral cavity, the most common neoplasm that arises from the residual odontogenic epithelium is ameloblastoma. Although rare, accounting for about 1% of all jaw tumors [1,2], they represent the second-most common odontogenic tumor, with a pooled incidence rate of 0.92 per million person-years [3]. The term “ameloblastoma” originates from the early English “amel”, which means enamel, and the Greek word “blastos”, meaning germ. They are composed of the epithelium of ectodermal origin, deriving from the cells localized at the tooth root, or in close approximation, resulting from the ectoderm germ layer. Most ameloblastomas are benign and slow-growing, with locally aggressive behavior. The patients may experience no symptoms until the tumor is of a larger size. Signs most commonly reported are as follows: abnormal growth in the jaw or sinus area, painless swelling in the jaw, delayed tooth eruption. The abnormal cell growth easily infiltrates local tissue, typically bone. Surgical excision is usually needed to treat this disorder, which has a high propensity for local recurrence even with an appropriate surgical management and requires lifelong follow-up for surveillance.
According to the most recent classification of the World Health Organization, drawn up in 2022 and updated in 2024, ameloblastoma is classified into five clinical types: conventional (solid/multicystic), unicystic, adenoid, metastasizing, and peripheral/extraosseous [4,5].
Peripheral ameloblastoma (PA), also known as extraosseous ameloblastoma, soft tissue ameloblastoma, ameloblastoma of mucosal origin, or ameloblastoma of the gingiva, is a rare, unusual, benign, and extraosseous odontogenic tumor, which affects soft tissues and accounts for 1–10% of all ameloblastomas [2,6,7,8]. This term was first coined by Kuru in 1911, but the first complete description of this condition was provided by Stanley and Krogh in 1959 [2,6,8] and, until 2014, less than 200 cases of PA have been reported in the literature [6] (Table 1).
The 5th WHO histological classification of tumors of the oral cavity (2024) [5,9] included the extraosseous/peripheral ameloblastoma as one of the variants of ameloblastoma, suggesting that PA derives from the same histological structures related to the classic type. The tumor cell arises from the cell rest of Serres, the remnants of reduced enamel epithelium, and the basal cells of the surface epithelium [2,6].
However, differently from intraosseous ameloblastoma that presents at a younger age, PA arises between the third and the sixth decade of life (with an average age at presentation of 50 years old). PA is a rare tumor with a prevalence of 1–5% and mild male predilection (male/female ratio of 1.9:1); it occurs mainly in the gingival soft tissue [1,6,10,11,12].
Clinically, PA is a solid, painless, exophytic, extraosseous, sessile, and gradually growing lesion, arising from the soft tissue of tooth-bearing areas. It predominantly occurs in the gingiva (for this reason it is also named as “gingival ameloblastoma”) and it can be misinterpreted as other lesions with similar appearance, including pyogenic granuloma, peripheral ossifying fibroma, peripheral giant cell granuloma, and squamous papilloma. The lingual mandibular gingiva of the premolar area is the most involved site. This tumor does not generally invade the underlying bone; therefore, PA has been considered as a hamartomatous lesion, less aggressive than the conventional ameloblastoma [1,2,6,8,10,11,13].
The aim of this report is to describe the case of a middle-aged man diagnosed with peripheral ameloblastoma and to provide an updated overview of the current literature on this condition.
Table 1.
Analysis of case reports and case series.
| Author | Year | Patient, Age | Treatment | Follow-Up (Months) | Recurrence |
|---|---|---|---|---|---|
| Braunstein et al. [14] | 1949 | 1 patient | Excision of soft-tissue lesion (blunt dissection) | 4 | No |
| Klinar et al. [15] | 1969 | 1 patient | Surgical excision by extraoral approach, wide margins | 2–3–5 | No |
| Gardner et al. [16] | 1977 | 21 patients | Excision (13 cases), electrocautery, extraction of teeth, removal of small amounts of bone, wide resection of the mandible with retention of the inferior border (1 case) | 11–60 | 3 |
| Patrikiou et al. [17] | 1983 | 1 patient | Excision under general anesthesia with curettage of underlying bone | 8 | No |
| Atkinson et al. [18] | 1984 | 10 patients | Megavoltage radiotherapy (4500 rads in 4 weeks) and 3 cases received adjuvant surgery | - | 1 |
| Schaberg et al. [19] | 1985 | 1 patient | Excision with small margin of normal tissue, subsequent re-excision with larger margin of normal tissue | 42 | No |
| Buchner et al. [20] | 1987 | 13 patients | Excision (initially incomplete in 4 cases but repeat excision performed) | - | 1, peripheral ameloblastic carcinoma from recurrent lesion |
| Ficarra et al. [21] | 1987 | 1 patient | Excision | 60 | No |
| Woo et al. [22] | 1987 | 1 patient | Excision by intraoral approach | 9 | No |
| El-Mofty et al. [23] | 1991 | 11 patients | Excision of the lesion down to the periosteum with small amount of normal tissue | - | 1 |
