Abstract
The C-terminal repeat domain of Clostridium difficile toxin A harbors toxin-neutralizing epitopes and is considered to be a candidate component of a vaccine against C. difficile-associated disease (CDAD). Fourteen of the 38 C-terminal toxin A repeats (14CDTA) were cloned into pTECH-1 in frame with the immunogenic fragment C of tetanus toxin (TETC) to generate plasmid p56TETC. Expression of the TETC-14CDTA fusion protein was driven from the anaerobically inducible nirB promoter within attenuated Salmonella typhimurium BRD509 (aroA aroD). The TETC-14CDTA fusion protein was purified and shown to bind to known toxin A receptors found on the surface of rabbit erythrocytes. Intranasal (i.n.) and intragastric (i.g.) immunization with 107 and 1010 CFU, respectively, of BRD509(p56TETC) generated significant (P < 0.05) anti-toxin A serum responses after a single dose. Antibody titers were elevated following a boosting dose with either live vaccine or a subcutaneous injection of 0.5 μg of purified 14CDTA protein. Importantly, serum from mice immunized with BRD509(p56TETC) neutralized toxin A cytotoxicity. Both i.n. and i.g. immunizations also generated toxin A-specific immunoglobulin A on the pulmonary and intestinal mucosa, respectively. Intranasal vaccination induced consistently higher serum and mucosal anti-toxin A antibody responses. Significant anti-tetanus toxoid serum and mucosal antibodies were also generated by both immunization routes. The availability of live attenuated Salmonella typhi for human use may allow the development of a multivalent mucosal vaccine against CDAD, tetanus, and typhoid.
Clostridium difficile-associated disease (CDAD) is a major nosocomial health care problem which can lead to patient isolation or even ward closures (3, 39). Disease is characteristically preceded by broad-spectrum antibiotic use, with symptoms ranging from mild diarrhea to the life-threatening syndrome of pseudomembranous colitis (18, 32). The population at risk includes all patients on antimicrobial agents, especially the immunocompromised and the elderly (45). A relatively high patient relapse rate of approximately 20% (55) coupled with an ever-growing elderly population means that improved immunoprophylactic treatments are required.
Common pathological features of CDAD include fluid accumulation, inflammation, and necrosis of the gastrointestinal mucosa (40, 41). The main C. difficile virulence-associated determinants which promote tissue damage are toxin A (308 kDa) and toxin B (270 kDa). Studies carried out in various animal models of CDAD have shown toxin A, which can bind to human intestinal epithelial cells (46), to be the primary mediator of tissue damage within the intestine (34, 35, 38). However, toxin B is extremely cytotoxic for several cell lines in vitro (20) and promotes colonic mucosal damage in organ culture (43).
A striking feature of the predicted amino acid sequences of both toxin A and toxin B is the repetitive nature of the C termini (2, 12). In the case of toxin A, there are 38 tandem repeat amino acid sequences classified on both size and sequence homology. These repeat sequences encode a receptor-binding domain of toxin A (42) and harbor epitopes that can induce antibodies that neutralize the cytotoxic activity of whole toxin (33). A conserved decapeptide from one of these repeat sequences, the class IIB repeat, can promote cellular attachment and stimulate also the production of toxin-neutralizing antibodies (56). Thus, the C-terminal repeat region appears to be a candidate component of future CDAD vaccines.
Parenteral immunization with either small amounts of toxin A (25) or a recombinant protein expressing 33 of the 38 C-terminal repeats (33) can generate a toxin-neutralizing systemic antibody response which will partially protect against toxin challenge. The induction of a local anti-toxin A antibody response at the site of action of the toxin, such as the intestinal mucosa, could enhance the level of protection. Indeed, toxin-specific immunoglobulin A (IgA) antibodies that inhibit toxin A from binding to brush border membranes have been detected on the human colonic mucosa (24).
The ability to induce a local immune response at mucosal surfaces is compromised by the inherent unresponsiveness of the mucosal immune system to most antigens (36). Vaccines based on attenuated Salmonella typhimurium are capable of delivering bacterial (9, 15), viral (5, 21), and protozoal (4, 26) antigens to the mucosal immune system. In a previous study we expressed 8, 14, 20, and 36 C-terminal toxin A repeats within an attenuated S. typhimurium vaccine strain, BRD915 (53). The construct containing the 14 toxin A repeats (14CDTA) was shown to be optimum for retention of receptor-binding function and also the induction of an anti-toxin A antibody response in mice (53). In the present study, 14CDTA was expressed in an attenuated S. typhimurium aroA aroD vaccine strain, BRD509, as a fusion to the immunogenic, nontoxic fragment C of tetanus toxin (TETC). This approach to protein expression and mucosal delivery has been used by others to promote stable heterologous antigen expression in vivo (6, 37) and to potentially optimize antigen delivery to the mucosal immune system (23, 27). Both intragastric (i.g.) and intranasal (i.n.) results of immunization were shown to be efficient at generating anti-toxin A antibodies that could neutralize the cytotoxicity of whole toxin A. Importantly, mucosal local anti-toxin A IgA responses were also induced by both immunization routes.
MATERIALS AND METHODS
DNA manipulation.
Restriction enzymes and DNA ligase were purchased from Promega (Southampton, United Kingdom) and used according to the manufacturer’s instructions. DNA which had been subjected to restriction enzyme treatment was purified by using either S-300 HR Microspin columns (Pharmacia) or Prep-A-Gene purification resin (Bio-Rad, Hemel Hempstead, United Kingdom). PCR was carried out with a Perkin-Elmer 9600 cycle sequencer and Taq DNA polymerase used as described by the manufacturer (Appligene Oncor, Watford, United Kingdom). DNA cycle sequencing was performed with an ABI PRISM reaction kit (Perkin-Elmer Applied Biosystems, Warrington, United Kingdom) and analyzed with an Applied Biosystems 373A DNA sequencer. Chromosomal DNA was isolated from C. difficile VPI 10463 by the method of Wren and Tabaqchali (57).
Bacterial strains, plasmids, and growth conditions.
S. typhimurium LB5010 (galE), S. typhimurium BRD509 (aroA aroD), and plasmid pTECH-1 have been described elsewhere (27). Bacteria were routinely cultivated either in Luria broth (LB) or on LB agar with or without ampicillin (100 μg/ml). For aerobic growth, cultures were incubated for 18 h with shaking at 37°C. For anaerobic growth, cultures were incubated for between 36 and 72 h in an atmosphere composed of 80% (vol/vol) N2 and 10% (vol/vol) each CO2 and H2 (Mark III work station; Don Whitley Scientific Limited, Shipley, United Kingdom).
