Abstract
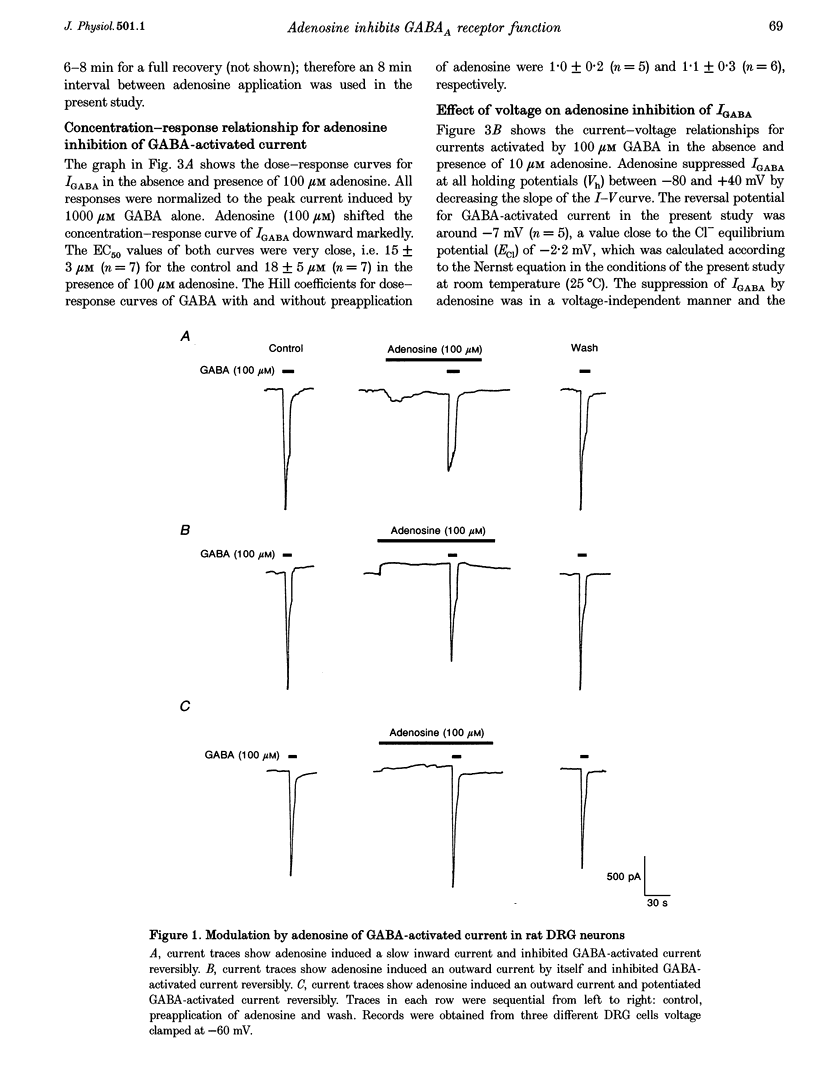
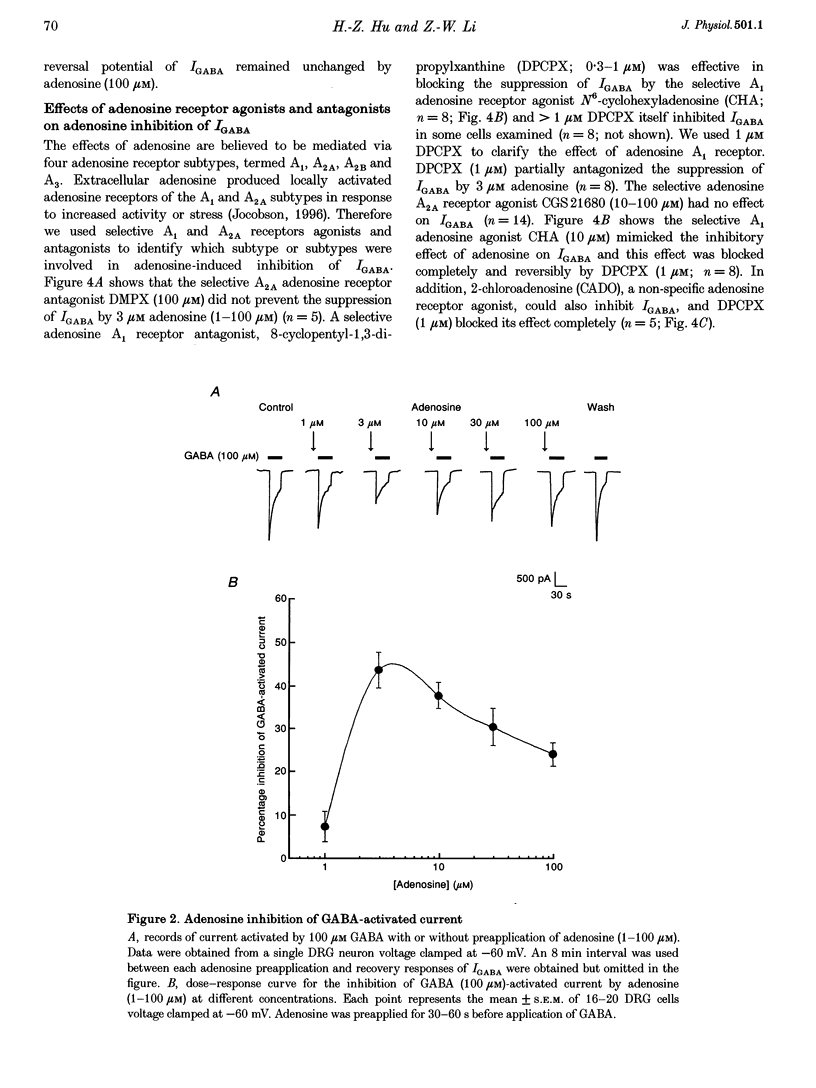
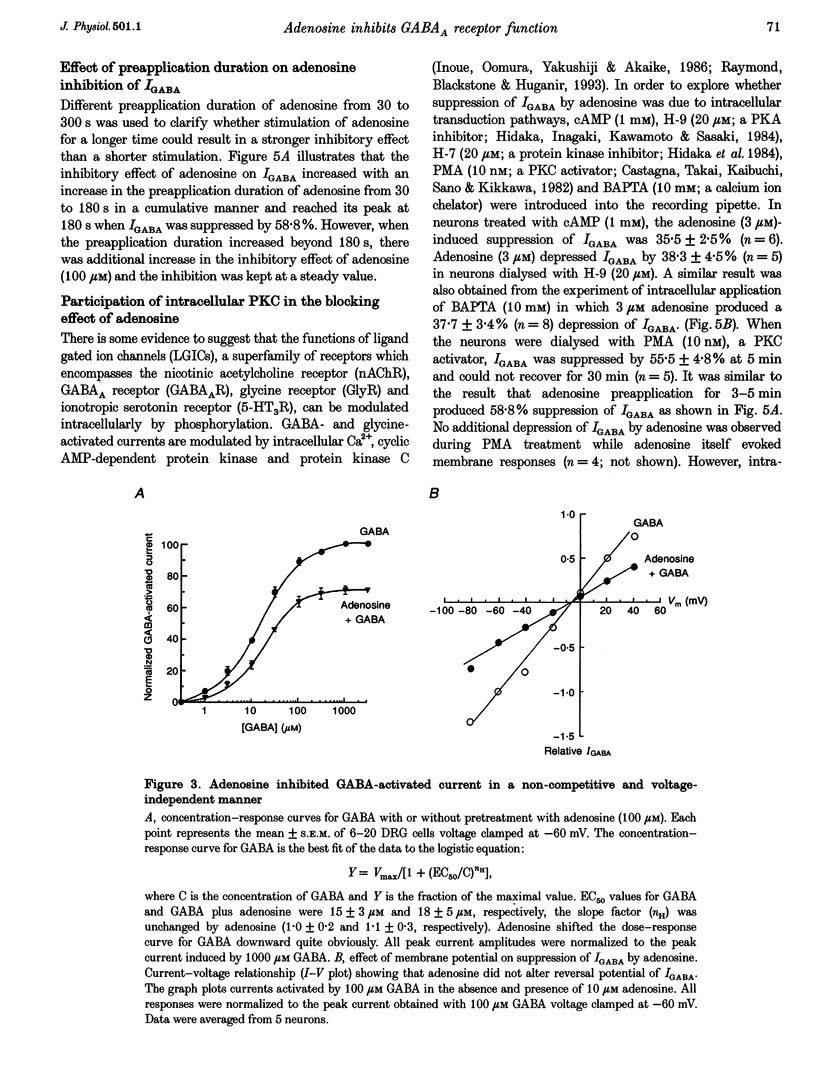
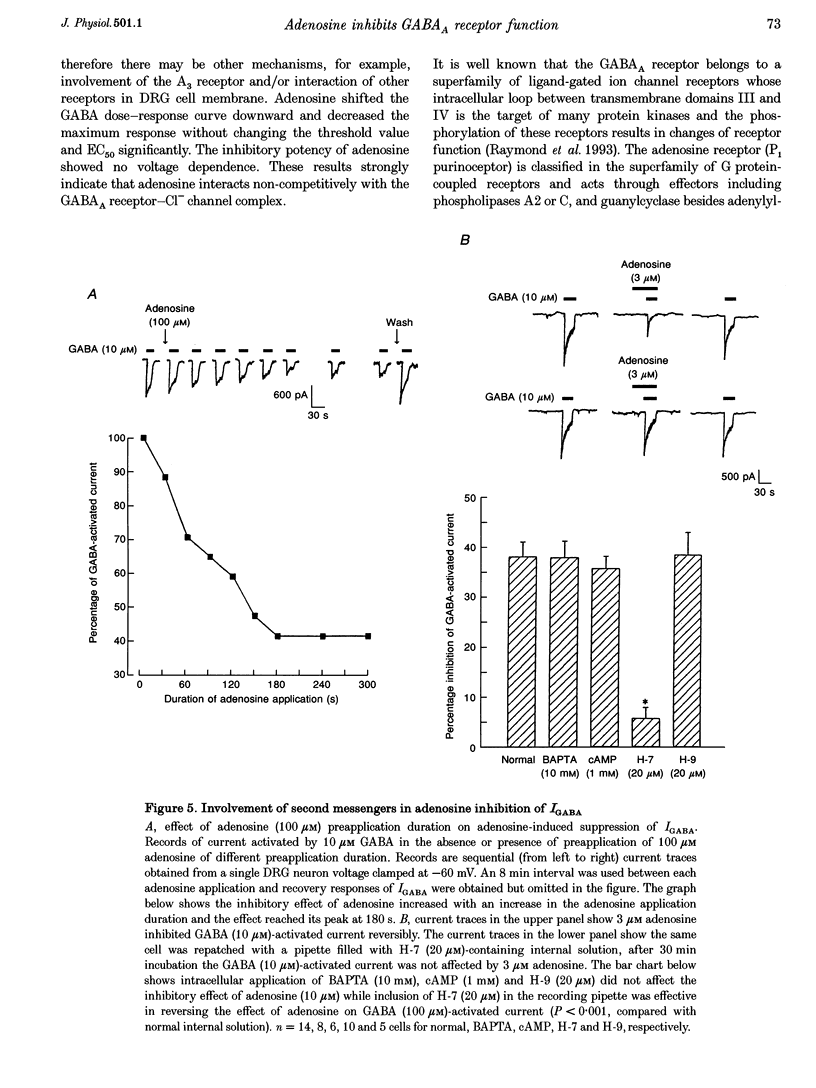
1. The modulation by adenosine of GABA-activated current (IGADA) was studied in freshly isolated rat dorsal root ganglion (DRG) neurons using the whole-cell patch-clamp technique. 2. In most of the DRG neurons examined (68/90, 75.5%) adenosine (1-10 microM) suppressed IGABA, while in some neurons examined, it potentiated (16/90, 17.8%) IGABA. It exerted no effects on IGABA in a few cells (6/90, 6.7%). 