| Nauta et al. [24] | 1992 | Male, 32 | Excision with an en bloc resection of the adjacent bone of the alveolar process | 12 | No |
| Hernandez et al. [25] | 1992 | 1 patient | Excision down to level of bone (2 lesions) | 24 | 1 |
| Baden et al. [26] | 1993 | 1 patient | Excision | 60 | 2, ameloblastic carcinomas |
| Gurol et al. [27] | 1995 | 8 patients | Complete excision through the periosteum without removing bone or teeth | 3 cases with no follow-up reported, 2 cases 6 months, 1 case 24 m, 1 case 108 m, 1 case 120 m | No |
| Zhu et al. [28] | 1995 | 1 patient | Excision including overlying gingiva and thin lingual alveolar bone | 36 | No |
| Califano et al. [29] | 1996 | 1 patient | Surgical resection of the left maxilla with excision of bone surrounding the tumor | 12 | No |
| Wettan et al. [30] | 2001 | 1 patient | Excision | 36 | 2, with dysplastic change |
| Philipsen et al. [31] | 2001 | 160 patients, 65% males and 35% female (male/female ratio 1.9:1), average age 52.1 years | Conservative supraperiosteal surgical excision with adequate disease-free margins | - | Recurrence rate 16–19% |
| Marucci et al. [32] | 2004 | 1 patient | Radical surgical excision | - | - |
| Lopez-Jornet et al. [33] | 2005 | 1 patient | Excisional biopsy with curettage of the affected mandibular bone | 24 | No |
| Martelli-Jùnior et al. [34] | 2005 | 1 patient | Excision with narrow margin including underlying periosteum | 12 | No |
| Yanamoto et al. [35] | 2005 | 1 patient | En bloc excision together with the maxillary canine and underlying alveolar bone, under local anesthesia; layer of exposed bony surface shaved with a round burr | 180 | No |
| Curtis et al. [36] | 2006 | 1 patient | Resection of the lesion, buccal pad of fat and a mucosal flap for reconstruction | 36 | No |
| LeCorn et al. [37] | 2006 | 1 patient | Excision | 4 | No |
| Gomes et al. [38] | 2007 | 1 patient | Excisional biopsy | 9 | No |
| Yamanishi et al. [39] | 2007 | 1 patient | Complete surgical excision by intraoral approach (blunt dissection) | 7 | No |
| Vanoven et al. [1] | 2008 | Man, 73 | Resection of the tumor en bloc with partial maxillectomy with obturator reconstruction | - | No |
| Ide et al. [40] | 2009 | 1 patient | Excision | 12 | No |
| Beena et al. [41] | 2012 | 1 patient | Excisional biopsy of soft-tissue lesion | - | - |
| Bertossi et al. [42] | 2014 | 1 patient | Resection of lesion with surrounding bone, extraction of the second molar, flap for closure | 24 | No |
| Bhat et al. [43] | 2014 | 1 patient | Excised with a 5 mm margin using diathermy under general anesthesia | 12 | No |
| Lascane et al. [44] | 2014 | 1 patient | Excisional biopsy under local anesthesia | 12 | No |
| Goda et al. [7] | 2015 | 1 patient, female, 69 | Complete surgical excision by intraoral approach (blunt dissection) | 30 | No |
| Borrello et al. [10] | 2016 | Female, 78 | Excisional biopsy | 12 | No |
| Kandagal et al. [45] | 2016 | 1 patient | Complete surgical excision of soft tissue lesion | 24 | No |
| Zhang et al. [8] | 2018 | 25 patients, 22 males and 3 females (M:F = 7.3:1), average age 48.3 years (range 11–81 years, 1 case in pediatric patient) | Excision with or without partial bone resection | Periodic, every 6 months in the first 2 years and at least every 12 months, 3–180 (mean 61) |
1, maybe due to incomplete removal of primary lesion |
| Janardhanan et al. [13] | 2018 | Male, 33 | Excision | 24 | No |
| Nurik et al. [46] | 2018 | 1 patient | Complete surgical excision | - | - |
| Upadhyaya et al. [47] | 2018 | 18 patients, 6 females and 12 males | 12 excisional biopsies, 5 incisional biopsies, 1 unknown | - | - |
| Cadavid et al. [48] | 2019 | 2 patients | Treated conservatively with enucleation plus curettage or cryotherapy | 120 | No |
| On et al. [49] | 2019 | 1 patient | Excisional biopsy of the lesion after the 5 cm incision and dissection of lateral wall of oropharynx by intraoral approach under general anesthesia | - | - |
| Ülker et al. [2] | 2020 | Female, 34 | Excision | 3 | No |
| Vezhavendhan et al. [6] | 2020 | Male, 72 | Excision | - | 1, 6 years after the first excision |
2. Materials and Methods
Case Presentation
In November 2020, a 48-year-old Asian man was referred to the Oral Medicine Department (S.C. Odontostomatology II) of the St Paolo and Carlo Hospital in Milan because of a gingival growth at a right lower premolar area. The patient reported a previous diagnosis of PA of the lesion, made throughout incisional biopsy in another clinical unit.
On extraoral examination, no swelling, asymmetry, or lymphadenopathy were evident.
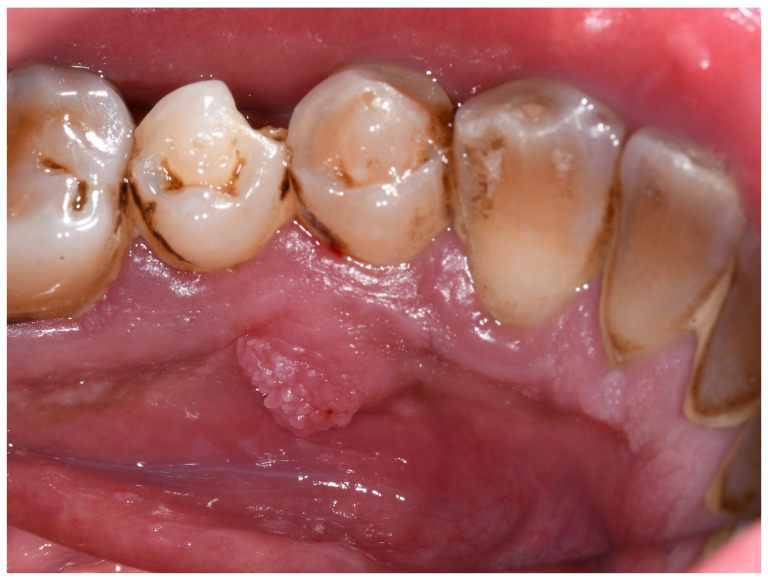
Intraoral examination revealed a dome-shaped exophytic lesion, with cobblestone surface, and pink color, with a major axis of about 1 cm located on the lingual alveolar mucosa, in correspondence of tooth 4.4 (Figure 1). Tooth 4.4 was normoresponsive to vitality test, painless on percussion, and free from pathological periodontal probing, as were the adjacent teeth.
Figure 1.
Intraoral photography: dome-shaped exophytic lesion with cobblestone surface and normal color, of 1 cm× 1 cm, located on the lingual aspect of the right mandibular premolar area.
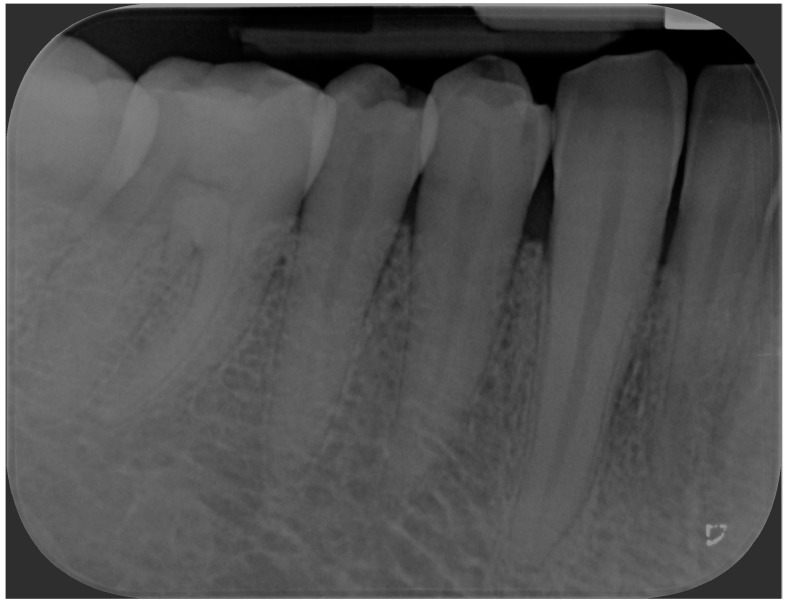
Periapical radiograph was taken, showing the absence of osseous alterations.