The 14 C-terminal toxin A repeats (bp 7159 through 8118) were amplified from chromosomal DNA isolated from C. difficile VPI 10463 on a 960-bp fragment, using primers 5′-ACTTCTAGAGCCTCAACTGGTTATACAAGT-3′ (sense strand) and 5′-ATAACTAGTAGGGGCTTTTACTCCATCAAC-3′ (antisense strand), which were based on the published toxin A sequence (12). Underlining denotes an XbaI restriction site in the sense-strand primer and a SpeI restriction site in antisense-strand primer. The amplified toxin A sequence was subcloned into the pTAg cloning vector (R&D Systems Europe Ltd., Abingdon, United Kingdom), excised with XbaI-SpeI, and inserted into SpeI-digested pTECH-1 downstream of the TETC encoding sequence (27). The resulting plasmid, named p56TETC, was electroporated into S. typhimurium LB5010 (galE) (7) and then introduced into S. typhimurium BRD509 (aroA aroD) by P22 bacteriophage transduction (52).
SDS-PAGE and immunoblotting.
Proteins were separated on 10% (wt/vol) polyacrylamide gels and the discontinuous buffer system of Laemmli (31). Prior to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis, the protein content of samples was determined by the bicinchoninic acid assay (Pierce & Warriner Ltd., Chester, United Kingdom). Proteins separated by SDS-PAGE were transferred to Hybond C nitrocellulose membranes (Amersham Life Science Ltd., Amersham, United Kingdom) and processed as described previously (8). Membranes were probed with either monoclonal antibody PCG-4, specific for the C-terminal repeat region of toxin A, or a polyclonal rabbit anti-TETC antiserum.
Hemagglutination properties of cellular lysates.
Recombinant S. typhimurium BRD509 strains were grown under anaerobic conditions, and the soluble material was harvested by sonication and differential centrifugation as described by Ward et al. (54). Fractions (50 μg) of soluble material were serially diluted twofold in phosphate-buffered saline (PBS) in a 96-well U-bottomed plate (Sterilin) and reacted with 25 μl of a 2% (vol/vol) suspension of washed rabbit erythrocytes at 4°C (TCS Microbiology, Botolph Claydon, United Kingdom). Wells containing the highest dilution of protein able to promote 100% agglutination of erythrocytes were taken as the endpoint, and each assay was performed in duplicate.
Animals and immunization.
To generate the inoculum, bacteria were grown statically for 16 h at 37°C in LB with or without ampicillin, washed twice with cold PBS, and finally resuspended in sterile PBS to give a final concentration of approximately 1011 CFU/ml. Six- to eight-week-old female BALB/c mice (Harlan Olac, Bichester, United Kingdom) were randomized into groups of 10. For i.g. inoculation, each mouse received a 200-μl inoculum containing 1 × 1010 to 2 × 1010 CFU via gavage tube under anesthesia (Halothane; Rhone Merieux). For i.n. inoculation, each mouse received 1 × 107 to 2 × 107 CFU in 30 μl via the nares under slight anesthesia (Halothane; Rhone Merieux). Colony counts were performed on all inocula to verify viable cell numbers. Five of the animals from each immunization group were exsanguinated by cardiac puncture after 27 days, while the remaining five animals received a second identical dose. After 64 days, these animals were boosted subcutaneously (s.c.) with 0.5 μg of purified 14CDTA protein. The purified protein was generated by cloning the entire 14 C-terminal toxin A repeats into the pRSET-A vector (Invitrogen, De Schelp, The Netherlands) and expressing them in Escherichia coli BL21(DE3) (Novagen) with a polyhistidine N-terminal tag. The 14 toxin A repeats were then purified to high levels by bovine thyroglobulin affinity chromatography as described by Krivan and Wilkins (29). All surviving mice were killed after 83 days. Serum samples were collected from the tail vein of each animal 1 day prior to immunization. Nasal, lung, and intestinal lavage were carried out with 0.1% (wt/vol) bovine serum albumin in PBS as described by Douce et al. (11) on all killed animals. Intestinal lavage samples were stored with 1 mM phenylmethylsulfonyl fluoride to inhibit intestinal proteases.
ELISA.
Both the anti-toxin A and anti-tetanus toxoid (TT) antibody responses in individual serum samples were determined as described by Douce et al. (10). Serum samples were serially diluted fivefold in PBS and incubated in wells of an enzyme immunoassay/radioimmunoassay 96-well plate (Corning Costar, High Wycombe, United Kingdom) previously coated with either whole toxin A (0.15 μg/well in 0.1 M NaHCO3 [pH 9.5]) or TT (0.5 μg/well in PBS [pH 7.2]). Bound antibody was visualized by using a polyvalent anti-mouse immunoglobulin–horseradish peroxidase conjugate (Sigma) and o-phenylenediamine as a substrate. Enzyme-linked immunosorbent assay (ELISA) titers were determined as the reciprocal of the highest serum dilution which gave an absorbance value of 0.5 U above the background for toxin A, and 0.3 U above the background for TT. All titers were standardized against either monoclonal antibody PCG-4 or polyclonal anti-TETC positive control antiserum.
Measurement of antitoxin antibody levels in mucosal secretions.
The levels of both anti-toxin A- and anti-TT IgA within the mucosal lavage samples were determined by ELISA using the method of Douce et al. (11). After incubation of the antigen-coated plates with diluted lavage samples, anti-mouse α-chain-specific biotin-conjugated antibody (Sigma) was incubated in the wells, and bound antibody was detected with horseradish peroxidase-conjugated streptavidin (Dako) and o-phenylenediamine. ELISA titers were calculated as the reciprocal of the highest dilution which gave an absorbance 0.5 U above the background for toxin A and 0.3 U above the background for TT. The total IgA content of each lavage sample was also determined by using plates coated with anti-mouse α-chain-specific immunoglobulin (0.25 μg/well; Sigma) and comparing endpoint titers to known amounts of myeloma murine IgA (ICN Biomedicals) (11). ELISA units were expressed as ELISA titer per microgram of total IgA per sample.
Antibody-mediated toxin A neutralization.
The toxin A-neutralizing properties of serum antibody were determined by using CHO-K1 cells (51) and thyroglobulin affinity-purified toxin A. Freshly trypsinized CHO-K1 cells (passages 26 to 38) were seeded into 96-well trays (Corning Costar) at 3 × 104 cells per well and allowed to recover for 24 h at 37°C in a 5% CO2 (vol/vol) atmosphere. The sera were serially diluted twofold in growth medium and allowed to react with toxin A (0.6 μg/ml, final concentration) for 90 min at 37°C. The toxin A-antiserum mixtures were then added to the CHO-K1 cells to give a final total volume of 100 μl, and the cellular morphology was noted after a 24-h incubation at 37°C and 5% (vol/vol) CO2. The neutralizing titer was taken as the highest dilution of sample to prevent 100% cellular rounding. All samples were tested in duplicate, and individual assays were standardized against a control antiserum specific for toxin A (56).
Statistical analysis.
Unpaired Student’s t test was used to compare unrelated groups of data. If the standard deviations (SD) were found to be significantly different, the Mann-Whitney nonparametric test was used to determine the statistical relatedness of the data groups. Probability values of <0.05 were taken as significant. Statistical analysis was carried out with the InStat statistical package (Sigma).
