Abstract
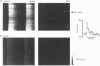
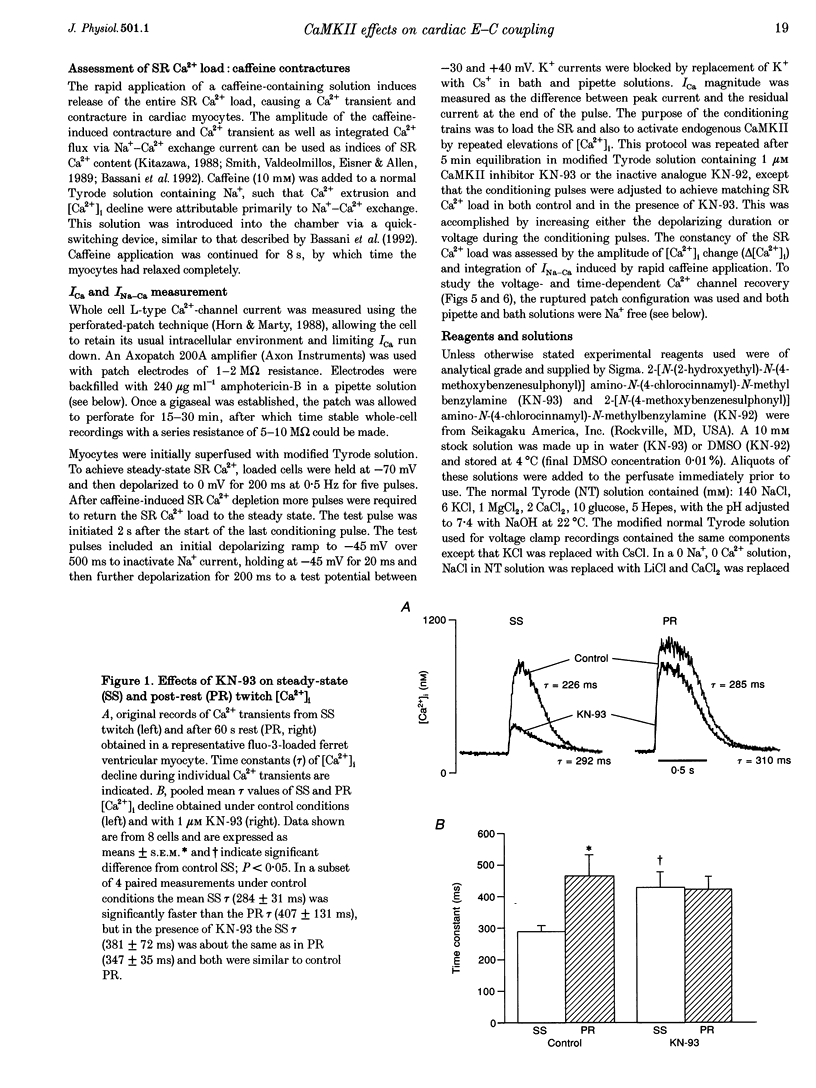
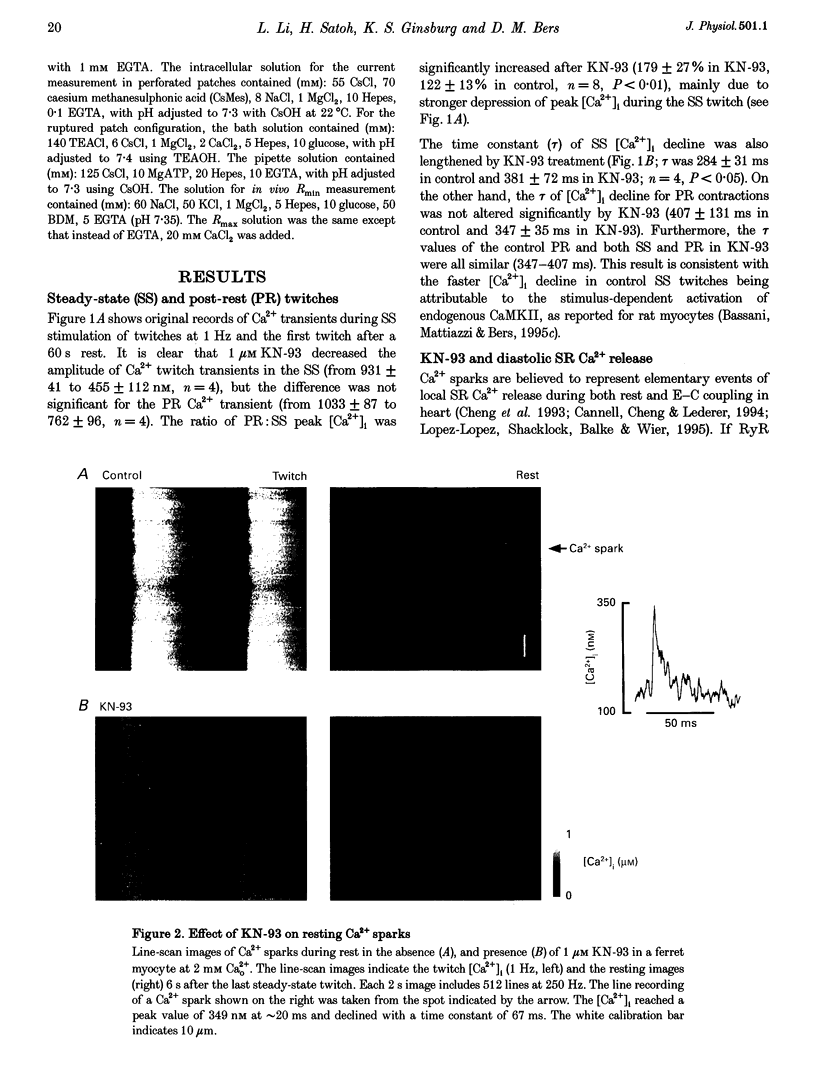
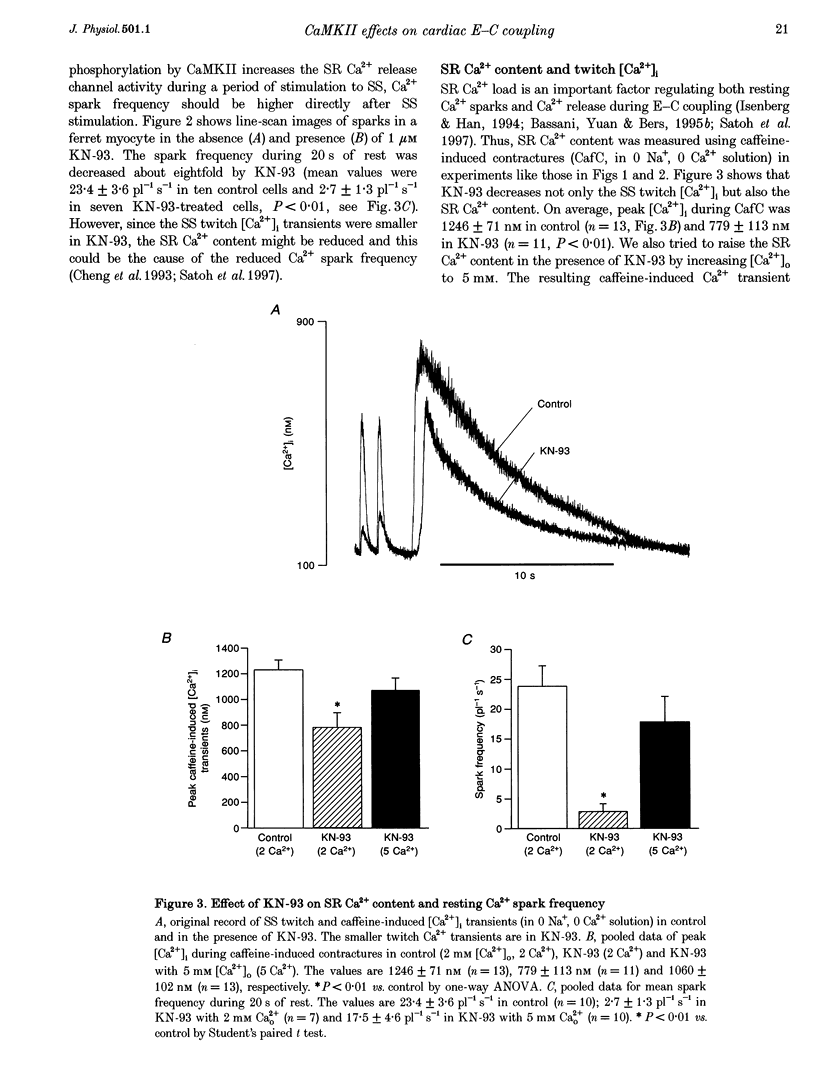
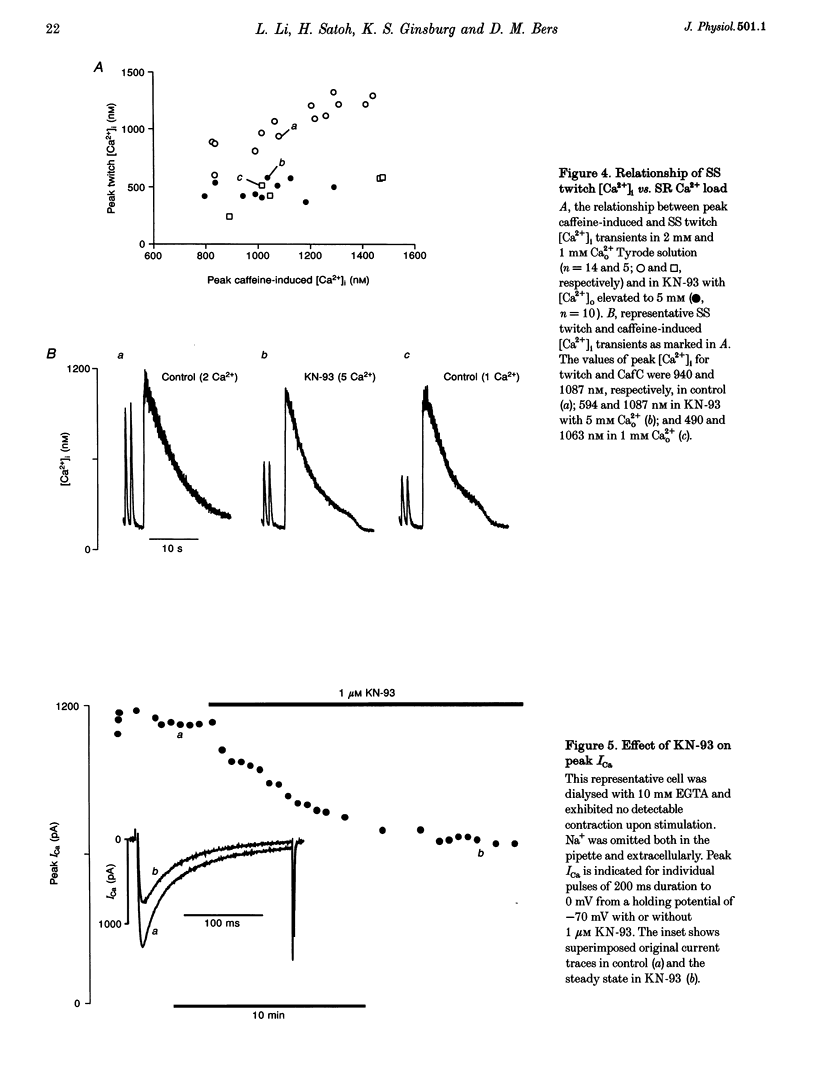
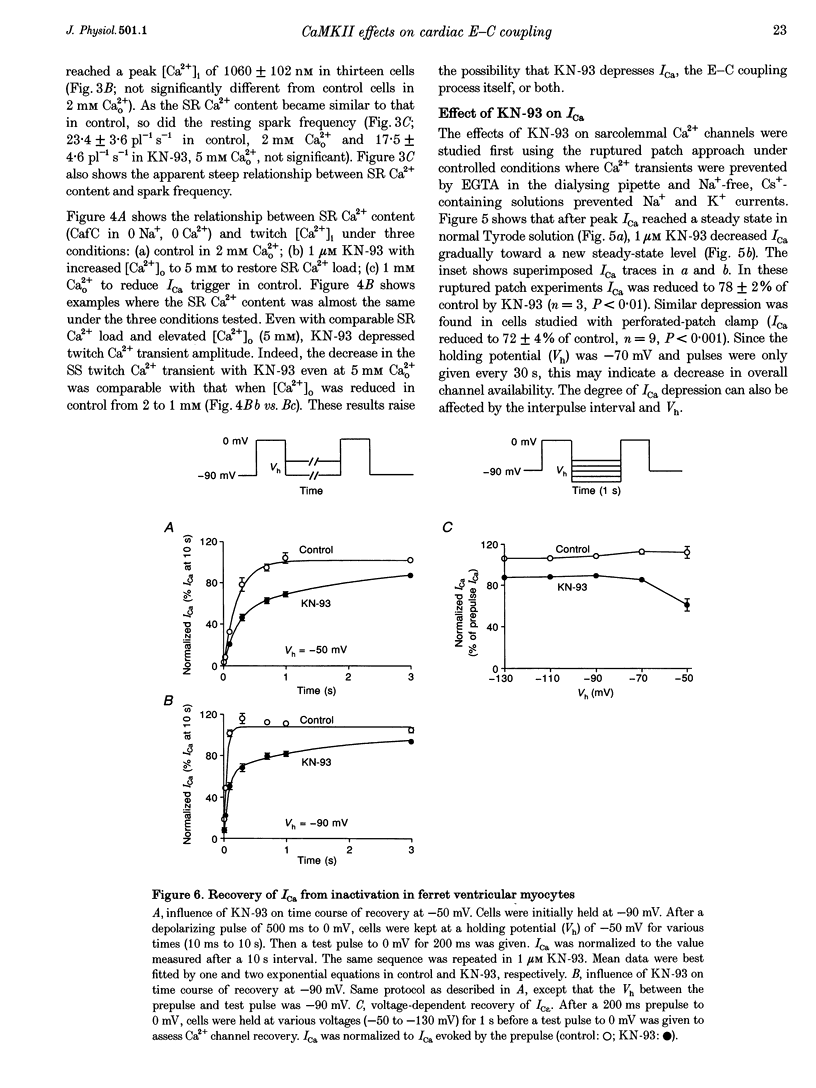
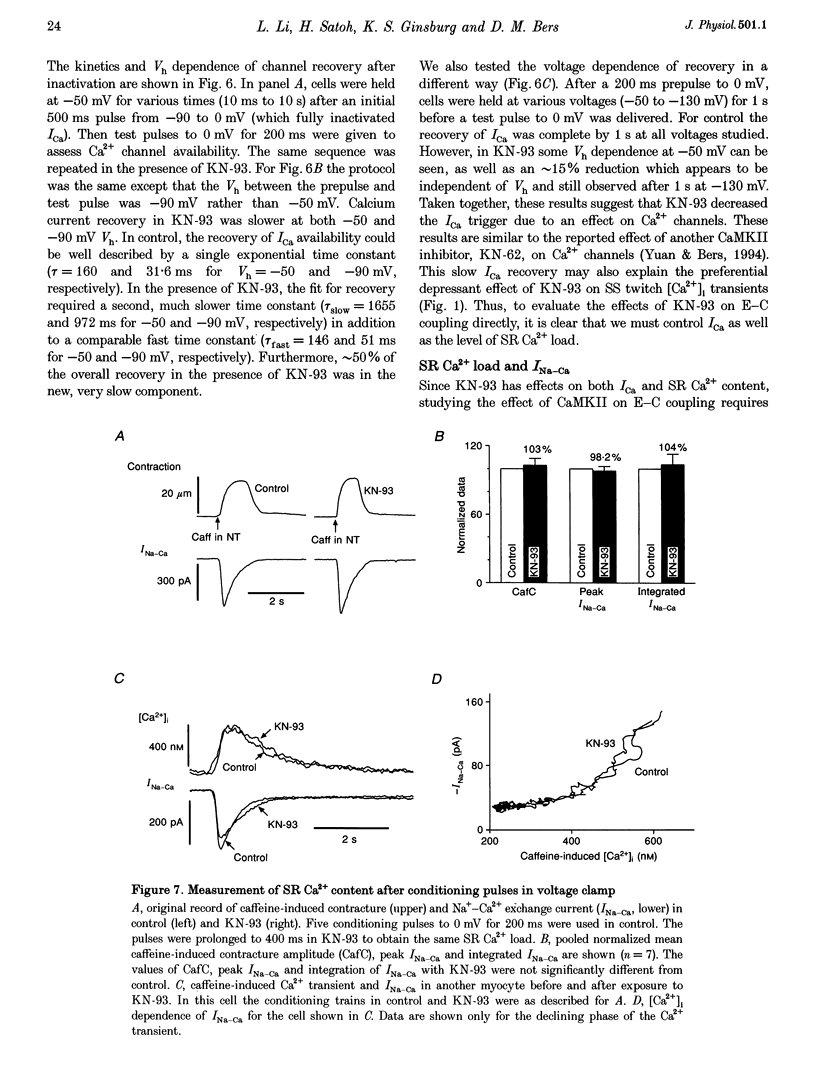
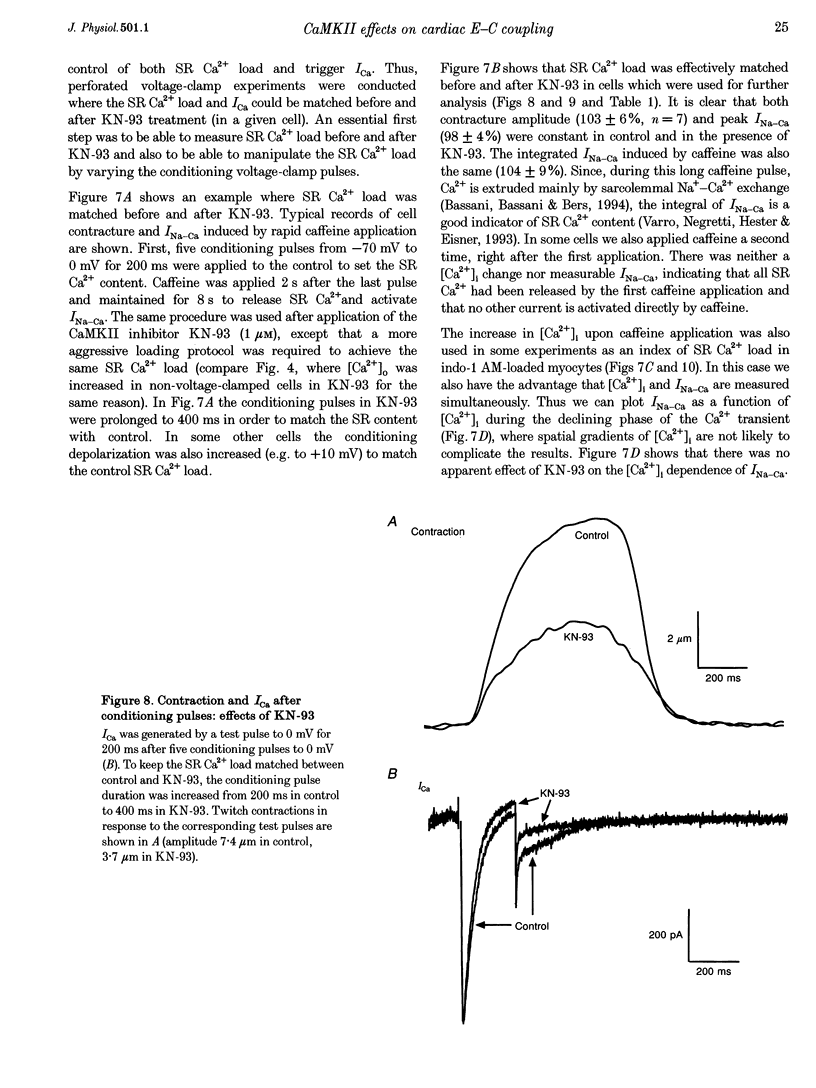
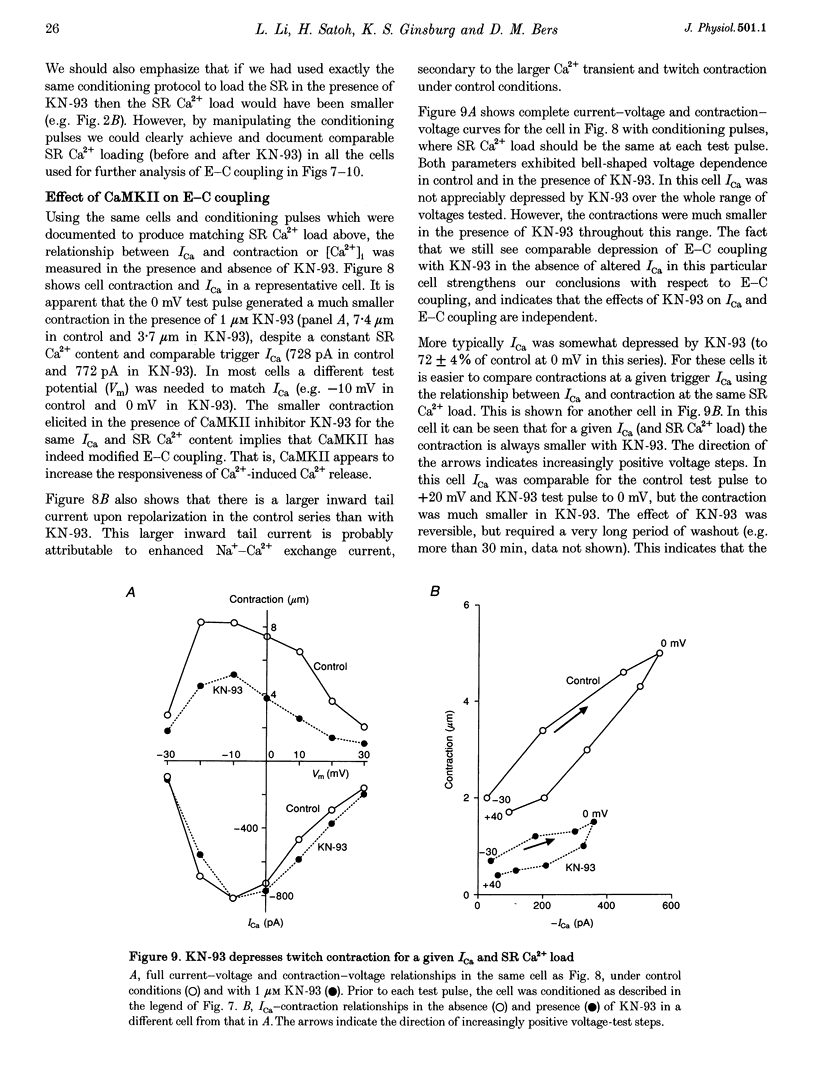
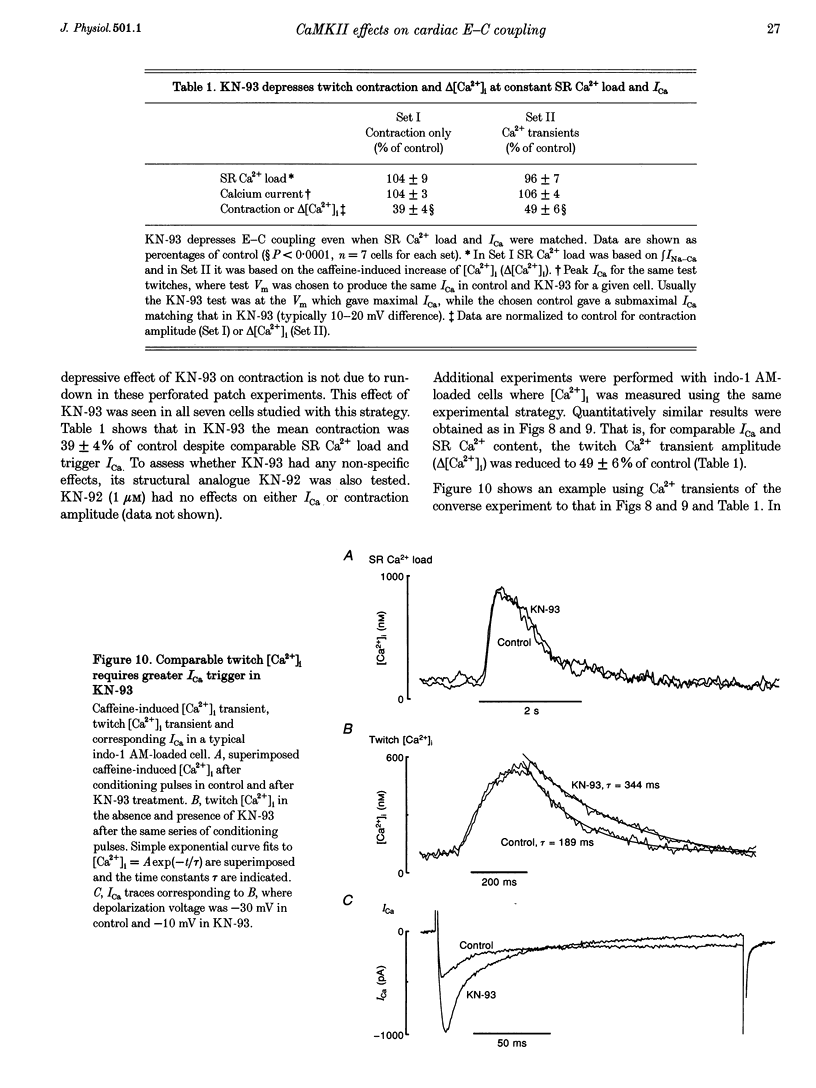
1. The effect of Ca(2+)-calmodulin-dependent protein kinase II (CaMKII) on excitation-contraction coupling (E-C coupling) was studied in intact ferret cardiac myocytes using the selective inhibitor KN-93, KN-93 decreased steady-state (SS) twitch [Ca2+]i (by 51%), resting Ca2+ spark frequency (by 88%) and SS sarcoplasmic reticulum (SR) Ca2+ content evaluated by eaffeine application (by 37.5%). 2. Increasing extracellular Ca2+ concentration ([Ca2+]o) to 5 mM in KN-93 restored SR Ca2+ load and Ca2+ spark frequency towards that in control (2 mM Ca2+o), but SS twitch [Ca2+]i was still significantly depressed by KN-93. 3. KN-93 decreased Ca2+ transient amplitude of SS twitches much more strongly than the amplitude of post-rest (PR) twitches. In the control, the time constant (Tau) of [Ca2+]i decline of SS twitches was faster than that for PR twitches. This stimulation-dependent acceleration of [Ca2+]i decline was abolished by KN-93. 4. Voltage-clamp experiments demonstrated that KN-93 significantly inhibited sarcolemmal L-type Ca2+ current (ICa) during repetitive pulses by slowing recovery from inactivation. This may explain the preferential action of KN-93 to suppress SS vs. PR twitches. 