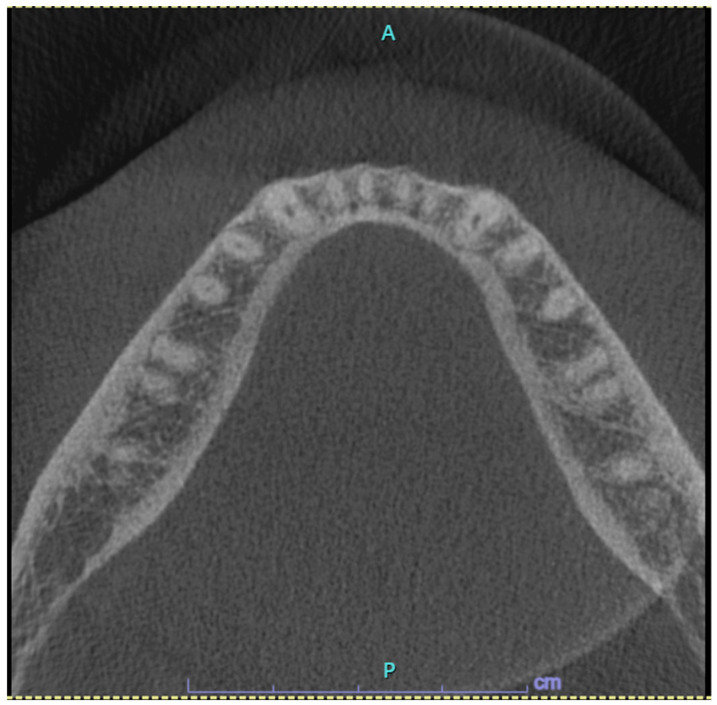
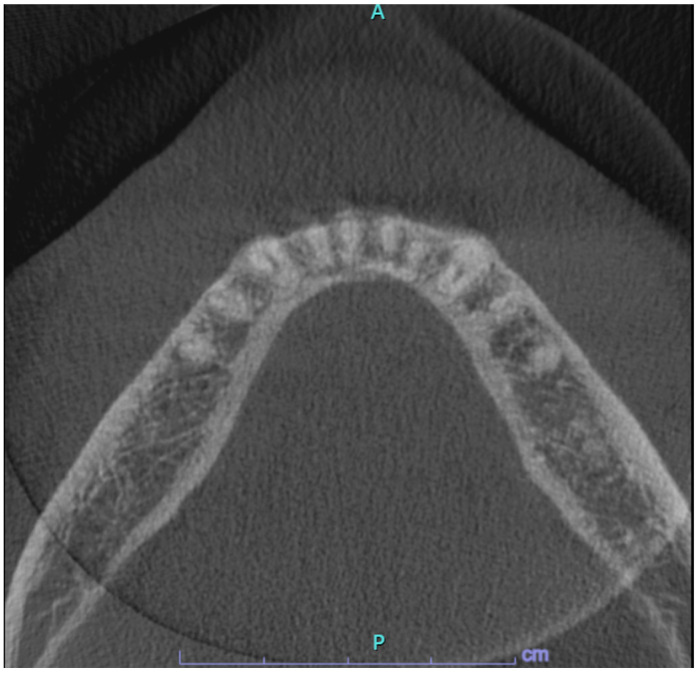
After patient’s informed consent, the diagnostic excisional biopsy of the lesion with histopathological investigation was performed, which confirmed the presence of PA. Axial Cone beam computed tomography (CBCT) scan revealed the absence of radiographic pathological alterations in the lingual mandibular cortical bone and of periosteal involvement and the absence of swelling at the site of the lesion (Figure 2).
Figure 2.
Axial Cone beam computed tomography (CBCT) sections showed the absence of altered density of trabecular bone, radiolucency, bone expansion, or changes in the mandibular lingual cortical bone in the right premolar area.
Therefore, surgical excision of the lesion was carried out under local anesthesia with mepivacaine 2% with vasoconstrictor (adrenaline 1:100.000); non-absorbable 4/0 silk sutures were applied and hemostasis by compression was achieved. The specimen was fixed in formalin and sent to the Pathological Anatomy Department with a request for histopathological examination. No intraoperative and postoperative complications occurred. Postoperative instructions were provided and antiseptic and analgesic therapies were prescribed.
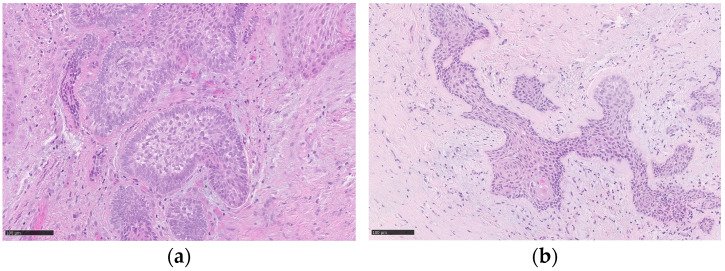
The 3-week follow-up visit showed the complete mucosal healing at the surgical site and the absence of recurrence of the lesion. Histopathological findings revealed the presence of a lesion consisting of epithelial islands characterized by peripheral basal cells with nuclear palisade and reverse polarization of the nuclei and central cells with a lighter cytoplasm that were loosely arranged. Furthermore, v-raf murine sarcoma viral oncogene homolog B (BRAF) immunohistochemistry was performed and it was negative. The histopathological report confirmed the presence of “mucosal fragment, coherent with oral/periodontal mucosa, with underlying multinodular odontogenic epithelial proliferation and resection margins in healthy tissue, with the morphologic features of peripheral ameloblastoma, with plexiform aspects and squamous metaplasia” (Figure 3).
Figure 3.
Histopathological findings show (a) the lesion involving the oral stroma, consisting of epithelial islands characterized by peripheral basal cells with nuclear palisade and reverse polarization of the nuclei; the cells present in the center of the islands are loosely arranged and have a lighter cytoplasm than that of the cells of the basal layer. (b) In other deeper islands, the aspects described previously are less evident and below, in the center of an island, a focus of squamous differentiation is present. The black scale bars represent, in both the images, 100 µm.
