Abstract
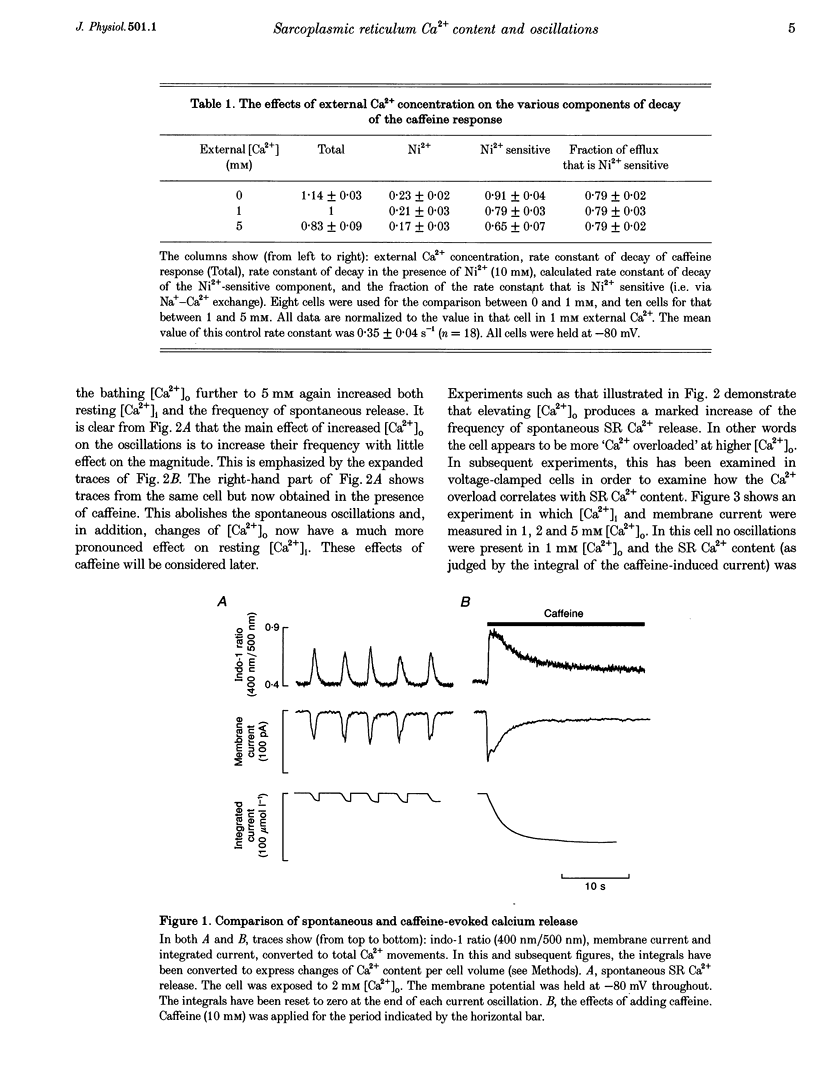
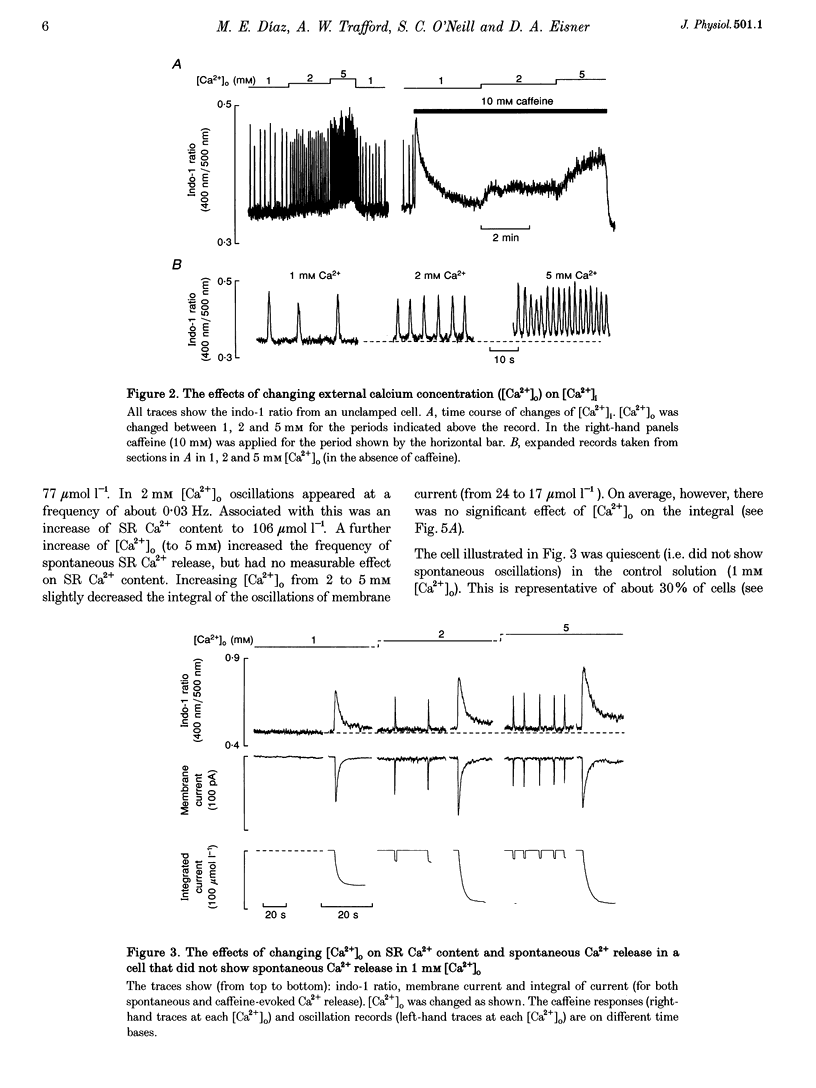
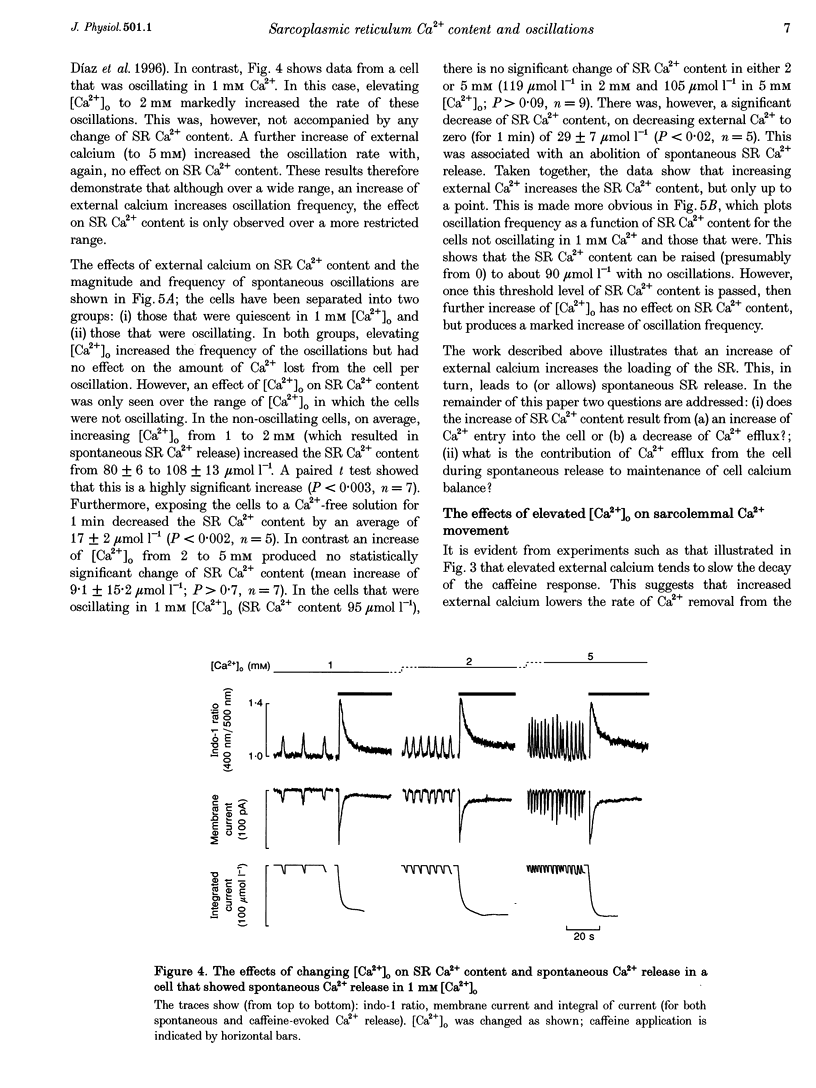
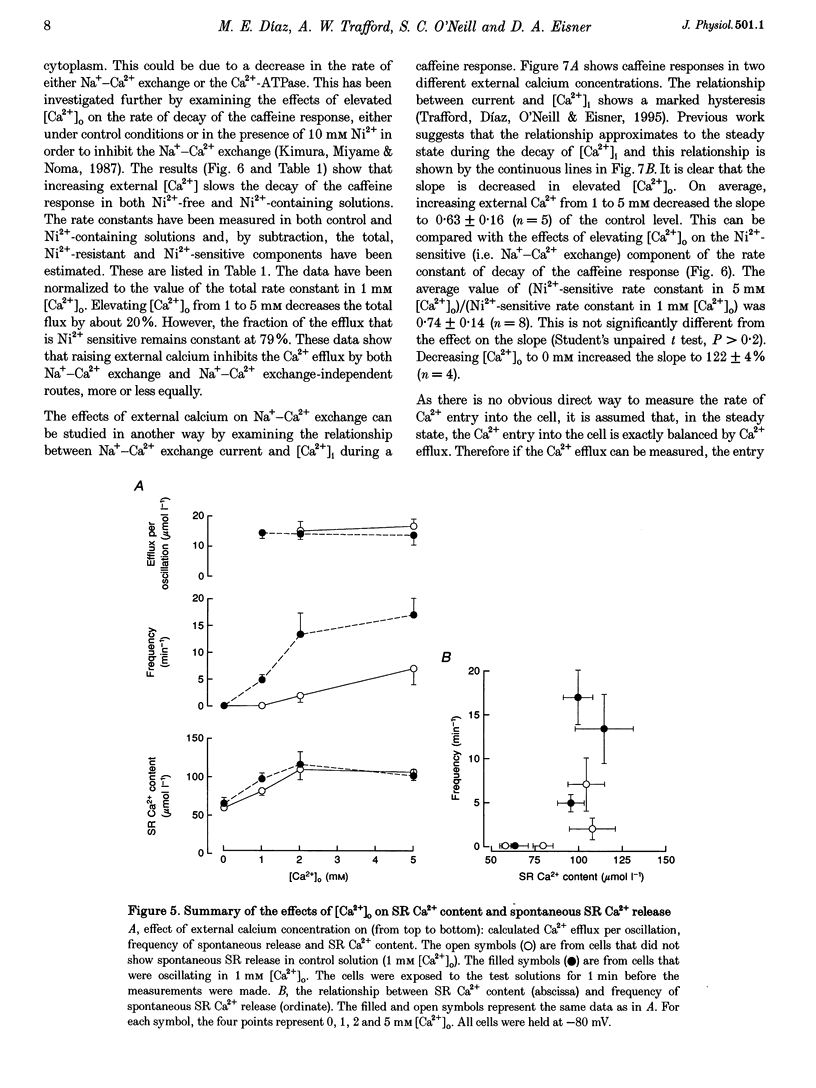
1. Intracellular calcium concentration ([Ca2+]i) and Na(+)-Ca2+ exchange currents were measured in calcium-overloaded voltage-clamped rat ventricular myocytes loaded with the Ca(2+)-sensitive fluorescent indicator indo-1. Sarcoplasmic reticulum (SR) Ca2+ content was measured from the integral of the caffeine-evoked current. In cells that had spontaneous SR Ca2+ release in 1 mM external Ca2+ concentration ([Ca2+]o)i raising [Ca2+]o increased the frequency of release with no effect on SR Ca2+ content. In quiescent cells, increased [Ca2+]o produced spontaneous Ca2+ release associated with increased SR Ca2+ content. Further increase of [Ca2+]o had no effect on SR Ca2+ content. The amount of Ca2+ leaving the cell during each release was constant over a wide range of frequencies and [Ca2+]o values. It appears there is a maximum level of SR Ca2+ content, perhaps because spontaneous Ca2+ release results when the content reaches a threshold. 2. From the relationship between [Ca2+]i and Na(+)-Ca2+ exchange current during a caffeine response, it is possible to estimate the changes in Na(+)-Ca2+ exchange current expected from a change of [Ca2+]i. The data show that the calcium oscillations contribute a significant fraction of the total extra Ca2+ efflux induced by increasing [Ca2+]o. Raising [Ca2+]o decreased the rate of calcium removal from the cell as measured from the rate of decay of the caffeine response, suggesting that both inhibition of Ca2+ efflux and increased Ca2+ entry account for the Ca2+ overload at elevated [Ca2+]o. 3. Inhibiting spontaneous SR Ca2+ release increases resting [Ca2+]i. The Ca2+ efflux is identical to that in the presence of release. It is concluded that spontaneous release of calcium, although potentially arrhythmogenic, is an effective way to activate Ca2+ efflux in overloaded conditions and minimizes any increase of diastolic tension.