RESULTS
Expression and in vitro characterization of the TETC-14CDTA fusion protein.
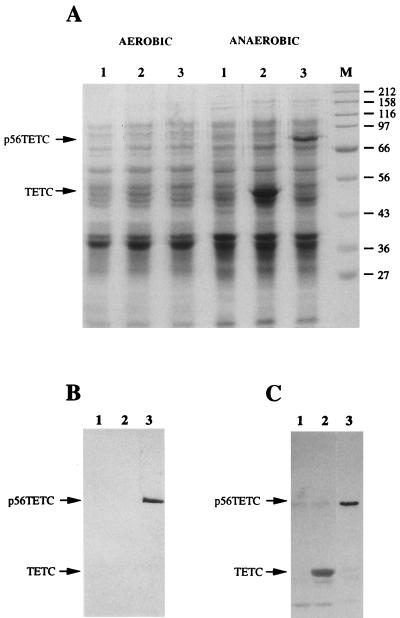
DNA encoding the 14 C-terminal repeats of C. difficile toxin A was inserted into pTECH-1 downstream and in frame with the TETC coding sequence to generate p56TETC. Transduction of p56TETC into attenuated S. typhimurium BRD509 generated recombinant bacteria with an unaltered smooth lipopolysaccharide phenotype as determined by SDS-PAGE and silver staining (data not shown). SDS-PAGE analysis of cell lysates prepared from BRD509(pTECH-1) showed the presence of an additional polypeptide, not present in similarly prepared BRD509 lysates, with an apparent molecular mass of 50 kDa within cells grown under anaerobic conditions (Fig. 1A). This polypeptide migrated on gels with purified TETC and was absent from lysates prepared from bacterial cells grown under aerobic conditions, as expected for nirB-controlled heterologous protein expression. A similar analysis of BRD509(p56TETC) identified a unique polypeptide within lysates prepared from anaerobically grown cells with an apparent molecular mass of 80 kDa (Fig. 1A). This band corresponded to the TETC-14CDTA fusion protein.
FIG. 1.
(A) SDS-PAGE analysis (10% [wt/vol] polyacrylamide gel) of S. typhimurium BRD509 cellular lysates grown under aerobic and anaerobic conditions and stained with Coomassie brilliant blue G. Sample 1, BRD509; sample 2, BRD509(pTECH-1); sample 3, BRD509(p56TETC). Molecular weight markers (M) are also shown. Apparent molecular masses are shown in kilodaltons. (B and C) Immunoblots of anaerobically grown BRD509 cellular lysates with toxin A-specific monoclonal antibody PCG-4 (B) and anti-TETC polyclonal antiserum (C). Approximately 40 μg of lysate was loaded per lane.
To validate the identity of the TETC-14CDTA protein, lysates prepared from anaerobically grown bacterial cells were subjected to immunoblotting with monoclonal antibody PCG-4, which is specific for the toxin A repeat region. The candidate TETC-14CDTA fusion protein of 80 kDa reacted strongly with monoclonal antibody PCG-4 (Fig. 1B). No cross-reaction was seen between PCG-4 and either S. typhimurium cellular antigens or TETC. The 80-kDa TETC-14CDTA fusion protein also reacted strongly with a polyclonal antiserum specific for TETC (Fig. 1C). A much weaker immunoreactive band of 50-kDa apparent molecular mass was also seen. This band corresponded to the TETC domain and probably resulted from the translation machinery stalling at the hinge region built into pTECH-1, as has been previously reported (27). Both the SDS-PAGE and immunoblot data showed the p56TETC fusion protein to be expressed at a lower level than TETC alone in BRD509. To determine the solubility of TETC-14CDTA, we fractionated BRD509(p56TETC) cells by differential centrifugation and analyzed by immunoblotting both the soluble and insoluble, cell envelope-associated material. Using these techniques, we found the TETC-14CDTA fusion protein to be located in the soluble fraction (data not shown).
The TETC-14CDTA fusion protein was also analyzed for the ability to bind to the Galα1-3 Galβ1-4 GlcNAc toxin A receptor present on the surface of rabbit erythrocytes (28). The soluble fraction of BRD509(p56TETC) (50 μg of soluble cellular protein serially diluted twofold in PBS and incubated with 25 μl of a 2% [vol/vol] solution of rabbit erythrocytes at 4°C for 18 h) was found to hemagglutinate rabbit erythrocytes even at a dilution of 1:427 ± 1:148, whereas the same amount of material harvested from BRD509(pTECH-1) did not hemagglutinate. For toxin A (5 μg tested), hemagglutinating activity was found at a dilution of 1:21 ± 1:9. (Values represent means ± standard errors of the means from three separate experiments, each performed in duplicate.)
Anti-toxin A serum responses after i.n. or i.g. immunization.
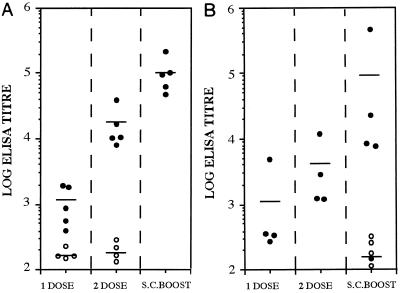
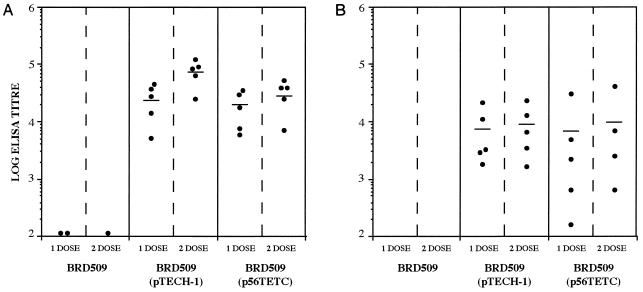
Groups of BALB/c mice were immunized i.n. with 107 CFU of either BRD509(p56TETC) or BRD509(pTECH-1), and the resulting antiserum was reacted with native toxin A in ELISA. Mice immunized with BRD509(p56TETC) generated an anti-toxin A antibody response after one dose that was significantly (P < 0.05) higher than the titers seen with the control mice immunized with BRD509(pTECH-1) (Fig. 2A). Mice were successfully boosted with a second i.n. dose of BRD509(p56TETC), generating antibody titers over 100-fold higher than the control titers (P < 0.05). In an attempt to increase the anti-toxin A response even further, all immunized mice received a small s.c. dose of 0.5 μg of purified 14CDTA protein. The s.c. boost was shown to be effective, significantly (P < 0.05) increasing the mean anti-toxin A titer a further sixfold. All control mice immunized with BRD509(pTECH-1) did not show any increase in anti-toxin A antibody levels after the s.c. boost.
