Abstract
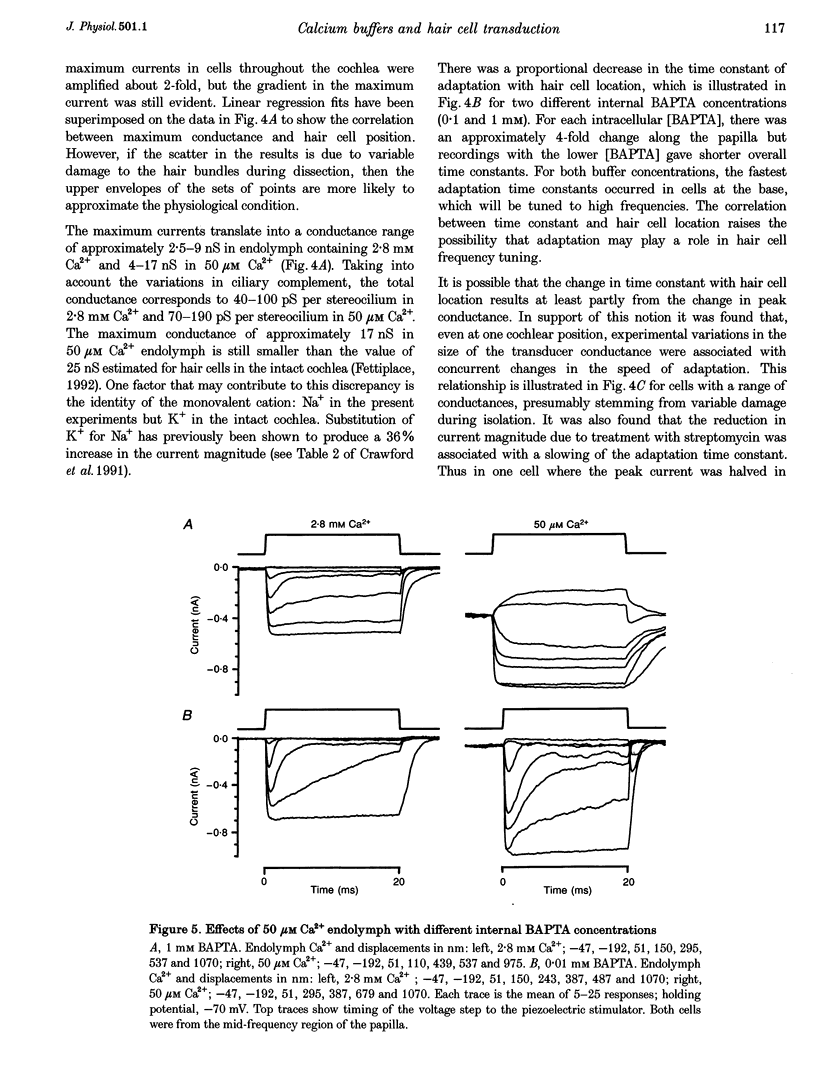