3. Adenosine shifted the GABA concentration-response curve downward with no significant change of the EC50. The maximal response to GABA was suppressed by 29.6 +/- 2.6%. The adenosine-induced inhibition of IGABA showed no voltage dependence. 4. 8-Cyclopentyl-1,3-dimethylxanthine (DPCPX; 1 microM), a selective A1 adenosine receptor antagonist, partially reversed adenosine inhibition of IGABA and completely blocked N6-cyclo-hexyladenosine (CHA; an A1 adenosine receptor agonist) inhibition of IGABA. DPCPX (1 microM) also blocked the suppression of IGABA by 2-chloroadenosine (CADO). CGS21680, a selective A2A adenosine receptor agonist, did not inhibit IGABA and DMPX, a selective A2A adenosine receptor antagonist, did not prevent adenosine inhibition of IGABA. 5. Intracellular application of H-7 (20 microM; a protein kinase C inhibitor) reversed adenosine inhibition of IGABA while inclusion of cAMP (1 mM), H-9 (20 microM; a protein kinase A inhibitor) and BAPTA (10 mM; a chelator of calcium ions) in the recording pipette did not affect the depression of IGABA by adenosine. IGABA was also suppressed by internal perfusion of PMA, a protein kinase C activator. 6. The results suggest that adenosine, as a neuromodulator, exerts a modulatory effect on the GABA-induced presynaptic inhibition in primary sensory transmission.