5. In KN-93, even when both ICa and SR Ca2+ load were matched to the control levels by manipulation of conditioning voltage-clamp pulses, contraction and twitch Ca2+ transients were still both significantly depressed (to 39 and 49% of control, respectively). 6. Since KN-93 reduced SR Ca2+ release channel (RyR) activity during E-C coupling, even for matched SR Ca2+ load and trigger ICa, we infer that endogenous CaMKII is an important modulator of E-C coupling in intact cardiac myocytes. Effects of KN-93 on ICa and SS twitch [Ca2+]i decline also indicate that endogenous CaMKII may have stimulatory effects on ICa and SR Ca2+ uptake.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson M. E., Braun A. P., Schulman H., Premack B. A. Multifunctional Ca2+/calmodulin-dependent protein kinase mediates Ca(2+)-induced enhancement of the L-type Ca2+ current in rabbit ventricular myocytes. Circ Res. 1994 Nov;75(5):854–861. doi: 10.1161/01.res.75.5.854. [DOI] [PubMed] [Google Scholar]
- Bassani J. W., Bassani R. A., Bers D. M. Calibration of indo-1 and resting intracellular [Ca]i in intact rabbit cardiac myocytes. Biophys J. 1995 Apr;68(4):1453–1460. doi: 10.1016/S0006-3495(95)80318-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassani J. W., Bassani R. A., Bers D. M. Relaxation in rabbit and rat cardiac cells: species-dependent differences in cellular mechanisms. J Physiol. 1994 Apr 15;476(2):279–293. doi: 10.1113/jphysiol.1994.sp020130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassani J. W., Yuan W., Bers D. M. Fractional SR Ca release is regulated by trigger Ca and SR Ca content in cardiac myocytes. Am J Physiol. 1995 May;268(5 Pt 1):C1313–C1319. doi: 10.1152/ajpcell.1995.268.5.C1313. [DOI] [PubMed] [Google Scholar]
