Abstract
1. Phorbol esters activate protein kinase C (PKC) and also increase the secretion of neurotransmitter substances by an unknown mechanism. To evaluate whether the stimulatory effects of such agents on acetylcholine (ACh) secretion occur as a consequence of stimulation of Ca2+ entry, we made electrophysiological measurements of ACh secretion (i.e. endplate potentials, EPPs) and the component of the prejunctional perineural voltage change associated with nerve terminal calcium currents (perineural calcium current) at frog neuromuscular junctions. 2. In the first series of experiments, modest concentrations of K+ channel blockers were employed so that simultaneous measurements of EPP amplitudes and perineural calcium currents could be made. In these experiments, 12-O-tetradecanoylphorbol 13-acetate (TPA; 162 nM) and phorbol 12,13-dibutyrate (PDBu; 100-200 nM) each increased ACh release but simultaneously decreased the calcium component of the prejunctional perineural current TPA and PDBu also inhibited perineural calcium currents in the presence of higher concentrations of K+ channel blockers. 3. Blockade of Ca2+ channels by Cd2+ prevented the action of PKC stimulators on perineural waveforms. 4. The inactive compound 4-alpha-phorbol 12-myristate 13-acetate (150 nM) did not affect EPP amplitudes or perineural currents. 5. The extracellular [Ca2+]-ACh release relationship was increased in maximum by PDBu without any change in the potency of Ca2+ to support evoked ACh release. 6. The results demonstrate that phorbol esters increase neurotransmitter secretion whilst simultaneously decreasing the nerve ending calcium currents that promote evoked release. The results, which suggest that the optimal control point for secretion might not be the calcium channel but rather a component of the secretory apparatus, are discussed in conjunction with the possible target sites for phorbol esters in the nerve ending.
Full text
PDF
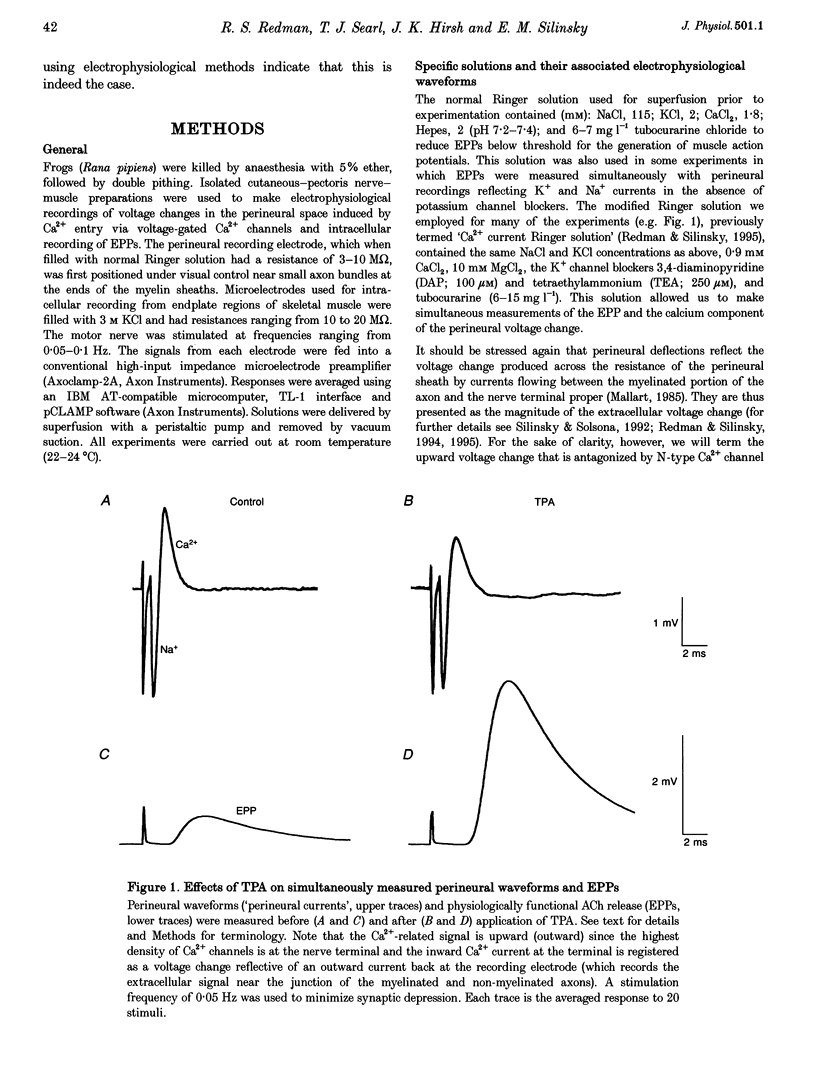
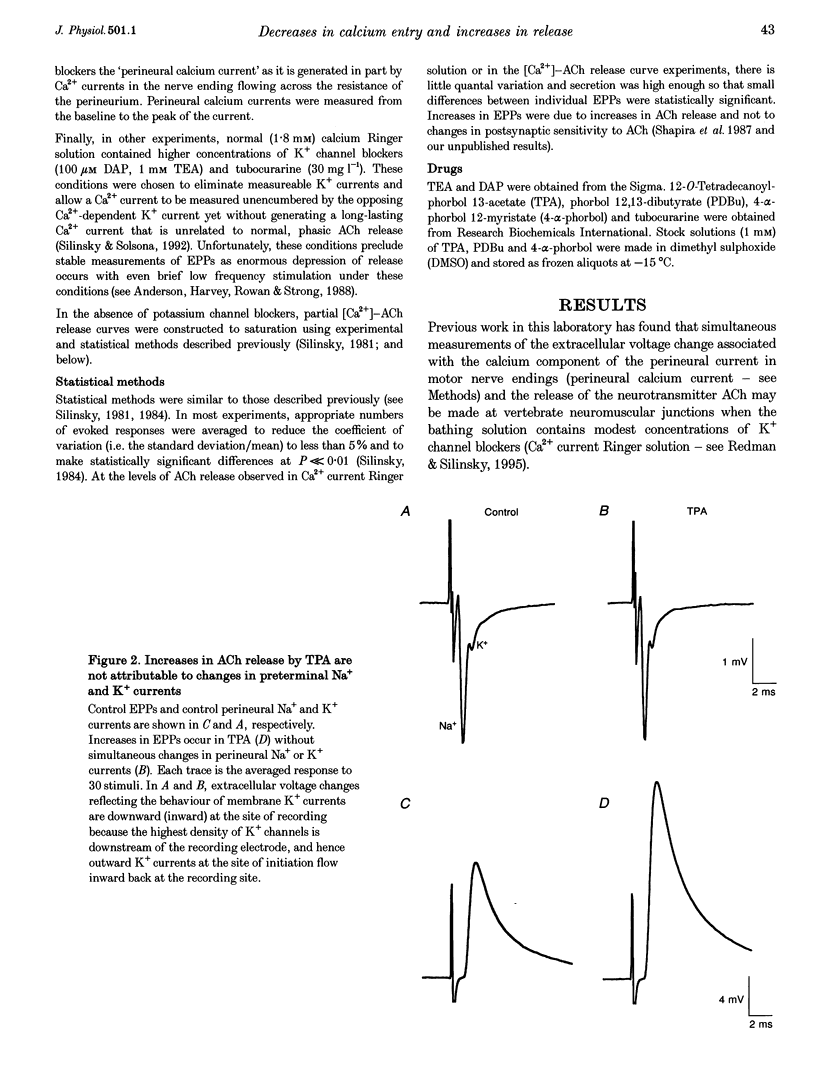
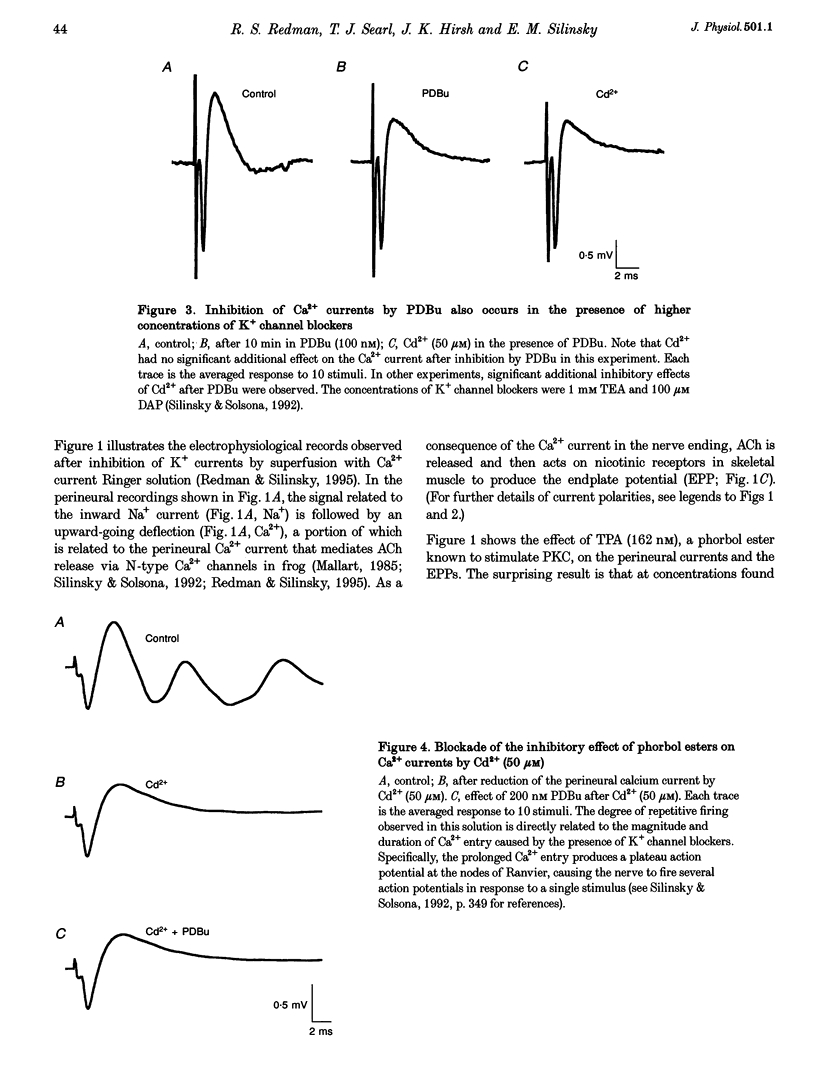