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen D. G., Eisner D. A., Pirolo J. S., Smith G. L. The relationship between intracellular calcium and contraction in calcium-overloaded ferret papillary muscles. J Physiol. 1985 Jul;364:169–182. doi: 10.1113/jphysiol.1985.sp015737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassani J. W., Yuan W., Bers D. M. Fractional SR Ca release is regulated by trigger Ca and SR Ca content in cardiac myocytes. Am J Physiol. 1995 May;268(5 Pt 1):C1313–C1319. doi: 10.1152/ajpcell.1995.268.5.C1313. [DOI] [PubMed] [Google Scholar]
- Bers D. M., Bridge J. H. Effect of acetylstrophanthidin on twitches, microscopic tension fluctuations and cooling contractures in rabbit ventricle. J Physiol. 1988 Oct;404:53–69. doi: 10.1113/jphysiol.1988.sp017278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callewaert G., Cleemann L., Morad M. Caffeine-induced Ca2+ release activates Ca2+ extrusion via Na+-Ca2+ exchanger in cardiac myocytes. Am J Physiol. 1989 Jul;257(1 Pt 1):C147–C152. doi: 10.1152/ajpcell.1989.257.1.C147. [DOI] [PubMed] [Google Scholar]
- Capogrossi M. C., Stern M. D., Spurgeon H. A., Lakatta E. G. Spontaneous Ca2+ release from the sarcoplasmic reticulum limits Ca2+-dependent twitch potentiation in individual cardiac myocytes. A mechanism for maximum inotropy in the myocardium. J Gen Physiol. 1988 Jan;91(1):133–155. doi: 10.1085/jgp.91.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H., Lederer W. J., Cannell M. B. Calcium sparks: elementary events underlying excitation-contraction coupling in heart muscle. Science. 1993 Oct 29;262(5134):740–744. doi: 10.1126/science.8235594. [DOI] [PubMed] [Google Scholar]