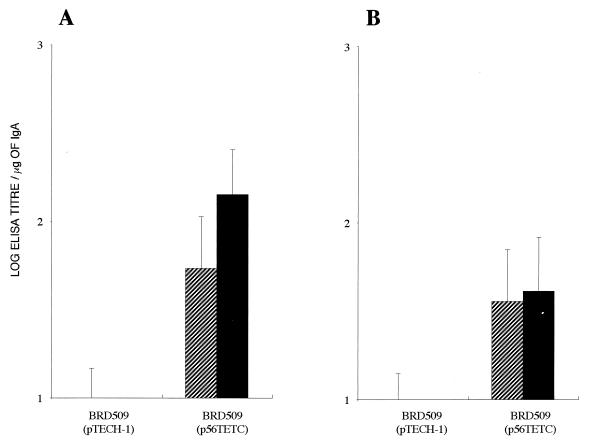
FIG. 2.
Anti-toxin A total immunoglobulin responses in sera of i.n. (A)- and i.g. (B)-immunized BALB/c mice. Antibody titers were measured by ELISA in serum taken after 1 dose (day 27) and 2 doses (day 63) of recombinant BRD509 and also after an s.c. boost (day 83) with 0.5 μg of purified 14CDTA protein. Filled markers represent individual responses from mice immunized with BRD509(p56TETC); open markers represent responses from BRD509(pTECH-1)-immunized mice. Each bar denotes the mean titer from five mice per group.
Similar groups of mice were also immunized i.g. with 1010 CFU of the same recombinant vaccine strains. A single dose of BRD509(p56TETC) generated a mean anti-toxin A serum response which was 120-fold higher than that for the control group (P < 0.05) (Fig. 2B). Although this response was boosted threefold with a second i.g. dose, the mean geometric anti-toxin A titer generated (3,464) was not as high as that seen after two i.n. doses (17,099). However, an additional s.c. boost with 0.5 μg of purified 14CDTA protein raised the anti-toxin A titers to a level which was statistically indistinguishable (P > 0.05) from that seen within i.n.-immunized mice, although the levels of anti-toxin A antibodies in individual i.g.-immunized mice were more varied than those for i.n.-immunized animals (Fig. 2).
Toxin A-specific mucosal IgA responses following i.n. and i.g. immunization.
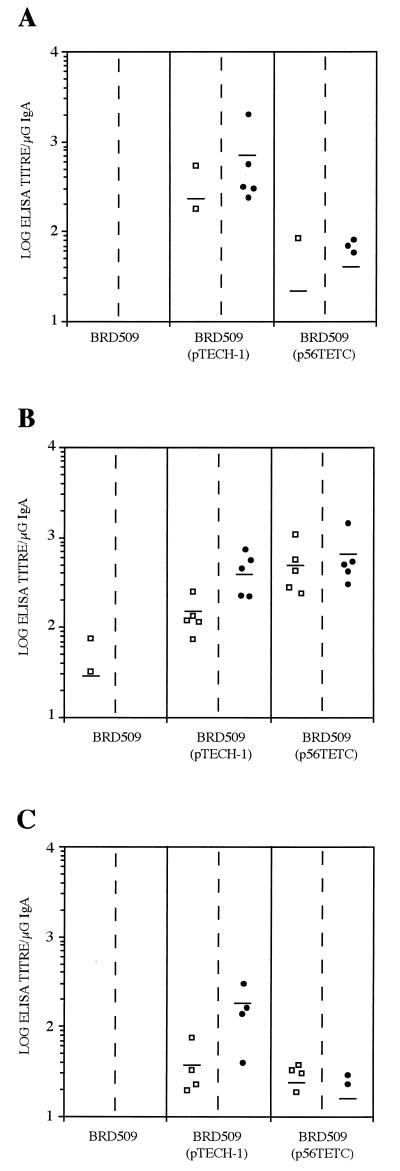
The ability of the recombinant Salmonella strains to induce a toxin A-specific local IgA response at the mucosal surface was also assessed. Pulmonary lavage samples taken from mice immunized i.n. with BRD509(p56TETC) harbored high levels of anti-toxin A IgA even after one dose (Fig. 3A). These responses were further elevated approximately threefold with a second i.n. dose followed by an s.c. boost with purified 14CDTA protein. In addition, BRD509(p56TETC) was also shown to stimulate toxin A-specific IgA responses at the mucosa of the small intestine following a single i.g. dose (Fig. 3B). There was no detectable difference in responses induced by one or two doses followed by an s.c. boost with purified protein.
FIG. 3.
Toxin A-specific mucosal IgA responses in pulmonary lavage samples after i.n. immunization (A) or in intestinal lavage after i.g. immunization (B) with BRD509(pTECH-1) or BRD509(p56TETC). Mean titers ± SD from five mice are shown after 1 dose (day 27) (hatched bars) or 2 doses and an s.c. boost with 14CDTA protein (day 83) (filled bars).
Antibody-mediated toxin A neutralization.
Postimmunization sera were tested for the ability to neutralize the cytotoxic activity of toxin A against CHO-K1 cells in vitro. Sera of all mice immunized i.n. with two doses of BRD509(p56TETC) followed by an s.c. injection of purified 14CDTA protein contained significant (P < 0.05) levels of toxin A neutralizing antibody (Table 1). Similarly, mice immunized with BRD509(p56TETC) i.g. generated a mean neutralizing titer which was comparable to that for the i.n.-immunized mice. However, not all of the i.g.-immunized animals produced detectable levels of neutralizing antibody. No toxin A-neutralizing activity was detected in the sera of mice after a single dose of BRD509(p56TETC) given either i.n. or i.g. (data not shown).
TABLE 1.
Toxin A-neutralizing properties of day 83 serum harvested from both i.g.- and i.n.-immunized micea
| Immunogen | i.n. immunization
|
i.g. immunization
|
||
|---|---|---|---|---|
| Individual neutralization titer | Mean neu-tralization titer | Individual neutralization titer | Mean neu-tralization titer | |
| BRD509(p56TETC) | 16 | 48 | ||
| 16 | 4 | |||
| 12 | 11 ± 6 | 4 | 11 ± 20 | |
| 6 | 0 | |||
| 4 | 0 | |||
| BRD509(pTECH-1) | 0 | 0 | 0 | 0 |
Toxin-neutralizing titers were scored as the highest dilution of antiserum to promote 100% neutralization of toxin A (60 ng/well) as measured against CHO-K1 cells in vitro. Individual neutralizing titers from each mice are shown along with mean titers ± SD for five mice.
Anti-TT responses in serum taken after i.n. or i.g. immunization.
In addition to toxin A-specific responses, serum samples were also tested for anti-TT antibody. All mice immunized i.n. with BRD509(p56TETC) generated high anti-TT responses even after a single dose (Fig. 4A). Mean antibody titers were comparable to those obtained with BRD509(pTECH-1) (P > 0.05). A second i.n. dose further increased antibody levels, most notably in BRD509(pTECH-1)-immunized mice. Similarly, i.g. immunization with BRD509(p56TETC) also generated significant anti-TT responses after one dose which were similar to those detected in BRD509(pTECH-1)-immunized mice (P > 0.05) (Fig. 4B). However, in contrast to the i.n.-immunized mice, antibody titers were not increased with a second i.g. dose. In addition, the mean antibody titers seen with i.g.-immunized mice were at least threefold lower than those found in i.n.-immunized mice. Strikingly, i.g. immunization also generated a wide range of individual responses in the immunized group.