- Bassani R. A., Bassani J. W., Bers D. M. Mitochondrial and sarcolemmal Ca2+ transport reduce [Ca2+]i during caffeine contractures in rabbit cardiac myocytes. J Physiol. 1992;453:591–608. doi: 10.1113/jphysiol.1992.sp019246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassani R. A., Mattiazzi A., Bers D. M. CaMKII is responsible for activity-dependent acceleration of relaxation in rat ventricular myocytes. Am J Physiol. 1995 Feb;268(2 Pt 2):H703–H712. doi: 10.1152/ajpheart.1995.268.2.H703. [DOI] [PubMed] [Google Scholar]
- Bers D. M., Berlin J. R. Kinetics of [Ca]i decline in cardiac myocytes depend on peak [Ca]i. Am J Physiol. 1995 Jan;268(1 Pt 1):C271–C277. doi: 10.1152/ajpcell.1995.268.1.C271. [DOI] [PubMed] [Google Scholar]
- Braun A. P., Schulman H. The multifunctional calcium/calmodulin-dependent protein kinase: from form to function. Annu Rev Physiol. 1995;57:417–445. doi: 10.1146/annurev.ph.57.030195.002221. [DOI] [PubMed] [Google Scholar]
- Brickey D. A., Bann J. G., Fong Y. L., Perrino L., Brennan R. G., Soderling T. R. Mutational analysis of the autoinhibitory domain of calmodulin kinase II. J Biol Chem. 1994 Nov 18;269(46):29047–29054. [PubMed] [Google Scholar]
- Cannell M. B., Cheng H., Lederer W. J. Spatial non-uniformities in [Ca2+]i during excitation-contraction coupling in cardiac myocytes. Biophys J. 1994 Nov;67(5):1942–1956. doi: 10.1016/S0006-3495(94)80677-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H., Lederer W. J., Cannell M. B. Calcium sparks: elementary events underlying excitation-contraction coupling in heart muscle. Science. 1993 Oct 29;262(5134):740–744. doi: 10.1126/science.8235594. [DOI] [PubMed] [Google Scholar]