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed S., Maruyama I. N., Kozma R., Lee J., Brenner S., Lim L. The Caenorhabditis elegans unc-13 gene product is a phospholipid-dependent high-affinity phorbol ester receptor. Biochem J. 1992 Nov 1;287(Pt 3):995–999. doi: 10.1042/bj2870995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akers R. F., Lovinger D. M., Colley P. A., Linden D. J., Routtenberg A. Translocation of protein kinase C activity may mediate hippocampal long-term potentiation. Science. 1986 Feb 7;231(4738):587–589. doi: 10.1126/science.3003904. [DOI] [PubMed] [Google Scholar]
- Anderson A. J., Harvey A. L., Rowan E. G., Strong P. N. Effects of charybdotoxin, a blocker of Ca2+-activated K+ channels, on motor nerve terminals. Br J Pharmacol. 1988 Dec;95(4):1329–1335. doi: 10.1111/j.1476-5381.1988.tb11772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brose N., Hofmann K., Hata Y., Südhof T. C. Mammalian homologues of Caenorhabditis elegans unc-13 gene define novel family of C2-domain proteins. J Biol Chem. 1995 Oct 20;270(42):25273–25280. doi: 10.1074/jbc.270.42.25273. [DOI] [PubMed] [Google Scholar]
- Campenot R. B., Draker D. D., Senger D. L. Evidence that protein kinase C activities involved in regulating neurite growth are localized to distal neurites. J Neurochem. 1994 Sep;63(3):868–878. doi: 10.1046/j.1471-4159.1994.63030868.x. [DOI] [PubMed] [Google Scholar]
- Dekker L. V., De Graan P. N., Gispen W. H. Transmitter release: target of regulation by protein kinase C? Prog Brain Res. 1991;89:209–233. doi: 10.1016/s0079-6123(08)61724-0. [DOI] [PubMed] [Google Scholar]
- Doerner D., Pitler T. A., Alger B. E. Protein kinase C activators block specific calcium and potassium current components in isolated hippocampal neurons. J Neurosci. 1988 Nov;8(11):4069–4078. doi: 10.1523/JNEUROSCI.08-11-04069.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen J. S., Spruyt M. A., Brown A. M., Sahasrabudhe S. R., Blume A. J., Vitek M. P., Muenkel H. A., Sonnenberg-Reines J. The release of Alzheimer's disease beta amyloid peptide is reduced by phorbol treatment. J Biol Chem. 1994 Mar 18;269(11):8376–8382. [PubMed] [Google Scholar]
- Kazanietz M. G., Lewin N. E., Bruns J. D., Blumberg P. M. Characterization of the cysteine-rich region of the Caenorhabditis elegans protein Unc-13 as a high affinity phorbol ester receptor. Analysis of ligand-binding interactions, lipid cofactor requirements, and inhibitor sensitivity. J Biol Chem. 1995 May 5;270(18):10777–10783. doi: 10.1074/jbc.270.18.10777. [DOI] [PubMed] [Google Scholar]