FIG. 4.
Individual anti-TT total immunoglobulin responses in sera of i.n. (A)- and i.g. (B)-immunized BALB/c mice taken after 1 dose (day 27) and 2 doses (day 83) of BRD509, BRD509(pTECH-1), or BRD509(p56TETC). Each bar denotes the mean titer from five mice per group.
Anti-TT local IgA responses induced by i.n. or i.g. immunization.
Anti-TT IgA was found in the nasal cavities of mice immunized i.n. with BRD509(p56TETC) (Fig. 5A). However, not all of the mice responded, even after two doses. In contrast, all mice immunized i.n. with BRD509(p56TETC) harbored high levels of anti-TT IgA in the lung mucosa (Fig. 5B). These mean antibody titers were significantly higher (P < 0.05) than the responses detected with BRD509(pTECH-1) after one dose and also significantly higher (P < 0.05) than those seen at the nasal mucosa.
FIG. 5.
Individual anti-TT mucosal IgA responses from BALB/c mice after 1 dose (day 27) (squares) or 2 doses (day 83) (circles) of BRD509, BRD509(pTECH-1), or BRD509(p56TETC). Responses in nasal (A) and pulmonary (B) lavage samples taken from i.n.-immunized mice are shown, along with responses from intestinal lavage samples collected from i.g.-immunized mice (C). Each bar denotes the mean titer from five mice per group.
Four of the five mice immunized i.g. with BRD509(p56TETC) were also shown to exhibit significant (P < 0.05) anti-TT IgA responses at the intestinal mucosa after a single dose (Fig. 5C). In contrast to BRD509(pTECH-1)-immunized animals, these responses were not boosted with a second dose.
DISCUSSION
In this study, 14 of the 38 C-terminal repeats of C. difficile toxin A were expressed as a fusion to the C terminus of TETC in the attenuated S. typhimurium vaccine strain BRD509 (aroA aroD). Expression of the p56TETC fusion protein was under control of the nirB promoter. The nirB promoter was selected since it is optimally activated within the intracellular environment of epithelial cells and macrophages, therefore limiting heterologous antigen expression to sites that are important in stimulating the mucosal immune system (14). The production of soluble recombinant protein in Salmonella cannot always be achieved, as some highly expressed foreign antigens accumulate within the cell as inclusion bodies (1). This self-aggregation can alter or destroy protective epitopes within the heterologous protein and severely reduce vaccine efficacy. The TETC-14CDTA fusion protein was shown to accumulate within E. coli BRD509 cells in a predominantly soluble form. The 14 C-terminal toxin A amino acid repeats also appeared to be folded in a conformation that facilitated binding to target eukaryotic cells, as the TETC-14CDTA fusion bound effectively to known toxin A receptors present on the surface of rabbit erythrocytes.
Salmonella strains typically infect via the oral route, crossing the intestinal epithelial layer through microfold or M cells found within Peyer’s patches as well as enterocytes (22), whence they disseminate into deeper lymphoid tissues such as the liver and spleen. Thus, many studies using S. typhimurium strain as vaccine delivery vehicles have utilized the oral route of immunization. However, S. typhimurium can also gain entry into the host through the nasal mucosa (16), and interestingly, several recent reports have demonstrated immune responses against guest antigen after immunization with recombinant Salmonella strains via the i.n. route (9, 17, 21). Here, we compared the i.n. and i.g. routes of immunization using TETC-14CDTA as the guest antigen. Sera of mice immunized i.g. or i.n. with BRD509(p56TETC) exhibited significant anti-toxin A antibody responses after a single dose. The serum antibody titers detected in the i.n.-immunized mice following a second boosting dose were significantly higher than in those immunized i.g. In addition, the number of immunized animals which seroconverted to TETC-14CDTA following i.n. vaccination was higher, with all mice responding equally well. This was in contrast to the large variation in endpoint titers seen in sera from i.g.-vaccinated mice. Anderson et al. (1) showed that mice primed with a single oral dose of BRD509 expressing P.69 pertactin from Bordetella pertussis could be boosted with an s.c. injection of a small amount of purified P.69 pertactin protein. In an attempt to maximize anti-toxin A antibody titers, we used this approach and reimmunized both the i.n.- and i.g.-immunized mice s.c. with 0.5 μg of purified 14CDTA protein. This booster injection successfully raised anti-toxin A responses in both immunized groups, particularly in i.g.-immunized animals.
The ability to stimulate a toxin A-neutralizing response may be a key property of any future C. difficile toxin-based vaccine. The importance of toxin A-neutralizing antibody has been highlighted by Torres et al. (50), who have correlated protection against lethal C. difficile challenge in the hamster infection model with the presence of neutralizing antibody. In this study, we have shown toxin-neutralizing activity in sera from mice which had received two doses of BRD509(p56TETC), either i.n. or i.g., plus an s.c. boost with purified 14CDTA protein. Mean neutralization titers were similar for i.n.- and i.g.-vaccinated groups. However, only in the i.n.-immunized group did we detect responses in all animals. Serum samples which gave the highest neutralization titers also contained the highest levels of anti-toxin A antibody as measured by ELISA (data not shown). Significant neutralizing responses were seen only after two doses of BRD509(p56TETC) followed by a boost with 14CDTA protein (data not shown).
Oral administration of attenuated Salmonella is known to be an efficient way of stimulating intestinal mucosal immunity against guest antigens (13, 21). Bacterial antigens sampled by M cells interact with the B lymphocytes of the gut-associated lymphoid tissue, which differentiate within the mesenteric lymph nodes into plasma cells. These then seed the secondary effector tissues of the body, such as the lamina propria of the gut, where they produce IgA, which is subsequently transported to the mucosal surface (reviewed in reference 36). We have shown that i.g. immunization with a single dose of BRD509(p56TETC) is sufficient to stimulate significant levels of toxin A-specific IgA on the intestinal surface. In a similar fashion to the gut-associated lymphoid tissue GALT being the major inductive site within the gut, the nasal-associated lymphoid tissue appears to be an important mucosal inductive site for the upper respiratory tract (19, 30). Our results show significant anti-toxin A responses on the lung mucosa following i.n. vaccination with one dose of BRD509(p56TETC). The mucosal lymphoid tissues are interconnected to form a common mucosal immune system with antibody-secreting plasma cells migrating from the immunization site to various effector sites located throughout the host (47). However, we were unable to detect toxin A-specific IgA in either nasal or pulmonary lavage samples following i.g. vaccination, nor did we detect it at the intestinal mucosa in i.n.-immunized mice. This indicates a preference of BRD509(p56TETC)-primed B cells for colonizing effector sites adjacent to the induction site. Alternatively, the levels of IgA at these distal sites may have been too low for detection in our assay due to the sample dilution which is inherent within the mucosal lavage procedure. Indeed, these distal surfaces may well have been primed by our mucosal immunization regimens. Not all studies on C. difficile vaccine development have incorporated attempts to induce or monitor mucosal antitoxin responses (25, 33, 50) even though toxin A-specific mucosal IgA has been shown to neutralize the tissue-damaging activity of toxin A at the intestinal surface (24). We believe that the use of Salmonella-based mucosal delivery systems may warrant more detailed investigations.