- Coronado R., Morrissette J., Sukhareva M., Vaughan D. M. Structure and function of ryanodine receptors. Am J Physiol. 1994 Jun;266(6 Pt 1):C1485–C1504. doi: 10.1152/ajpcell.1994.266.6.C1485. [DOI] [PubMed] [Google Scholar]
- Edman C. F., Schulman H. Identification and characterization of delta B-CaM kinase and delta C-CaM kinase from rat heart, two new multifunctional Ca2+/calmodulin-dependent protein kinase isoforms. Biochim Biophys Acta. 1994 Mar 10;1221(1):89–101. doi: 10.1016/0167-4889(94)90221-6. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Györke S., Fill M. Ryanodine receptor adaptation: control mechanism of Ca(2+)-induced Ca2+ release in heart. Science. 1993 May 7;260(5109):807–809. doi: 10.1126/science.8387229. [DOI] [PubMed] [Google Scholar]
- Hain J., Onoue H., Mayrleitner M., Fleischer S., Schindler H. Phosphorylation modulates the function of the calcium release channel of sarcoplasmic reticulum from cardiac muscle. J Biol Chem. 1995 Feb 3;270(5):2074–2081. doi: 10.1074/jbc.270.5.2074. [DOI] [PubMed] [Google Scholar]
- Hanson P. I., Meyer T., Stryer L., Schulman H. Dual role of calmodulin in autophosphorylation of multifunctional CaM kinase may underlie decoding of calcium signals. Neuron. 1994 May;12(5):943–956. doi: 10.1016/0896-6273(94)90306-9. [DOI] [PubMed] [Google Scholar]