- Díaz M. E., Cook S. J., Chamunorwa J. P., Trafford A. W., Lancaster M. K., O'Neill S. C., Eisner D. A. Variability of spontaneous Ca2+ release between different rat ventricular myocytes is correlated with Na(+)-Ca2+ exchange and [Na+]i. Circ Res. 1996 May;78(5):857–862. doi: 10.1161/01.res.78.5.857. [DOI] [PubMed] [Google Scholar]
- Eisner D. A., Nichols C. G., O'Neill S. C., Smith G. L., Valdeolmillos M. The effects of metabolic inhibition on intracellular calcium and pH in isolated rat ventricular cells. J Physiol. 1989 Apr;411:393–418. doi: 10.1113/jphysiol.1989.sp017580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisner D. A., Valdeolmillos M. A study of intracellular calcium oscillations in sheep cardiac Purkinje fibres measured at the single cell level. J Physiol. 1986 Mar;372:539–556. doi: 10.1113/jphysiol.1986.sp016024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A. Calcium-induced release of calcium from the cardiac sarcoplasmic reticulum. Am J Physiol. 1983 Jul;245(1):C1–14. doi: 10.1152/ajpcell.1983.245.1.C1. [DOI] [PubMed] [Google Scholar]
- Fedida D., Noble D., Rankin A. C., Spindler A. J. The arrhythmogenic transient inward current iTI and related contraction in isolated guinea-pig ventricular myocytes. J Physiol. 1987 Nov;392:523–542. doi: 10.1113/jphysiol.1987.sp016795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrier G. R., Saunders J. H., Mendez C. A cellular mechanism for the generation of ventricular arrhythmias by acetylstrophanthidin. Circ Res. 1973 May;32(5):600–609. doi: 10.1161/01.res.32.5.600. [DOI] [PubMed] [Google Scholar]