Full text
PDF

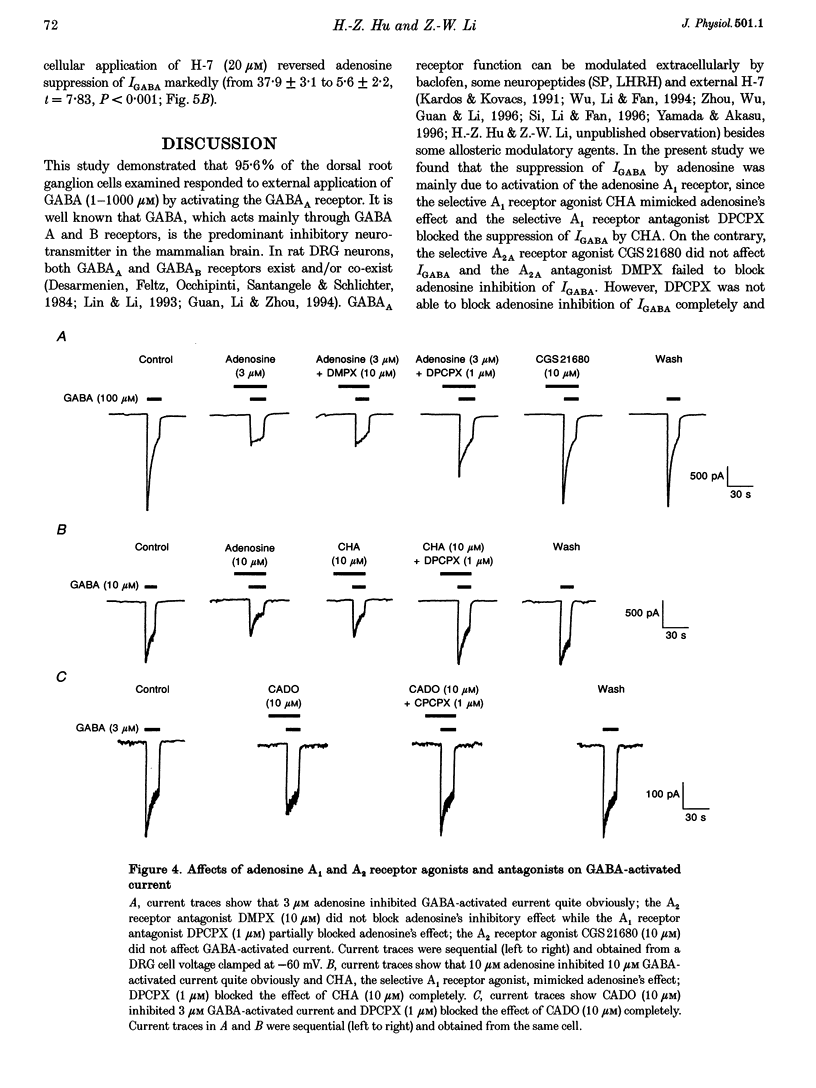


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akhondzadeh S., Stone T. W. Interaction between adenosine and GABAA receptors on hippocampal neurones. Brain Res. 1994 Dec 5;665(2):229–236. doi: 10.1016/0006-8993(94)91342-0. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Overview. Purinergic mechanisms. Ann N Y Acad Sci. 1990;603:1–18. doi: 10.1111/j.1749-6632.1990.tb37657.x. [DOI] [PubMed] [Google Scholar]
- Castagna M., Takai Y., Kaibuchi K., Sano K., Kikkawa U., Nishizuka Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem. 1982 Jul 10;257(13):7847–7851. [PubMed] [Google Scholar]
- Christofi F. L., McDonald T. J., Cook M. A. Adenosine receptors are coupled negatively to release of tachykinin(s) from enteric nerve endings. J Pharmacol Exp Ther. 1990 Apr;253(1):290–295. [PubMed] [Google Scholar]
- DeLander G. E., Wahl J. J. Behavior induced by putative nociceptive neurotransmitters is inhibited by adenosine or adenosine analogs coadministered intrathecally. J Pharmacol Exp Ther. 1988 Aug;246(2):565–570. [PubMed] [Google Scholar]
- Doi T., Kuzuna S., Maki Y. Spinal antinociceptive effects of adenosine compounds in mice. Eur J Pharmacol. 1987 Jun 4;137(2-3):227–231. doi: 10.1016/0014-2999(87)90226-3. [DOI] [PubMed] [Google Scholar]
- Dolphin A. C., Prestwich S. A. Pertussis toxin reverses adenosine inhibition of neuronal glutamate release. Nature. 1985 Jul 11;316(6024):148–150. doi: 10.1038/316148a0. [DOI] [PubMed] [Google Scholar]
- Désarmenien M., Feltz P., Occhipinti G., Santangelo F., Schlichter R. Coexistence of GABAA and GABAB receptors on A delta and C primary afferents. Br J Pharmacol. 1984 Feb;81(2):327–333. doi: 10.1111/j.1476-5381.1984.tb10082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredholm B. B., Abbracchio M. P., Burnstock G., Daly J. W., Harden T. K., Jacobson K. A., Leff P., Williams M. Nomenclature and classification of purinoceptors. Pharmacol Rev. 1994 Jun;46(2):143–156. [PMC free article] [PubMed] [Google Scholar]
- Hidaka H., Inagaki M., Kawamoto S., Sasaki Y. Isoquinolinesulfonamides, novel and potent inhibitors of cyclic nucleotide dependent protein kinase and protein kinase C. Biochemistry. 1984 Oct 9;23(21):5036–5041. doi: 10.1021/bi00316a032. [DOI] [PubMed] [Google Scholar]
- Jahr C. E., Jessell T. M. ATP excites a subpopulation of rat dorsal horn neurones. Nature. 1983 Aug 25;304(5928):730–733. doi: 10.1038/304730a0. [DOI] [PubMed] [Google Scholar]