- Harkins A. B., Kurebayashi N., Baylor S. M. Resting myoplasmic free calcium in frog skeletal muscle fibers estimated with fluo-3. Biophys J. 1993 Aug;65(2):865–881. doi: 10.1016/S0006-3495(93)81112-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn R., Marty A. Muscarinic activation of ionic currents measured by a new whole-cell recording method. J Gen Physiol. 1988 Aug;92(2):145–159. doi: 10.1085/jgp.92.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hryshko L. V., Stiffel V., Bers D. M. Rapid cooling contractures as an index of sarcoplasmic reticulum calcium content in rabbit ventricular myocytes. Am J Physiol. 1989 Nov;257(5 Pt 2):H1369–H1377. doi: 10.1152/ajpheart.1989.257.5.H1369. [DOI] [PubMed] [Google Scholar]
- Isenberg G., Han S. Gradation of Ca(2+)-induced Ca2+ release by voltage-clamp pulse duration in potentiated guinea-pig ventricular myocytes. J Physiol. 1994 Nov 1;480(Pt 3):423–438. doi: 10.1113/jphysiol.1994.sp020372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy M. B., Greengard P. Two calcium/calmodulin-dependent protein kinases, which are highly concentrated in brain, phosphorylate protein I at distinct sites. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1293–1297. doi: 10.1073/pnas.78.2.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitazawa T. Caffeine contracture in guinea-pig ventricular muscle and the effect of extracellular sodium ions. J Physiol. 1988 Aug;402:703–729. doi: 10.1113/jphysiol.1988.sp017230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lokuta A. J., Rogers T. B., Lederer W. J., Valdivia H. H. Modulation of cardiac ryanodine receptors of swine and rabbit by a phosphorylation-dephosphorylation mechanism. J Physiol. 