BRD509(p56TETC) was also assessed for the ability to generate serum antibodies against TETC. Significant anti-TT titers were detected after a single dose administered by both i.n. and i.g. routes. These results are in line with those of other studies using Salmonella-expressed TETC (6). As with anti-toxin A responses, the antibody titers were higher after i.n. immunization than after i.g. vaccination. In addition, the individual antibody responses were also consistently greater in i.n.-immunized mice. Significant anti-TT IgA responses were also detected in the lungs of all i.n.-immunized mice after two doses. The titers detected were similar to those generated following immunization with BRD509(pTECH-1). In contrast, much lower responses were detected at the intestinal mucosa following BRD509(p56TETC) i.g. immunization. In these experiments, we have simultaneously immunized mice with antigens derived from three different pathogens, opening up the exciting possibility of developing multivalent mucosal vaccination regimens.
Ryan et al. (44) have expressed 33 of the 38 C-terminal toxin A repeats of C. difficile as a secreted protein from an attenuated Vibrio cholerae strain. Although no significant local IgA antibody responses were detected, oral immunization with these derivatives did provide some protection against toxin A-mediated damage of rabbit ileal loops. Our results show that a smaller fragment of the C-terminal region expressed in S. typhimurium BRD509 can induce high levels of toxin A-specific serum antibody and significant mucosal IgA responses. Importantly, the toxin-specific antibodies could neutralize the cytotoxicity of whole toxin A. Intragastric immunization was more proficient in generating anti-toxin A IgA at the gut mucosa, the site of action of C. difficile toxin A. However, i.n. immunization generated higher titers of both serum antibody and toxin-specific IgA, especially in the lung. These findings could have important implications for developing immunization strategies to protect against other systemic or mucosal pathogens. An attenuated Salmonella typhi vaccine expressing TETC is currently being evaluated in clinical trials as an oral vaccine against both tetanus and typhoid (49). A novel mucosal tetanus vaccine would be of value for immunizing populations in the developing world (48). Our results suggest that a multivalent antityphoid vaccine which will also protect against two clostridial pathogens may be possible.
ACKNOWLEDGMENTS
Monoclonal antibody PCG-4 and purified toxin A were generously donated by D. M. Lyerly, TechLab, Inc., Blacksburg, Va. We also thank L. Batty for technical assistance.
S. J. Ward was funded by the Wellcome Trust, grant 042686/Z/94. G. Douce was supported by a Wellcome Trust program grant to G. Dougan.
REFERENCES
- 1.Anderson R, Dougan G, Roberts M. Delivery of the pertactin/P.69 polypeptide of Bordetella pertussis using an attenuated Salmonella typhimurium vaccine strain: expression levels and immune response. Vaccine. 1996;14:1384–1390. doi: 10.1016/s0264-410x(96)00036-9. [DOI] [PubMed] [Google Scholar]
- 2.Barroso L A, Wang S Z, Phelps C J, Johnson J L, Wilkins T D. Nucleotide sequence of Clostridium difficile toxin B gene. Nucleic Acids Res. 1990;18:4004. doi: 10.1093/nar/18.13.4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cartmill T D I, Panigrahi H, Worsley M A, McCann D C, Nice C N, Keith E. Management and control of a large outbreak of diarrhoea due to Clostridium difficile. J Hosp Infect. 1994;27:1–15. doi: 10.1016/0195-6701(94)90063-9. [DOI] [PubMed] [Google Scholar]
- 4.Chabalgoity J A, Harrison J A, Esteves A, Demarco de Hormaeche R, Ehrlich R, Khan C M, Hormaeche C E. Expression and immunogenicity of an Echinococcus granulosus fatty acid-binding protein in live attenuated Salmonella vaccine strains. Infect Immun. 1997;65:2402–2412. doi: 10.1128/iai.65.6.2402-2412.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chabalgoity J A, Khan C M, Nash A A, Hormaeche C E. A Salmonella typhimurium htrA live vaccine expressing multiple copies of a peptide comprising amino acids 8–23 of herpes simplex virus glycoprotein D as a genetic fusion to tetanus toxin fragment C protects mice from herpes simplex virus infection. Mol Microbiol. 1996;19:791–801. doi: 10.1046/j.1365-2958.1996.426965.x. [DOI] [PubMed] [Google Scholar]
- 6.Chatfield S N, Charles I G, Makoff A J, Oxer M D, Dougan G, Pickard D, Slater D, Fairweather N F. Use of the nirB promoter to direct the stable expression of heterologous antigens in Salmonella oral vaccine strains: development of a single-dose oral tetanus vaccine. Bio/Technology. 1992;10:888–892. doi: 10.1038/nbt0892-888. [DOI] [PubMed] [Google Scholar]
- 7.Chatfield S N, Fairweather N, Charles I, Pickard D, Levine M, Hone D, Posada M, Strugnell R A, Dougan G. Construction of a genetically defined Salmonella typhi Ty2 aroA, aroC mutant for the engineering of a candidate oral typhoid-tetanus vaccine. Vaccine. 1992;10:53–60. doi: 10.1016/0264-410x(92)90420-o. [DOI] [PubMed] [Google Scholar]
- 8.Christodoulides M, McGuiness B T, Heckels J E. Immunisation with synthetic peptides containing epitopes of the class 1 outer membrane protein of Neisseria meningitidis: production of bactericidal antibodies on immunisation with a cyclic peptide. J Gen Microbiol. 1993;139:1729–1738. doi: 10.1099/00221287-139-8-1729. [DOI] [PubMed] [Google Scholar]