- Kardos J., Kovacs I. Binding interaction of gamma aminobutyric acid A and B receptors in cell culture. Neuroreport. 1991 Sep;2(9):541–543. doi: 10.1097/00001756-199109000-00011. [DOI] [PubMed] [Google Scholar]
- Klishin A., Lozovaya N., Krishtal O. A1 adenosine receptors differentially regulate the N-methyl-D-aspartate and non-N-methyl-D-aspartate receptor-mediated components of hippocampal excitatory postsynaptic current in a Ca2+/Mg(2+)-dependent manner. Neuroscience. 1995 Apr;65(4):947–953. doi: 10.1016/0306-4522(94)00518-a. [DOI] [PubMed] [Google Scholar]
- Krishtal O. A., Marchenko S. M., Pidoplichko V. I. Receptor for ATP in the membrane of mammalian sensory neurones. Neurosci Lett. 1983 Jan 31;35(1):41–45. doi: 10.1016/0304-3940(83)90524-4. [DOI] [PubMed] [Google Scholar]
- Li C., Peoples R. W., Li Z., Weight F. F. Zn2+ potentiates excitatory action of ATP on mammalian neurons. Proc Natl Acad Sci U S A. 1993 Sep 1;90(17):8264–8267. doi: 10.1073/pnas.90.17.8264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Z. W., Li Z. W. [Responses mediated by GABAA and GABAB receptors on the somatic membrane of toad dorsal root ganglion neurones]. Sheng Li Xue Bao. 1993 Apr;45(2):117–123. [PubMed] [Google Scholar]
- MacDonald R. L., Skerritt J. H., Werz M. A. Adenosine agonists reduce voltage-dependent calcium conductance of mouse sensory neurones in cell culture. J Physiol. 1986 Jan;370:75–90. doi: 10.1113/jphysiol.1986.sp015923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayfield R. D., Suzuki F., Zahniser N. R. Adenosine A2a receptor modulation of electrically evoked endogenous GABA release from slices of rat globus pallidus. J Neurochem. 1993 Jun;60(6):2334–2337. doi: 10.1111/j.1471-4159.1993.tb03526.x. [DOI] [PubMed] [Google Scholar]
- Nagy J. I., Buss M., LaBella L. A., Daddona P. E. Immunohistochemical localization of adenosine deaminase in primary afferent neurons of the rat. Neurosci Lett. 1984 Jul 27;48(2):133–138. doi: 10.1016/0304-3940(84)90008-9. [DOI] [PubMed] [Google Scholar]
- Otsuka M., Yanagisawa M. Pain and neurotransmitters. Cell Mol Neurobiol. 1990 Sep;10(3):293–302. doi: 10.1007/BF00711176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petcoff D. W., Cooper D. M. Adenosine receptor agonists inhibit inositol phosphate accumulation in rat striatal slices. Eur J Pharmacol. 1987 Jun 4;137(2-3):269–271. doi: 10.1016/0014-2999(87)90234-2. [DOI] [PubMed] [Google Scholar]
- Raymond L. A., Blackstone C. D., Huganir R. L. Phosphorylation of amino acid neurotransmitter receptors in synaptic plasticity. Trends Neurosci. 1993 Apr;16(4):147–153. doi: 10.1016/0166-2236(93)90123-4. [DOI] [PubMed] [Google Scholar]