1995 Sep 15;487(Pt 3):609–622. doi: 10.1113/jphysiol.1995.sp020904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-López J. R., Shacklock P. S., Balke C. W., Wier W. G. Local calcium transients triggered by single L-type calcium channel currents in cardiac cells. Science. 1995 May 19;268(5213):1042–1045. doi: 10.1126/science.7754383. [DOI] [PubMed] [Google Scholar]
- Mamiya N., Goldenring J. R., Tsunoda Y., Modlin I. M., Yasui K., Usuda N., Ishikawa T., Natsume A., Hidaka H. Inhibition of acid secretion in gastric parietal cells by the Ca2+/calmodulin-dependent protein kinase II inhibitor KN-93. Biochem Biophys Res Commun. 1993 Sep 15;195(2):608–615. doi: 10.1006/bbrc.1993.2089. [DOI] [PubMed] [Google Scholar]
- Satoh H., Blatter L. A., Bers D. M. Effects of [Ca2+]i, SR Ca2+ load, and rest on Ca2+ spark frequency in ventricular myocytes. Am J Physiol. 1997 Feb;272(2 Pt 2):H657–H668. doi: 10.1152/ajpheart.1997.272.2.H657. [DOI] [PubMed] [Google Scholar]
- Schiefer A., Meissner G., Isenberg G. Ca2+ activation and Ca2+ inactivation of canine reconstituted cardiac sarcoplasmic reticulum Ca(2+)-release channels. J Physiol. 1995 Dec 1;489(Pt 2):337–348. doi: 10.1113/jphysiol.1995.sp021055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulman H., Hanson P. I., Meyer T. Decoding calcium signals by multifunctional CaM kinase. Cell Calcium. 1992 Jun-Jul;13(6-7):401–411. doi: 10.1016/0143-4160(92)90053-u. [DOI] [PubMed] [Google Scholar]
- Smith G. L., Valdeolmillos M., Eisner D. A., Allen D. G. Effects of rapid application of caffeine on intracellular calcium concentration in ferret papillary muscles. J Gen Physiol. 1988 Sep;92(3):351–368. doi: 10.1085/jgp.92.