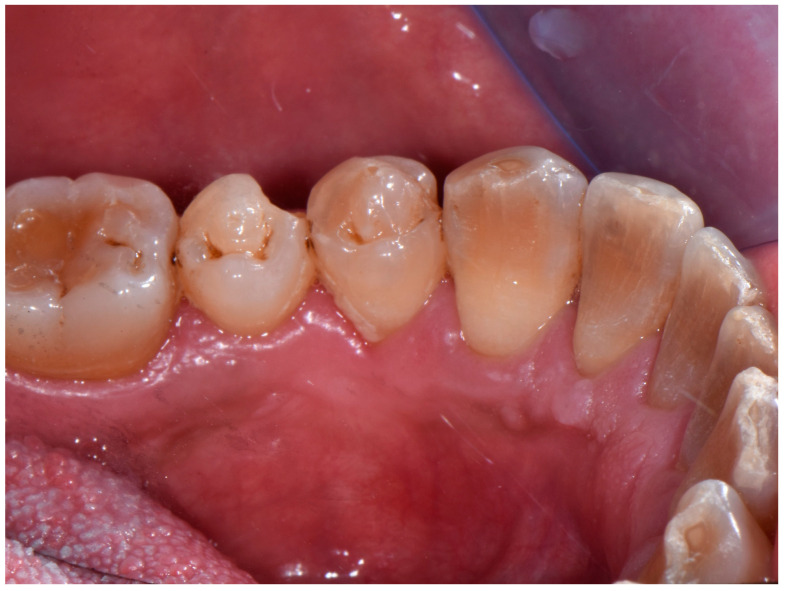
At 6 months recall, the patient was still asymptomatic with oral mucosae free from pathological lesions; good healing of the surgical site and the absence of recurrence were observed (Figure 4).
Figure 4.
Clinical image showing the complete mucosal healing, without local recurrence.
During the visit, a periapical radiograph of teeth 4.3, 4.4 and 4.5 was performed, from which no radiographic bone alterations of pathologic significance were evident (Figure 5).
Figure 5.
Periapical radiograph of the mandibular premolar right region after 6-months follow-up, showing no pathological alteration of the bone.
Further follow-up examinations were scheduled at 1 and 2 years after the excisional biopsy for a clinical and radiological revaluation with CBCT scans in order to evaluate the integrity of the lingual cortical bone (Figure 6). The patient was asymptomatic and, at intraoral examination, no signs of recurrence could be observed: the mucosa showed a physiological appearance, pinkish and not ulcerated. The last follow-up after 3 years still showed clinical and radiographic stability of the picture. The patient is currently under active annual follow-up to early detect any recurrences.
Figure 6.
Axial CBCT sections at 2-years follow-up showing the absence of pathological changes.
3. Discussion
Ameloblastoma is the most frequent epithelial odontogenic tumor and accounts for about 11% of all odontogenic tumors [11]. According to the most recent classification of the World Health Organization, five subtypes exist: conventional (solid/multicystic), unicystic, metastasizing, adenoid, and peripheral ameblastomas (PA) [4]. Conventional ameloblastoma is the most common type of ameloblastoma, representing 57–63.8% of cases [50]; it mainly occurs in the mandible [51], without a clear sex predilection or ethnicity preference, in particular between the second and the fourth decade of life. Clinically, conventional ameloblastoma appears as a slow and asymptomatic cortical expansion; in cases of large dimension, tooth mobility, facial asymmetry, masticatory dysfunction, and pain can occur. Radiographic images include a multilocular radiolucent lesion, with well-defined and scalloped margins, sometimes also described as a “soap-bubble” appearance [52]. Differently from other types of ameloblastoma, the conventional type shows a more aggressive behavior and a higher recurrence rate; radical surgery is the most effective therapy [53]. Unicystic ameloblastoma is a rare variant of ameloblastoma, characterized by slow growth and relative local aggressiveness [54]. Radiographically, the typical finding is an expansive unilocular radiolucency with well-defined edges, sometimes associated with an impacted tooth [54]. Generally, the unicystic variant is considered less aggressive than the solid one; thus, the treatment of choice is enucleation or curettage, unless in the case of the unicystic ameloblastoma mural subtype, for which marginal resection is recommended [54]. The metastasizing type of ameloblastoma is an aggressive variant with the ability to metastasize mainly in the lungs and cervical lymph nodes [55]. Radiologically, there is a radiolucent/hypodense multiloculated lesion with irregular edges [55]. The first-choice treatment is a conservative surgery, associated with, when necessary, adjuvant therapies (such as radiation, chemotherapy, combination therapy, and neck dissection) [55]. Early and multiple recurrences, occurring post-treatment, are relatively common and they may signal a poor prognosis; for this reason, a long-term follow-up is necessary [55]. Adenoid ameloblastoma was recognized as a separate entity from the conventional ameloblastoma by the WHO in 2022, and it shows a slight predilection for females. The most common radiographic features are represented by ill-defined radiolucent and usually unilocular lesions, which can be associated with cortical perforation [56]. Histopathological findings include for this variant the presence of ductal structures, a cribriform architecture, epithelial whorls, and enamel knot-like structures; less often are found ghost cells and dentinoid [57]. This adenoid subtype has a locally aggressive behavior, with a high recurrence rate. Surgical resection with adequate disease-free margins and long-term follow-up are, also in this case, necessary [56].
PA, which is the subtype identified in our clinical case, is the rare extraosseous variant of ameloblastoma, characterized by a benign behavior and a minimal bony involvement; it represents 1–10% of all ameloblastomas [2,6,7,8]. Etiopathogenesis has been correlated with the cell rest of Serres, the remnants of reduced enamel epithelium, the basal cells of the surface epithelium, or with the pluripotent cells of minor salivary glands [6].
From a clinical point of view, PA appears as an exophytic slowly growing mass, either sessile or pedunculated, with a firm consistency [10,24,31]. The surface can be smooth, granular, warty, or papillary, and the color can range from normal mucosa to dark red [1]. The most affected intraoral site is the lingual gingiva of the canine/premolar region, followed by the anterior mandible and the maxillary tuber [6,24,31]. The maxilla is less involved than the mandible with an estimated ratio of 1:2.5 [6,24,31]. Extragingival localization is extremely rare, but some cases in the buccal mucosa, in the oral floor, and in the pterygomandibular space have been documented [6,24,31]. Even though the mass itself is usually painless, teeth migration can occur in case involving the interdental papilla [1].
The bone involvement of PA is absent or negligible, and the tumor usually remains superficial to the cortical bone [1,10], since the dense fibrous tissue of the gingiva and the periosteum may act as a barrier for the infiltration [8]. Nevertheless, a few cases in the literature have shown some bone involvement, named as “cupping” or “saucerization”, which appears as a small depression on the bone surface due to the local compression of the tumor [1,8]. For this reason, the use of 3D imaging, such as magnetic resonance (MRI) or computed tomography (CT), is useful to identify the lesion profile more precisely [11]. Our patient showed a pinkish-colored exophytic lesion, with papillary surface, localized on the lingual gingiva of the premolar region, without bone involvement or saucerization, as investigated by radiographic images.