- 9.Corthesy-Theulaz I E, Hopkins S, Bachmann D, Saldinger P F, Porta N, Haas R, Zheng-Xin Y, Meyer T, Bouzourene H, Blum A L, Kraehenbuhl J P. Mice are protected from Helicobacter pylori infection by nasal immunization with attenuated Salmonella typhimurium phoPc expressing urease A and B subunits. Infect Immun. 1998;66:581–586. doi: 10.1128/iai.66.2.581-586.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Douce G, Fontana M, Pizza M, Rappuoli R, Dougan G. Intranasal immunogenicity and adjuvanticity of site-directed mutant derivatives of cholera toxin. Infect Immun. 1997;65:2821–2828. doi: 10.1128/iai.65.7.2821-2828.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Douce G, Turcotte C, Cropley I, Roberts M, Pizza M, Domenghini M, Rappuoli R, Dougan G. Mutants of Escherichia coli heat-labile toxin lacking ADP-ribosyltransferase activity act as nontoxic, mucosal adjuvants. Proc Natl Acad Sci USA. 1995;92:1644–1648. doi: 10.1073/pnas.92.5.1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dove C H, Wang S Z, Price S B, Phelps C J, Lyerly D M, Wilkins T D, Johnson J L. Molecular characterization of the Clostridium difficile toxin A gene. Infect Immun. 1990;58:480–488. doi: 10.1128/iai.58.2.480-488.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dusek D M, Progulske-Fox A, Brown T A. Systemic and mucosal immune responses in mice orally immunized with avirulent Salmonella typhimurium expressing a cloned Porphyromonas gingivalis hemagglutinin. Infect Immun. 1994;62:1652–1657. doi: 10.1128/iai.62.5.1652-1657.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Everest P, Frankel G, Li J, Lund P, Chatfield S, Dougan G. Expression of LacZ from the htrA, nirB and groE promoters in a Salmonella vaccine strain: influence of growth in mammalian cells. FEMS Microb Lett. 1995;126:97–102. doi: 10.1111/j.1574-6968.1995.tb07398.x. [DOI] [PubMed] [Google Scholar]
- 15.Fairweather N F, Chatfield S N, Makoff A J, Strugnell R A, Bester J, Maskell D J, Dougan G. Oral vaccination of mice against tetanus by use of a live attenuated Salmonella carrier. Infect Immun. 1990;58:1323–1326. doi: 10.1128/iai.58.5.1323-1326.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fedorka-Cray P J, Kelley C, Stabel T J, Gray J T, Laufer J A. Alternative routes of invasion may affect pathogenesis of Salmonella typhimurium in swine. Infect Immun. 1995;63:2658–2664. doi: 10.1128/iai.63.7.2658-2664.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galen J E, Gomez-Duarte O G, Losonsky G A, Halpern J L, Lauderbaugh C S, Kaintuck S, Reymann M K, Levine M M. A murine model of intranasal immunization to assess the immunogenicity of attenuated Salmonella typhi live vector vaccines in stimulating serum antibody responses to expressed foreign antigens. Vaccine. 1997;15:700–708. doi: 10.1016/s0264-410x(96)00227-7. [DOI] [PubMed] [Google Scholar]
- 18.George R H, Symonds J M, Dimock F, Brown J D, Arabi Y, Shinagawa N, Keighley M R B, Alexander Williams J, Burdon D W. Identification of Clostridium difficile as a cause of pseudomembranous colitis. Br Med J. 1978;1:695. doi: 10.1136/bmj.1.6114.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giannasca P J, Boden J A, Monath T P. Targeted delivery of antigen to hamster nasal lymphoid tissue with M-cell-directed lectins. Infect Immun. 1997;65:4288–4298. doi: 10.1128/iai.65.10.4288-4298.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hecht G, Koutsouris A, Pothoulakis C, LaMont J T, Madara J L. Clostridium difficile toxin B disrupts the barrier function of T84 monolayers. Gastroenterology. 1992;102:416–423. doi: 10.1016/0016-5085(92)90085-d. [DOI] [PubMed] [Google Scholar]
- 21.Hopkins S, Kraehenbuhl J P, Schodel F, Potts A, Peterson D, De Grandi P, Nardelli-Haefliger D. A recombinant Salmonella typhimurium vaccine induces local immunity by four different routes of immunization. Infect Immun. 1995;63:3279–3286. doi: 10.1128/iai.63.9.3279-3286.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones B D, Ghori N, Falkow S. Salmonella typhimurium initiates murine infection by penetrating and destroying the specialized epithelial M cells of the Peyer’s patches. J Exp Med. 1994;180:15–23. doi: 10.1084/jem.180.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karem K L, Chatfield S, Nuklin K, Rouse B T. Differential induction of carrier antigen-specific immunity by Salmonella typhimurium live-vaccine strains after single mucosal or intravenous immunization of BALB/c mice. Infect Immun. 1995;63:4557–4563. doi: 10.1128/iai.63.12.4557-4563.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kelly C P, Pothoulakis C, Orellana J, LaMont J T. Human colonic aspirates containing immunoglobulin A antibody to Clostridium difficile toxin A inhibit toxin A-receptor binding. Gastroenterology. 1992;102:35–40. doi: 10.1016/0016-5085(92)91781-x. [DOI] [PubMed] [Google Scholar]
- 25.Ketley J M, Mitchell T J, Candy D C, Burdon D W, Stephen J. The effects of Clostridium difficile crude toxins and toxin A on ileal and colonic loops in immune and non-immune rabbits. J Med Microbiol. 1987;24:41–52. doi: 10.1099/00222615-24-1-41. [DOI] [PubMed] [Google Scholar]
- 26.Khan C M, Villarreal-Ramos B, Pierce R J, Demarco de Hormaeche R, McNeill H, Ali T, Chatfield S, Capron A, Dougan G, Hormaeche C E. Construction, expression, and immunogenicity of multiple tandem copies of the Schistosoma mansoni peptide 115-131 of the P28 glutathione S-transferase expressed as C-terminal fusions to tetanus toxin fragment C in a live aro-attenuated vaccine strain of Salmonella. J Immunol. 1994;153:5634–5642. [PubMed] [Google Scholar]
- 27.Khan C M, Villarreal-Ramos B, Pierce R J, Riveau G, Demarco de Hormaeche R, McNeill H, Ali T, Fairweather N, Chatfield S, Capron A, et al. Construction, expression, and immunogenicity of the Schistosoma mansoni P28 glutathione S-transferase as a genetic fusion to tetanus toxin fragment C in a live Aro attenuated vaccine strain of Salmonella. Proc Natl Acad Sci USA. 1994;91:11261–11265. doi: 10.1073/pnas.91.23.11261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krivan H C, Clark G F, Smith D F, Wilkins T D. Cell surface binding site for Clostridium difficile enterotoxin: evidence for a glycoconjugate containing the sequence Galα1-3Galβ1-4GlcNAc. Infect Immun. 1986;53:573–581. doi: 10.1128/iai.53.3.573-581.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krivan H C, Wilkins T D. Purification of Clostridium difficile toxin A by affinity chromatography on immobilized thyroglobulin. Infect Immun. 1987;55:1873–1877. doi: 10.1128/iai.55.8.1873-1877.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuper C F K, Koornstra P J, Hameleers D M H, Biewenga J, Spit B J, Duijvestijn A M, van Breda Vriesman P J C, Sminia T. The role of nasopharyngeal lymphoid tissue. Immunology Today. 1992;13:219–224. doi: 10.1016/0167-5699(92)90158-4. [DOI] [PubMed] [Google Scholar]
- 31.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 32.Larson H E, Borriello S P. Quantitative study of antibiotic-induced susceptibility to Clostridium difficile enterocecitis in hamsters. Antimicrob Agents Chemother. 1990;34:1348–1353. doi: 10.1128/aac.34.7.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lyerly D M, Johnson J L, Frey S M, Wilkins T D. Vaccination against lethal Clostridium difficile enterocolitis with a nontoxic recombinant peptide of toxin A. Curr Microbiol. 1990;21:29–32. [Google Scholar]