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. K., Colbran R. J., Brickey D. A., Soderling T. R. Functional determinants in the autoinhibitory domain of calcium/calmodulin-dependent protein kinase II. Role of His282 and multiple basic residues. J Biol Chem. 1992 Jan 25;267(3):1761–1768. [PubMed] [Google Scholar]
- Stern M. D. Theory of excitation-contraction coupling in cardiac muscle. Biophys J. 1992 Aug;63(2):497–517. doi: 10.1016/S0006-3495(92)81615-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumi M., Kiuchi K., Ishikawa T., Ishii A., Hagiwara M., Nagatsu T., Hidaka H. The newly synthesized selective Ca2+/calmodulin dependent protein kinase II inhibitor KN-93 reduces dopamine contents in PC12h cells. Biochem Biophys Res Commun. 1991 Dec 31;181(3):968–975. doi: 10.1016/0006-291x(91)92031-e. [DOI] [PubMed] [Google Scholar]
- Takasago T., Imagawa T., Furukawa K., Ogurusu T., Shigekawa M. Regulation of the cardiac ryanodine receptor by protein kinase-dependent phosphorylation. J Biochem. 1991 Jan;109(1):163–170. doi: 10.1093/oxfordjournals.jbchem.a123339. [DOI] [PubMed] [Google Scholar]
- Valdivia H. H., Kaplan J. H., Ellis-Davies G. C., Lederer W. J. Rapid adaptation of cardiac ryanodine receptors: modulation by Mg2+ and phosphorylation. Science. 1995 Mar 31;267(5206):1997–2000. doi: 10.1126/science.7701323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varro A., Negretti N., Hester S. B., Eisner D. A. An estimate of the calcium content of the sarcoplasmic reticulum in rat ventricular myocytes. Pflugers Arch. 1993 Apr;423(1-2):158–160. doi: 10.1007/BF00374975. [DOI] [PubMed] [Google Scholar]