- Redman R. S., Silinsky E. M. A selective adenosine antagonist (8-cyclopentyl-1,3-dipropylxanthine) eliminates both neuromuscular depression and the action of exogenous adenosine by an effect on A1 receptors. Mol Pharmacol. 1993 Oct;44(4):835–840. [PubMed] [Google Scholar]
- Sawynok J., Reid A., Isbrucker R. Adenosine mediates calcium-induced antinociception and potentiation of noradrenergic antinociception in the spinal cord. Brain Res. 1990 Aug 6;524(2):187–195. doi: 10.1016/0006-8993(90)90689-9. [DOI] [PubMed] [Google Scholar]
- Sebastião A. M., Ribeiro J. A. Adenosine A2 receptor-mediated excitatory actions on the nervous system. Prog Neurobiol. 1996 Feb;48(3):167–189. doi: 10.1016/0301-0082(95)00035-6. [DOI] [PubMed] [Google Scholar]
- Sebastião A. M., Ribeiro J. A. Interactions between adenosine and phorbol esters or lithium at the frog neuromuscular junction. Br J Pharmacol. 1990 May;100(1):55–62. doi: 10.1111/j.1476-5381.1990.tb12051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams M. Purine nucleosides and nucleotides as central nervous system modulators. Adenosine as the prototypic paracrine neuroactive substance. Ann N Y Acad Sci. 1990;603:93–107. doi: 10.1111/j.1749-6632.1990.tb37664.x. [DOI] [PubMed] [Google Scholar]
- Yamada K., Akasu T. Substance P suppresses GABAA receptor function via protein kinase C in primary sensory neurones of bullfrogs. J Physiol. 1996 Oct 15;496(Pt 2):439–449. doi: 10.1113/jphysiol.1996.sp021697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon K. W., Rothman S. M. Adenosine inhibits excitatory but not inhibitory synaptic transmission in the hippocampus. J Neurosci. 1991 May;11(5):1375–1380. doi: 10.1523/JNEUROSCI.11-05-01375.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X. P., Wu X. P., Guan B. C., Li Z. W. Modulatory effects of gonadorelin on GABA-induced depolarization and GABA-activated current in rat spinal ganglion neurons. Zhongguo Yao Li Xue Bao. 1996 Jan;17(1):31–34. [PubMed] [Google Scholar]
- Zhu X., Zhu H., Bao Y. D. [Glutamate receptor and GABA receptor expressed in amphibian oocytes after injection of chicken retina mRNA]. Sheng Li Xue Bao. 1994 Oct;46(5):417–426. [PubMed] [Google Scholar]
- de Mendonça A., Sebastião A. M., Ribeiro J. A. Inhibition of NMDA receptor-mediated currents in isolated rat hippocampal neurones by adenosine A1 receptor activation. Neuroreport. 1995 May 30;6(8):1097–1100. doi: 10.1097/00001756-199505300-00006. [DOI] [PubMed] [Google Scholar]