- Friel D. D., Tsien R. W. Phase-dependent contributions from Ca2+ entry and Ca2+ release to caffeine-induced [Ca2+]i oscillations in bullfrog sympathetic neurons. Neuron. 1992 Jun;8(6):1109–1125. doi: 10.1016/0896-6273(92)90132-w. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Han S., Schiefer A., Isenberg G. Ca2+ load of guinea-pig ventricular myocytes determines efficacy of brief Ca2+ currents as trigger for Ca2+ release. J Physiol. 1994 Nov 1;480(Pt 3):411–421. doi: 10.1113/jphysiol.1994.sp020371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn R., Marty A. Muscarinic activation of ionic currents measured by a new whole-cell recording method. J Gen Physiol. 1988 Aug;92(2):145–159. doi: 10.1085/jgp.92.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai H., Petersen O. H. Spatial dynamics of second messengers: IP3 and cAMP as long-range and associative messengers. Trends Neurosci. 1994 Mar;17(3):95–101. doi: 10.1016/0166-2236(94)90112-0. [DOI] [PubMed] [Google Scholar]
- Kass R. S., Lederer W. J., Tsien R. W., Weingart R. Role of calcium ions in transient inward currents and aftercontractions induced by strophanthidin in cardiac Purkinje fibres. J Physiol. 1978 Aug;281:187–208. doi: 10.1113/jphysiol.1978.sp012416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura J., Miyamae S., Noma A. Identification of sodium-calcium exchange current in single ventricular cells of guinea-pig. J Physiol. 1987 Mar;384:199–222. doi: 10.1113/jphysiol.1987.sp016450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kort A. A., Lakatta E. G. Spontaneous sarcoplasmic reticulum calcium release in rat and rabbit cardiac muscle: relation to transient and rested-state twitch tension. Circ Res. 1988 Nov;63(5):969–979. doi: 10.1161/01.res.63.5.969. [DOI] [PubMed] [Google Scholar]