- Wang J., Best P. M. Inactivation of the sarcoplasmic reticulum calcium channel by protein kinase. Nature. 1992 Oct 22;359(6397):739–741. doi: 10.1038/359739a0. [DOI] [PubMed] [Google Scholar]
- Wier W. G. Cytoplasmic [Ca2+] in mammalian ventricle: dynamic control by cellular processes. Annu Rev Physiol. 1990;52:467–485. doi: 10.1146/annurev.ph.52.030190.002343. [DOI] [PubMed] [Google Scholar]
- Witcher D. R., Kovacs R. J., Schulman H., Cefali D. C., Jones L. R. Unique phosphorylation site on the cardiac ryanodine receptor regulates calcium channel activity. J Biol Chem. 1991 Jun 15;266(17):11144–11152. [PubMed] [Google Scholar]
- Xiao R. P., Cheng H., Lederer W. J., Suzuki T., Lakatta E. G. Dual regulation of Ca2+/calmodulin-dependent kinase II activity by membrane voltage and by calcium influx. Proc Natl Acad Sci U S A. 1994 Sep 27;91(20):9659–9663. doi: 10.1073/pnas.91.20.9659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu A., Hawkins C., Narayanan N. Phosphorylation and activation of the Ca(2+)-pumping ATPase of cardiac sarcoplasmic reticulum by Ca2+/calmodulin-dependent protein kinase. J Biol Chem. 1993 Apr 25;268(12):8394–8397. [PubMed] [Google Scholar]
- Yuan W., Bers D. M. Ca-dependent facilitation of cardiac Ca current is due to Ca-calmodulin-dependent protein kinase. Am J Physiol. 1994 Sep;267(3 Pt 2):H982–H993. doi: 10.1152/ajpheart.1994.267.3.H982. [DOI] [PubMed] [Google Scholar]
- duBell W. H., Lederer W. J., Rogers T. B. Dynamic modulation of excitation-contraction coupling by protein phosphatases in rat ventricular myocytes. J Physiol. 1996 Jun 15;493(Pt 3):793–800. doi: 10.1113/jphysiol.1996.sp021423. [DOI] [PMC free article] [PubMed] [Google Scholar]