Besides imaging, the final diagnosis of PA is made after the histopathologic examination [11,31,58], since the clinical presentation can be misdiagnosed with other conditions, such as pyogenic granuloma, fibrous epulis, peripheral ossifying fibroma, giant cell granuloma, inflammatory fibrous hyperplasia related to prosthesis, squamous papilloma, or intraoral basal cell carcinoma [1,2,59].
3.1. Differential Diagnosis of PA
Pyogenic granuloma is a benign reactive lesion, usually associated with chronic irritants such as plaque, calculus, or defective restoration margins. Clinically, it appears as a smooth or exophytic mass with a sessile or pedunculated base, characterized by different shades of color, from bright pink to red. The surface can sometimes be ulcerated, in particular in areas subjected to trauma. The most affected site is the gingiva, but it can also occur on the lips, buccal mucosa, and tongue. Pyogenic granuloma is usually asymptomatic, and the treatment of choice is surgical excision.
Fibrous epulis is another reactive lesion, which develops on the adherent gingiva as a response to food impaction, calculus, overhanging dental restorations, and other irritant factors. From a clinical point of view, it can be described as an exophytic, sessile, or pedunculated mass, with a more or less firm consistency and pink in color. The most frequent localization is at the interdental papilla, maybe due to the susceptibility of this area to the aggregation of food particles and plaque. The treatment consists in excising the lesion while removing local factors that can trigger its development.
Similarly, the peripheral ossifying fibroma (POF) is included in the differential diagnosis. It usually arises from interdental papilla and it shows a predilection for the anterior maxilla, especially incisor and canine areas. The histopathological findings show the presence of fibrous stroma in which mineralized tissues (such as bone and/or cementum-like) are present. Radiographs can show radiopaque opacification, occasionally associated with bone destruction.
The peripheral giant cell granuloma (PGCG) is a further reactive lesion in differential diagnosis; it is related to trauma or local irritation and it occurs mostly on the attached gingiva, on the alveolar mucosa, or on the crest of edentulous alveolar ridge. It appears as an exophytic mass, sessile or pedunculated, with a red and/or blue color, covered by a frequently ulcerated mucosa. Differently from the pyogenic granuloma, PGCG tends to affect in particular the molar area. The treatment includes complete surgical excision and the removal of causative factors.
The epulis fissuratum is an adaptive growth caused by chronic trauma and irritation from ill-fitting prosthesis. Considering its clinical features, it presents as a raised and sessile lesion in the form of folds, with a firm consistency. The overlying mucosa can be normal, erythematous, or ulcerated if it gets traumatized. The relining or remaking of the prosthesis and surgical excision are usually the choice treatment.
Squamous papilloma is one of the most common Human Papillomavirus (HPV)-related lesions, and it is mainly associated to genotype 6 and 11. Clinically, it appears as an exophytic growth with a characteristic warty surface, usually pedunculated, with color ranging from white to pink/red. The most affected intraoral sites are the palate, the tongue, and the labial mucosa. The average size of the squamous papilloma is normally less than 1 cm. Again, the therapy is the surgical removal of the lesion, also in order to decrease viral transmissibility.
Intraoral basal cell carcinoma is an extremely rare entity, which shares some common features with the PA. In fact, they both show a proliferation of basal cells, usually organized in nests, and intermixed with a fibrous stroma. Nevertheless, they can be distinguished relying on some microscopic and immunohistochemical findings: for example, the PA is positive for cytokeratin 19 and negative for Bcr-Ep4, while the opposite is seen in the intraoral basal cell carcinoma [1].
Considering its similarity, in terms of clinical aspect, to all the previous described conditions, the final diagnosis of PA is made after the histopathologic examination [2,11].
3.2. Histopathological Features of PA
Microscopically, PA is characterized by odontogenic epithelium organized in islands and chords, which show a follicular pattern and are similar to the odontogenic islands of the central ameloblastoma. Moreover, since the islands are found to be contiguous to the basal layer of the overlying surface epithelium, this arrangement can resemble the histopathological aspect of the basal cell carcinoma [4,6]. Considering the immunohistochemical features, PA shows a high positivity for cytokeratin 5, 14, and 19, as well as for calretinin and amelogenin; these findings may help the clinicians to rule out most of the odontogenic mesenchymal tumors, which are negative for these markers [1,6].
Immunohistochemistry may also highlight genetic mutation involved in the mitogen-activated protein kinase (MAPK) pathway, including BRAF, neuroblastoma RAS viral oncogene homolog (NRAS), and fibroblast growth factor receptor 2 (FGFR2) mutations. BRAF, in particular, is a member of the RAF kinase family and it plays an important role in the RAS-RAF-MAPK pathway, which regulates cell proliferation and differentiation. Overall, it is estimated that 80–90% of all subtypes of ameloblastoma are associated with the BRAF V600E mutation [60]. This latter occurs when valine (V) is substituted for glutamate (E) at codon 600. According to the current literature, the majority of ameloblastomas with this mutation are located in the mandible, in patients younger than 54 years old [61]. However, there is no statistically significant association according to histological variants or recurrence rate [61]. In our case, BRAF immunohistochemistry was was negative. Considering NRAS, a recent paper by Oh and Hong identified the NRAS G12D mutation in a case of peripheral ameloblastoma arising in the mandibular alveolar mucosa of a 65-year-old man [62].
3.3. Management of PA
The management of PA includes conservative local surgical excision with adequate disease-free margins, as preferential therapy [8,58]. However, due to the rarity of this condition, there is no consensus about the extent of the surgical margins [11]. After excision, the recurrence rate ranges from 16% to 19%, but some authors suggested that relapses might be attributed to incomplete excision of the primary lesion [6,11]. Although PA is considered a benign tumor, late recurrences (up to 10 years) or, more rarely, progressions to malignancy with recurrence as ameloblastic carcinoma have been documented [2,6,7,11]. In addition, a case of PA with malignant progression that also metastasized has been reported [1,7,46]. Therefore, a long-term periodic follow-up is highly suggested in order to detect any relapse or rare cases of malignant transformation [1,8].
Recently, with the elucidation of molecular markers of ameloblastoma, there have been attempts for the treatment of ameloblastoma with molecular targeted therapy, in particular used in patients with recurrent, metastatic, and malignant ameloblastic tumors [63,64]. Food and Drug Administration (FDA)-approved molecular targeted therapy for ameloblastomas include drugs able to inhibit the functions of mutated BRAF and mitogen-activated protein kinase kinase (MEK) [63,64].