- Lipp P., Pott L., Callewaert G., Carmeliet E. Simultaneous recording of Indo-1 fluorescence and Na+/Ca2+ exchange current reveals two components of Ca2(+)-release from sarcoplasmic reticulum of cardiac atrial myocytes. FEBS Lett. 1990 Nov 26;275(1-2):181–184. doi: 10.1016/0014-5793(90)81467-3. [DOI] [PubMed] [Google Scholar]
- López-López J. R., Shacklock P. S., Balke C. W., Wier W. G. Local, stochastic release of Ca2+ in voltage-clamped rat heart cells: visualization with confocal microscopy. J Physiol. 1994 Oct 1;480(Pt 1):21–29. doi: 10.1113/jphysiol.1994.sp020337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negretti N., Varro A., Eisner D. A. Estimate of net calcium fluxes and sarcoplasmic reticulum calcium content during systole in rat ventricular myocytes. J Physiol. 1995 Aug 1;486(Pt 3):581–591. doi: 10.1113/jphysiol.1995.sp020836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill S. C., Donoso P., Eisner D. A. The role of [Ca2+]i and [Ca2+] sensitization in the caffeine contracture of rat myocytes: measurement of [Ca2+]i and [caffeine]i. J Physiol. 1990 Jun;425:55–70. doi: 10.1113/jphysiol.1990.sp018092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orchard C. H., Eisner D. A., Allen D. G. Oscillations of intracellular Ca2+ in mammalian cardiac muscle. Nature. 1983 Aug 25;304(5928):735–738. doi: 10.1038/304735a0. [DOI] [PubMed] [Google Scholar]
- Page E. Quantitative ultrastructural analysis in cardiac membrane physiology. Am J Physiol. 1978 Nov;235(5):C147–C158. doi: 10.1152/ajpcell.1978.235.5.C147. [DOI] [PubMed] [Google Scholar]
- Sitsapesan R., Williams A. J. Regulation of the gating of the sheep cardiac sarcoplasmic reticulum Ca(2+)-release channel by luminal Ca2+. J Membr Biol. 1994 Feb;137(3):215–226. doi: 10.1007/BF00232590. [DOI] [PubMed] [Google Scholar]