- 34.Lyerly D M, Lockwood D E, Richardson S H, Wilkins T D. Biological activities of toxins A and B of Clostridium difficile. Infect Immun. 1982;35:1147–1150. doi: 10.1128/iai.35.3.1147-1150.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lyerly D M, Saum K E, MacDonald D K, Wilkins T D. Effects of Clostridium difficile toxins given intragastrically to animals. Infect Immun. 1985;47:349–352. doi: 10.1128/iai.47.2.349-352.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McGhee J R, Mestecky J, Dertzbaugh M T, Eldridge J H, Hirasawa M, Kiyono H. The mucosal immune system: from fundamental concepts to vaccine development. Vaccine. 1992;10:75–88. doi: 10.1016/0264-410x(92)90021-b. [DOI] [PubMed] [Google Scholar]
- 37.McSorley S J, Xu D, Liew F Y. Vaccine efficacy of Salmonella strains expressing glycoprotein 63 with different promoters. Infect Immun. 1997;65:171–178. doi: 10.1128/iai.65.1.171-178.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mitchell T J, Ketley J M, Burdon D W, Candy D C, Stephen J. The effects of Clostridium difficile crude toxins and purified toxin A on stripped rabbit ileal mucosa in Ussing chambers. J Med Microbiol. 1987;23:199–204. doi: 10.1099/00222615-23-3-199. [DOI] [PubMed] [Google Scholar]
- 39.Pierce P F, Wilson R, Silva J. Antibiotic-associated pseudomembranous colitis; an epidemic investigation of a cluster of cases. J Infect Dis. 1982;145:269–274. doi: 10.1093/infdis/145.2.269. [DOI] [PubMed] [Google Scholar]
- 40.Price A B, Davies D R. Pseudomembraneous colitis. J Clin Pathol. 1977;30:1–12. doi: 10.1136/jcp.30.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Price A B, Larson H E, Crow J. Morphology of experimental antibiotic-associated enterocolitis in the hamster: a model for human pseudomembraneous colitis and antibiotic-associated diarrhoea. Gut. 1979;20:467–475. doi: 10.1136/gut.20.6.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Price S B, Phelps C J, Wilkins T D, Johnson J L. Cloning of the carbohydrate-binding portion of the toxin A gene of Clostridium difficile. Curr Microbiol. 1987;16:82–86. [Google Scholar]
- 43.Riegler M, Sedivy R, Pothoulakis C, Hamilton G, Zacherl J, Bischof G, Cosentini E, Feil W, Schiessel R, LaMont J T, et al. Clostridium difficile toxin B is more potent than toxin A in damaging human colonic epithelium in vitro. J Clin Investig. 1995;95:2004–2011. doi: 10.1172/JCI117885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ryan E T, Butterton J R, Smith R N, Carroll P A, Crean T I, Calderwood S B. Protective immunity against Clostridium difficile toxin A induced by oral immunization with a live, attenuated Vibrio cholerae vector strain. Infect Immun. 1997;65:2941–2949. doi: 10.1128/iai.65.7.2941-2949.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Simor A E, Yake S L, Tsimidis K. Infection due to Clostridium difficile among elderly residents of a long-term care facility. Clin Infect Dis. 1993;17:672–678. doi: 10.1093/clinids/17.4.672. [DOI] [PubMed] [Google Scholar]
- 46.Smith J A, Cooke D L, Hyde S, Borriello S P, Long R G. Clostridium difficile toxin A binding to human intestinal epithelial cells. J Med Microbiol. 1997;46:953–958. doi: 10.1099/00222615-46-11-953. [DOI] [PubMed] [Google Scholar]
- 47.Staats H F, Jackson R J, Marinaro M, Takahashi I, Kiyono H, McGhee J R. Mucosal immunity to infection with implications for vaccine development. Curr Opin Immunol. 1994;6:572–583. doi: 10.1016/0952-7915(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 48.Stanfield J P, Galazka A. Neonatal tetanus in the third world today. Bull W H O. 1984;62:647–669. [PMC free article] [PubMed] [Google Scholar]
- 49.Tacket C O, Sztein M B, Losonsky G A, Wasserman S S, Nataro J P, Edelman R, Pickard D, Dougan G, Chatfield S N, Levine M M. Safety of live oral Salmonella typhi vaccine strains with deletions in htrA and aroC aroD and immune response in humans. Infect Immun. 1997;65:452–456. doi: 10.1128/iai.65.2.452-456.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Torres J F, Lyerly D M, Hill J E, Monath T P. Evaluation of formalin-inactivated Clostridium difficile vaccines administered by parenteral and mucosal routes of immunization in hamsters. Infect Immun. 1995;63:4619–4627. doi: 10.1128/iai.63.12.4619-4627.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tucker K D, Carrig P E, Wilkins T D. Toxin A of Clostridium difficile is a potent cytotoxin. J Clin Microbiol. 1990;28:869–871. doi: 10.1128/jcm.28.5.869-871.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Turner S J, Carbone F R, Strugnell R A. Salmonella typhimurium aroA aroD mutants expressing a foreign recombinant protein induce specific major histocompatibility complex class I-restricted cytotoxic T lymphocytes in mice. Infect Immun. 1993;61:5374–5380. doi: 10.1128/iai.61.12.5374-5380.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ward S J, Douce G, Dougan G, Wren B W. Delivery of non-toxic fragments of Clostridium difficile toxin A to the mucosal immune system. Rev Med Microbiol. 1997;8:S34–S36. [Google Scholar]
- 54.Ward S J, Scopes D, Christodoulides M, Clarke I N, Heckels J E. Expression of Neisseria meningitidis class I porin as a fusion protein in Escherichia coli: the influence of liposomes and adjuvants on the production of a bactericidal immune response. Microb Pathog. 1996;21:499–512. doi: 10.1006/mpat.1996.0079. [DOI] [PubMed] [Google Scholar]
- 55.Wilcox M H, Spencer R C. Clostridium difficile infection: responses, relapses and re-infections. J Hosp Infect. 1992;22:85–92. doi: 10.1016/0195-6701(92)90092-z. [DOI] [PubMed] [Google Scholar]
- 56.Wren B W, Russell R R, Tabaqchali S. Antigenic cross-reactivity and functional inhibition by antibodies to Clostridium difficile toxin A, Streptococcus mutans glucan-binding protein, and a synthetic peptide. Infect Immun. 1991;59:3151–3155. doi: 10.1128/iai.59.9.3151-3155.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wren B W, Tabaqchali S. Restriction endonuclease DNA analysis of Clostridium difficile. J Clin Microbiol. 1987;25:2402–2404. doi: 10.1128/jcm.25.12.2402-2404.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]