4. Conclusions
PA is a rare variant of the classic and more common intraosseous counterpart. Considering its clinical aspect, which lacks pathognomonic features, the diagnosis is usually made after histopathological analysis, associated with 3D computed tomography (CT) imaging. The treatment of choice is surgical excision. Even though it is a benign lesion, recurrences or rare malignant transformations have been reported; therefore, the recommended patient’s management includes, besides the surgical excision of the lesion, a long-term follow-up.
Author Contributions
S.D.: writing—original draft, investigation, conceptualization; M.Q.: writing—original draft, investigation; V.C.: writing—review and editing, investigation; L.M.: writing—review and editing, investigation; E.M.V.: writing—review and editing, investigation, conceptualization. All authors have read and agreed to the published version of the manuscript.
Informed Consent Statement
Written informed consent has been obtained from the patient to publish this paper.
Data Availability Statement
Data sharing is not applicable, since the article is a case report including a review of literature.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Vanoven B.J., Parker N.P., Petruzzelli G.J. Peripheral Ameloblastoma of the Maxilla: A Case Report and Literature Review. Am. J. Otolaryngol. 2008;29:357–360. doi: 10.1016/j.amjoto.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 2.Ülker E., Kirtiloğlu T., Taban B. Peripheral Ameloblastoma: A Case Report. J. Clin. Exp. Dent. 2020;12:e607–e609. doi: 10.4317/jced.56757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hendra F.N., Van Cann E.M., Helder M.N., Ruslin M., de Visscher J.G., Forouzanfar T., de Vet H.C.W. Global Incidence and Profile of Ameloblastoma: A Systematic Review and Meta-Analysis. Oral Dis. 2020;26:12–21. doi: 10.1111/odi.13031. [DOI] [PubMed] [Google Scholar]
- 4.Nosé V., Lazar A.J. Update from the 5th Edition of the World Health Organization Classification of Head and Neck Tumors: Familial Tumor Syndromes. Head Neck Pathol. 2022;16:143–157. doi: 10.1007/s12105-022-01414-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.WHO Classification of Tumours Editorial Board . Head and Neck Tumours. WHO Classification of Tumours. 5th ed. Volume 9. IARC WHO; Lyon, France: 2024. [Google Scholar]
- 6.Vezhavendhan N., Vidyalakshmi S., Muthukumaran R., Santhadevy A., Sivaramakrishnan M., Gayathri C. Peripheral Ameloblastoma of the Gingiva. Autops. Case Rep. 2020;10:e2019127. doi: 10.4322/acr.2019.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goda H., Nakashiro K., Ogawa I., Takata T., Hamakawa H. Peripheral Ameloblastoma with Histologically Low-Grade Malignant Features of the Buccal Mucosa: A Case Report with Immunohistochemical Study and Genetic Analysis. Int. J. Clin. Exp. Pathol. 2015;8:2085–2089. [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang X., Tian X., Hu Y., Zhang C., Wei C., Yang X. Oral Peripheral Ameloblastoma: A Retrospective Series Study of 25 Cases. Med. Oral Patol. Oral Cir. Bucal. 2018;23:e277–e281. doi: 10.4317/medoral.22225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wright J.M., Vered M. Update from the 4th Edition of the World Health Organization Classification of Head and Neck Tumours: Odontogenic and Maxillofacial Bone Tumors. Head Neck Pathol. 2017;11:68–77. doi: 10.1007/s12105-017-0794-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borrello R., Bettio E., Bacci C., Valente M., Sivolella S., Mazzoleni S., Berengo M. A Conservative Approach to a Peripheral Ameloblastoma. Case Rep. Dent. 2016;2016:8254571. doi: 10.1155/2016/8254571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anpalagan A., Tzortzis A., Twigg J., Wotherspoon R., Chengot P., Kanatas A. Current Practice in the Management of Peripheral Ameloblastoma: A Structured Review. Br. J. Oral Maxillofac. Surg. 2021;59:e1–e8. doi: 10.1016/j.bjoms.2020.08.084. [DOI] [PubMed] [Google Scholar]
- 12.Gardner D.G. Some Current Concepts on the Pathology of Ameloblastomas. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 1996;82:660–669. doi: 10.1016/S1079-2104(96)80441-0. [DOI] [PubMed] [Google Scholar]
- 13.Janardhanan M., Rakesh S., Savithri V., Aravind T. Peripheral Ameloblastoma with Neoplastic Osseous Invasion versus Peripheral Intraosseous Ameloblastoma: A Challenging Diagnosis. J. Oral Maxillofac. Pathol. JOMFP. 2018;22:396–400. doi: 10.4103/jomfp.JOMFP_130_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Braunstein E. Case report of an extraosseous adamantinoblastoma. Oral Surg. Oral Med. Oral Pathol. 1949;2:726–728. doi: 10.1016/0030-4220(49)90105-X. [DOI] [PubMed] [Google Scholar]
- 15.Klinar K., McManis J. Soft-tissue ameloblastoma. Oral Surg. Oral Med. Oral Pathol. 1969;28:266–272. doi: 10.1016/0030-4220(69)90296-5. [DOI] [PubMed] [Google Scholar]
- 16.Gardner D. Peripheral ameloblastoma: A study of 21 cases, including 5 reported as basal cell carcinoma of the gingiva. Cancer. 1977;39:1625–1633. doi: 10.1002/1097-0142(197704)39:4<1625::AID-CNCR2820390437>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 17.Patrikiou A., Papanicolaou S., Stylogianni E., Sotiriadou S. Peripheral ameloblastoma. Case report and review of the literature. Int. J. Oral Surg. 1983;12:51–55. doi: 10.1016/S0300-9785(83)80080-5. [DOI] [PubMed] [Google Scholar]
- 18.Atkinson C., Harwood A., Cummings B. Ameloblastoma of the jaw. A reappraisal of the role of megavoltage irradiation. Cancer. 1984;53:869–873. doi: 10.1002/1097-0142(19840215)53:4<869::AID-CNCR2820530409>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 19.Schaberg S.J., Antimarino R.F., Pierce G.L., Crawford B.E. Peripheral ameloblastoma. Report of a case. Int. J. Oral Surg. 1983;12:344–347. doi: 10.1016/S0300-9785(83)80024-6. [DOI] [PubMed] [Google Scholar]