- Klapstein G. J., Colmers W. F. 4-Aminopyridine and low Ca2+ differentiate presynaptic inhibition mediated by neuropeptide Y, baclofen and 2-chloroadenosine in rat hippocampal CA1 in vitro. Br J Pharmacol. 1992 Feb;105(2):470–474. doi: 10.1111/j.1476-5381.1992.tb14277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden D. J., Routtenberg A. cis-Fatty acids, which activate protein kinase C, attenuate Na+ and Ca2+ currents in mouse neuroblastoma cells. J Physiol. 1989 Dec;419:95–119. doi: 10.1113/jphysiol.1989.sp017863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallart A. A calcium-activated potassium current in motor nerve terminals of the mouse. J Physiol. 1985 Nov;368:577–591. doi: 10.1113/jphysiol.1985.sp015877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y. Studies and perspectives of protein kinase C. Science. 1986 Jul 18;233(4761):305–312. doi: 10.1126/science.3014651. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature. 1988 Aug 25;334(6184):661–665. doi: 10.1038/334661a0. [DOI] [PubMed] [Google Scholar]
- Rane S. G., Dunlap K. Kinase C activator 1,2-oleoylacetylglycerol attenuates voltage-dependent calcium current in sensory neurons. Proc Natl Acad Sci U S A. 1986 Jan;83(1):184–188. doi: 10.1073/pnas.83.1.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redman R. S., Silinsky E. M. ATP released together with acetylcholine as the mediator of neuromuscular depression at frog motor nerve endings. J Physiol. 1994 May 15;477(Pt 1):117–127. doi: 10.1113/jphysiol.1994.sp020176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redman R. S., Silinsky E. M. On the simultaneous electrophysiological measurements of neurotransmitter release and perineural calcium currents from frog motor nerve endings. J Neurosci Methods. 1995 Apr;57(2):151–159. doi: 10.1016/0165-0270(94)00133-2. [DOI] [PubMed] [Google Scholar]
- Scanziani M., Capogna M., Gähwiler B. H., Thompson S. M. Presynaptic inhibition of miniature excitatory synaptic currents by baclofen and adenosine in the hippocampus. Neuron. 1992 Nov;9(5):919–927. doi: 10.1016/0896-6273(92)90244-8. [DOI] [PubMed] [Google Scholar]
- Scholz K. P., Miller R. J. Inhibition of quantal transmitter release in the absence of calcium influx by a G protein-linked adenosine receptor at hippocampal synapses. Neuron. 1992 Jun;8(6):1139–1150. doi: 10.1016/0896-6273(92)90134-y. [DOI] [PubMed] [Google Scholar]