- Stern M. D., Kort A. A., Bhatnagar G. M., Lakatta E. G. Scattered-light intensity fluctuations in diastolic rat cardiac muscle caused by spontaneous Ca++-dependent cellular mechanical oscillations. J Gen Physiol. 1983 Jul;82(1):119–153. doi: 10.1085/jgp.82.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamatsu T., Wier W. G. Calcium waves in mammalian heart: quantification of origin, magnitude, waveform, and velocity. FASEB J. 1990 Mar;4(5):1519–1525. doi: 10.1096/fasebj.4.5.2307330. [DOI] [PubMed] [Google Scholar]
- Trafford A. W., Díaz M. E., O'Neill S. C., Eisner D. A. Comparison of subsarcolemmal and bulk calcium concentration during spontaneous calcium release in rat ventricular myocytes. J Physiol. 1995 Nov 1;488(Pt 3):577–586. doi: 10.1113/jphysiol.1995.sp020991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trafford A. W., O'Neill S. C., Eisner D. A. Factors affecting the propagation of locally activated systolic Ca transients in rat ventricular myocytes. Pflugers Arch. 1993 Oct;425(1-2):181–183. doi: 10.1007/BF00374521. [DOI] [PubMed] [Google Scholar]
- Varro A., Negretti N., Hester S. B., Eisner D. A. An estimate of the calcium content of the sarcoplasmic reticulum in rat ventricular myocytes. Pflugers Arch. 1993 Apr;423(1-2):158–160. doi: 10.1007/BF00374975. [DOI] [PubMed] [Google Scholar]
- Wier W. G., Cannell M. B., Berlin J. R., Marban E., Lederer W. J. Cellular and subcellular heterogeneity of [Ca2+]i in single heart cells revealed by fura-2. Science. 1987 Jan 16;235(4786):325–328. doi: 10.1126/science.3798114. [DOI] [PubMed] [Google Scholar]
- Wier W. G., Kort A. A., Stern M. D., Lakatta E. G., Marban E. Cellular calcium fluctuations in mammalian heart: direct evidence from noise analysis of aequorin signals in Purkinje fibers. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7367–7371. doi: 10.1073/pnas.80.23.7367. [DOI] [PMC free article] [PubMed] [Google Scholar]