- 20.Buchner A., Sciubba J.J. Peripheral epithelial odontogenic tumors: A review. Oral Surg. Oral Med. Oral Pathol. 1987;63:688–697. doi: 10.1016/0030-4220(87)90372-0. [DOI] [PubMed] [Google Scholar]
- 21.Ficarra G., Hansen L. Peripheral ameloblastoma. A case report. J. Craniomaxillofac. Surg. 1987;15:110–112. doi: 10.1016/S1010-5182(87)80029-X. [DOI] [PubMed] [Google Scholar]
- 22.Woo S., Smith-Williams J., Sciubba J., Lipper S. Peripheral ameloblastoma of the buccal mucosa: Case report and review of the English literature. Oral Surg. Oral Med. Oral Pathol. 1987;63:78–84. doi: 10.1016/0030-4220(87)90344-6. [DOI] [PubMed] [Google Scholar]
- 23.El-Mofty S., Gerard N., Farish S., Rodu B. Peripheral ameloblastoma: A clinical and histologic study of 11 cases. J. Oral Maxillofac. Surg. 1991;49:970–975. doi: 10.1016/0278-2391(91)90061-P. [DOI] [PubMed] [Google Scholar]
- 24.Nauta J.M., Panders A.K., Schoots C.J.F., Vermey A., Roodenburg J.L.N. Peripheral Ameloblastoma: A Case Report and Review of the Literature. Int. J. Oral Maxillofac. Surg. 1992;21:40–44. doi: 10.1016/S0901-5027(05)80451-5. [DOI] [PubMed] [Google Scholar]
- 25.Hernandez G., Sanchez G., Caballero T., Moskow B.S. A rare case of a multi-centric peripheral ameloblastoma of the gingiva. A light and electron microscopic study. J. Clin. Periodontol. 1992;19:281–287. doi: 10.1111/j.1600-051X.1992.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 26.Baden E., Doyle J., Petriella V. Malignant transformation of peripheral ameloblastoma. Oral Surg. Oral Med. Oral Pathol. 1993;75:214–219. doi: 10.1016/0030-4220(93)90096-M. [DOI] [PubMed] [Google Scholar]
- 27.Gurol M., Burkes E.J., Jr. Peripheral ameloblastoma. J. Periodontol. 1995;66:1065–1068. doi: 10.1902/jop.1995.66.12.1065. [DOI] [PubMed] [Google Scholar]
- 28.Zhu E., Okada N., Takagi M. Peripheral ameloblastoma: Case report and review of the literature. J. Oral Maxillofac. Surg. 1995;53:590–594. doi: 10.1016/0278-2391(95)90074-8. [DOI] [PubMed] [Google Scholar]
- 29.Califano. L., Maremonti P., Boscaino A., De Rosa G., Giardino C. Peripheral ameloblastoma: Report of a case with malignant aspect. Br. J. Oral Maxillofac. Surg. 1996;34:240–242. doi: 10.1016/S0266-4356(96)90277-6. [DOI] [PubMed] [Google Scholar]
- 30.Wettan H., Patella P., Freedman P. Peripheral ameloblastoma: Review of the literature and report of recurrence as severe dysplasia. J. Oral Maxillofac. Surg. 2001;59:811–815. doi: 10.1053/joms.2001.24302. [DOI] [PubMed] [Google Scholar]
- 31.Philipsen H.P., Reichart P.A., Nikai H., Takata T., Kudo Y. Peripheral Ameloblastoma: Biological Profile Based on 160 Cases from the Literature. Oral Oncol. 2001;37:17–27. doi: 10.1016/S1368-8375(00)00064-6. [DOI] [PubMed] [Google Scholar]
- 32.Marucci G., Betts C., Michal M., Foschini M.P. Peripheral ameloblastoma with Merkel cells. Virchows Arch. 2004;446:204–205. doi: 10.1007/s00428-004-1159-1. [DOI] [PubMed] [Google Scholar]
- 33.Lopez-Jornet P., Bermejo-Fenoll A. Peripheral ameloblastoma of the gingiva: The importance of diagnosis. J. Clin. Periodontol. 2005;32:12–15. doi: 10.1111/j.1600-051X.2004.00627.x. [DOI] [PubMed] [Google Scholar]
- 34.Martelli-Júnior H., Souza L., Santos L., Melo-Filho M.R., De Paula A.M. Peripheral ameloblastoma: A case report. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2005;99:e31–e33. doi: 10.1016/j.tripleo.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 35.Yanamoto S., Yamabe S., Kawasaki G., Mizuno A. Peripheral ameloblastoma in the maxillary canine region. Asian J. Oral Maxillofac. Surg. 2005;17:195–198. doi: 10.1016/S0915-6992(05)80051-2. [DOI] [Google Scholar]
- 36.Curtis N., Zoellner H. Surgical management of an ameloblastoma in soft tissues of the cheek. Br. J. Oral Maxillofac. Surg. 2006;44:495–496. doi: 10.1016/j.bjoms.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 37.LeCorn D.W., Bhattacharyya I., Vertucci F.J. Peripheral ameloblastoma: A case report and review of the literature. J. Endod. 2006;32:152–154. doi: 10.1016/j.joen.2005.10.028. [DOI] [PubMed] [Google Scholar]
- 38.Gomes C., Garcia B., Gomez R., de Freitas J.B., Mesquita R.A. A clinical case of peripheral ameloblastoma. Braz. J. Oral Sci. 2007;6:1364–1366. [Google Scholar]
- 39.Yamanishi T., Ando S., Aikawa T., Kishino M., Nakano Y., Sasai K., Isomura E., Tsuji T., Koizumi H., Iida S., et al. A case of extragingival peripheral ameloblasotoma in the buccal mucosa. J. Oral Pathol. Med. 2007;36:184–186. doi: 10.1111/j.1600-0714.2006.00480.x. [DOI] [PubMed] [Google Scholar]
- 40.Ide F., Mishima K., Miyazaki Y., Saito I., Kusama K. Peripheral ameloblastoma in-situ: An evidential fact of surface epithelium origin. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2009;108:763–767. doi: 10.1016/j.tripleo.2009.06.018. [DOI] [PubMed] [Google Scholar]
- 41.Beena V., Choudhary K., Heera R., Rajeev R., Sivakumar R., Vidhyadharan K. Peripheral ameloblastoma: A case report and review of literature. Case Rep. Dent. 2012;2012:571509. doi: 10.1155/2012/571509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bertossi D., Favero V., Albanese M., De-Santis D., Martano M., Padovano-di-Leva A., De-Florio I., Nocini P.F., Lo-Muzio L. Peripheral ameloblastoma of the upper gingiva: Report of a case and literature review. J. Clin. Exp. Dent. 2014;6:e180–e184. doi: 10.4317/jced.51124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bhat V., Bhandary S., Bhat S. Extraosseous ameloblastoma of maxillary gingiva- a case report. Indian J. Surg. Oncol. 2014;5:211–213. doi: 10.1007/s13193-014-0328-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lascane N., Sedassari B., Alves F., Gallottini M.H.C., Sousa S.C.O.M.D. Peripheral ameloblastoma with dystrophic calcification: An unusual feature in non-calcifying odontogenic tumors. Braz. Dent. J. 2014;25:253–256. doi: 10.1590/0103-6440201300024. [DOI] [PubMed] [Google Scholar]