- Sebastião A. M., Ribeiro J. A. Interactions between adenosine and phorbol esters or lithium at the frog neuromuscular junction. Br J Pharmacol. 1990 May;100(1):55–62. doi: 10.1111/j.1476-5381.1990.tb12051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapira R., Silberberg S. D., Ginsburg S., Rahamimoff R. Activation of protein kinase C augments evoked transmitter release. Nature. 1987 Jan 1;325(6099):58–60. doi: 10.1038/325058a0. [DOI] [PubMed] [Google Scholar]
- Silinsky E. M. On the calcium receptor that mediates depolarization-secretion coupling at cholinergic motor nerve terminals. Br J Pharmacol. 1981 Jun;73(2):413–429. doi: 10.1111/j.1476-5381.1981.tb10438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silinsky E. M. On the mechanism by which adenosine receptor activation inhibits the release of acetylcholine from motor nerve endings. J Physiol. 1984 Jan;346:243–256. doi: 10.1113/jphysiol.1984.sp015019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silinsky E. M., Solsona C. S. Calcium currents at motor nerve endings: absence of effects of adenosine receptor agonists in the frog. J Physiol. 1992 Nov;457:315–328. doi: 10.1113/jphysiol.1992.sp019380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silinsky E. M. The biophysical pharmacology of calcium-dependent acetylcholine secretion. Pharmacol Rev. 1985 Mar;37(1):81–132. [PubMed] [Google Scholar]
- Wardell C. F., Cunnane T. C. Biochemical machinery involved in the release of ATP from sympathetic nerve terminals. Br J Pharmacol. 1994 Apr;111(4):975–977. doi: 10.1111/j.1476-5381.1994.tb14837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werz M. A., Macdonald R. L. Phorbol esters: voltage-dependent effects on calcium-dependent action potentials of mouse central and peripheral neurons in cell culture. J Neurosci. 1987 Jun;7(6):1639–1647. doi: 10.1523/JNEUROSCI.07-06-01639.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yawo H. Noradrenaline modulates transmitter release by enhancing the Ca2+ sensitivity of exocytosis in the chick ciliary presynaptic terminal. J Physiol. 1996 Jun 1;493(Pt 2):385–391. doi: 10.1113/jphysiol.1996.sp021390. [DOI] [PMC free article] [PubMed] [Google Scholar]