- 45.Kandagal V.S., Chandrappa P.R., Desai D., Pandit S., Yadav S., Ingaleshwar P. Extraosseous ameloblastoma of maxillary gingiva: A rare case. Clin. Cancer Investig. J. 2016;5:49–51. [Google Scholar]
- 46.Nurkic T., Castillo-Jorge S., Schmalfuss I. Extra-gingival peripheral ameloblastoma. Appl. Radiol. 2018;47:38–40. doi: 10.37549/AR2517. [DOI] [Google Scholar]
- 47.Upadhyaya J.D., Bhattacharyya I., Fitzpatrick S.G., Cohen D.M., Islam M.N. Peripheral Ameloblastoma: A Study of 18 Cases and Usage of Ber-EP4 Immunohistochemistry to Rule out a Diagnosis of Intraoral Basal Cell Carcinoma. J. Oral Maxillofac. Surg. 2018;76:996–1004. doi: 10.1016/j.joms.2017.11.020. [DOI] [PubMed] [Google Scholar]
- 48.Cadavid A., Araujo J., Coutinho-Camillo C., Bologna S., Junior C.A.L., Lourenço S.V. Ameloblastomas: Current aspects of the new WHO classification in an analysis of 136 cases. Surg. Exp. Pathol. 2019;2:17. doi: 10.1186/s42047-019-0041-z. [DOI] [Google Scholar]
- 49.On D., Kang M., Ryu J., Kang M. Peripheral ameloblastoma of the pterygomandibular space: A case report. J. Oral Maxillofac. Surg. Med. Pathol. 2019;31:192–195. doi: 10.1016/j.ajoms.2019.01.003. [DOI] [Google Scholar]
- 50.Netto R., Peralta-Mamani M., de Freitas-Filho S.A., Moura L.L., Rubira C.M., Rubira-Bullen I.R. Segmental resection vs. partial resection on treating solid multicystic ameloblastomas of the jaws—Recurrence rates: A systematic review and meta-analysis. J. Clin. Exp. Dent. 2023;15:e518–e525. doi: 10.4317/jced.60502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hendra F.N., Helder M.N., Ruslin M., Van Cann E.M., Forouzanfar T. A network meta-analysis assessing the effectiveness of various radical and conservative surgical approaches regarding recurrence in treating solid/multicystic ameloblastomas. Sci. Rep. 2023;13:8445. doi: 10.1038/s41598-023-32190-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Figueiredo N.R., Dinkar A.D., Meena M., Satoskar S., Khorate M. Ameloblastoma: A clinicoradiographic and histopathologic correlation of 11 cases seen in Goa during 2008-2012. Contemp. Clin. Dent. 2014;5:160–165. doi: 10.4103/0976-237X.132305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Augustine D., Rao R.S., Surendra L., Patil S., Yoithapprabhunath T.R., Albogami S., Shamsuddin S., Basheer S.A., Sainudeen S. Histopathologic Feature of Hyalinization Predicts Recurrence of Conventional/Solid Multicystic Ameloblastomas. Diagnostics. 2022;12:1114. doi: 10.3390/diagnostics12051114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ali M., Qassem A., Tawashi S., Ahmad K., Khalil A.K. Unicystic ameloblastoma appeared as a massive multilocular entity: A case report with long-term follow-up. Int. J. Surg. Case Rep. 2024;120:109830. doi: 10.1016/j.ijscr.2024.109830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sarode G., Gondivkar S.M., Gore A., Anand R., Sengupta N., Mehta V., Sarode S.C. Clinico-pathological and prognostic overview of metastasizing ameloblastoma: An overview of the systematic reviews. J. Oral Biol. Craniofac. Res. 2023;13:751–757. doi: 10.1016/j.jobcr.2023.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Keshwar S., Raut T., Jain N., Shrestha A., Jaisani M.R. Histopathological Insight of a Case of Adenoid Ameloblastoma: A Rare Odontogenic Tumor. Case Rep. Dent. 2024;2024:8366045. doi: 10.1155/2024/8366045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Odell E.W., Gomes C.C., Thavaraj S. The evolving molecular characterisation, histological criteria and nomenclature of adenoid ameloblastoma as a World Health Organisation tumour type. Histopathology. 2024 doi: 10.1111/his.15270. Early View . [DOI] [PubMed] [Google Scholar]
- 58.Ghai S. Ameloblastoma: An Updated Narrative Review of an Enigmatic Tumor. Cureus. 2022;14:e27734. doi: 10.7759/cureus.27734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ide F., Ito Y., Miyazaki Y., Nishimura M., Kusama K., Kikuchi K. A New Look at the History of Peripheral Ameloblastoma. Head Neck Pathol. 2020;14:1052–1057. doi: 10.1007/s12105-020-01168-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mendez L.D., Wolsefer N.S., Asa S.L., Wasman J., Yoest J.M., Stojanov I.J. The diagnostic utility of BRAF VE1 mutation-specific im-munohistochemistry in ameloblastoma. Mod. Pathol. 2022;35:1570–1577. doi: 10.1038/s41379-022-01105-8. [DOI] [PubMed] [Google Scholar]
- 61.Mamat Yusof M.N., Ch’ng E.S., Radhiah Abdul Rahman N. BRAF V600E Mutation in Ameloblastoma: A Systematic Review and Meta-Analysis. Cancers. 2022;14:5593. doi: 10.3390/cancers14225593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hong S.D. Novel NRAS G12D mutation in extraosseous/peripheral ameloblastoma: Case report and review of mitogen-activated protein kinase pathway mutations in extraosseous/peripheral and intraosseous ameloblastomas. J. Dent. Sci. 2024;19:1892–1893. doi: 10.1016/j.jds.2024.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Oh K.Y. Treatment options for advanced ameloblastoma in the era of precision medicine: A brief review. Oral Oncol. 2023;146:106585. doi: 10.1016/j.oraloncology.2023.106585. [DOI] [PubMed] [Google Scholar]
- 64.Grynberg S., Vered M., Shapira-Frommer R., Asher N., Ben-Betzalel G., Stoff R., Steinberg Y., Amariglio N., Greenberg G., Barshack I., et al. Neoadjuvant BRAF-targeted therapy for ameloblastoma of the mandible: An organ preservation approach. J. Natl. Cancer Inst. 2024;116:539–546. doi: 10.1093/jnci/djad232. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable, since the article is a case report including a review of literature.