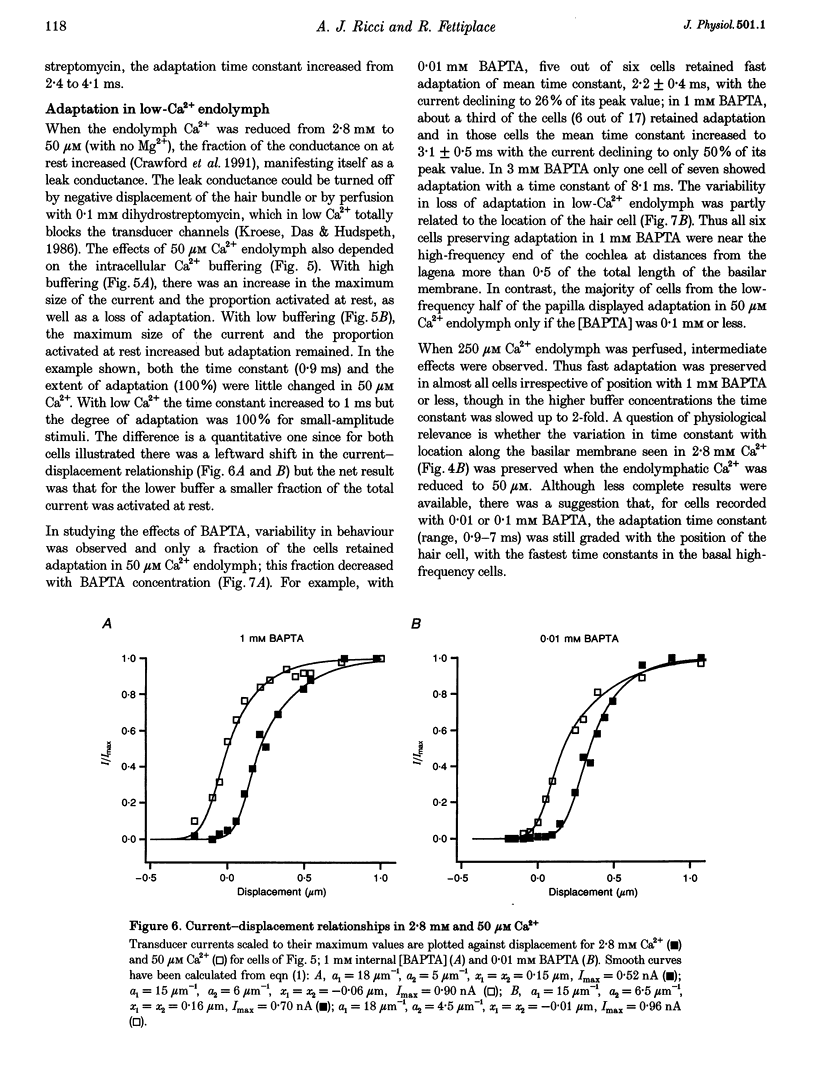
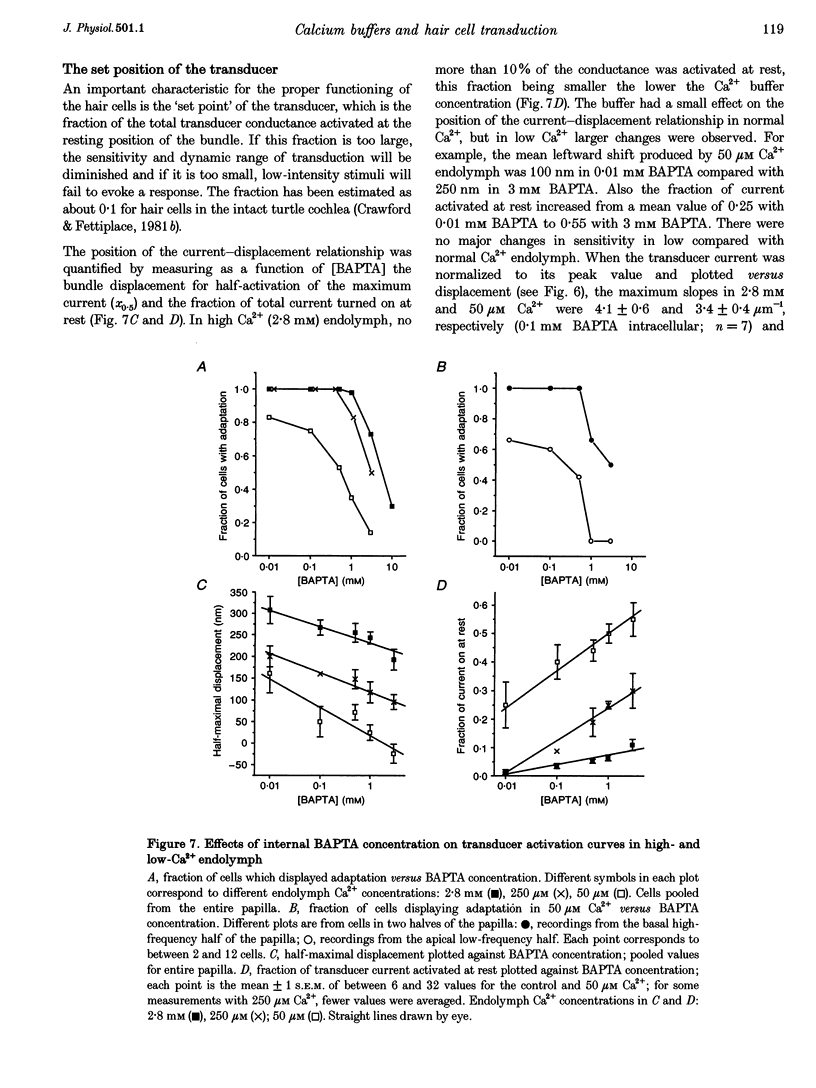
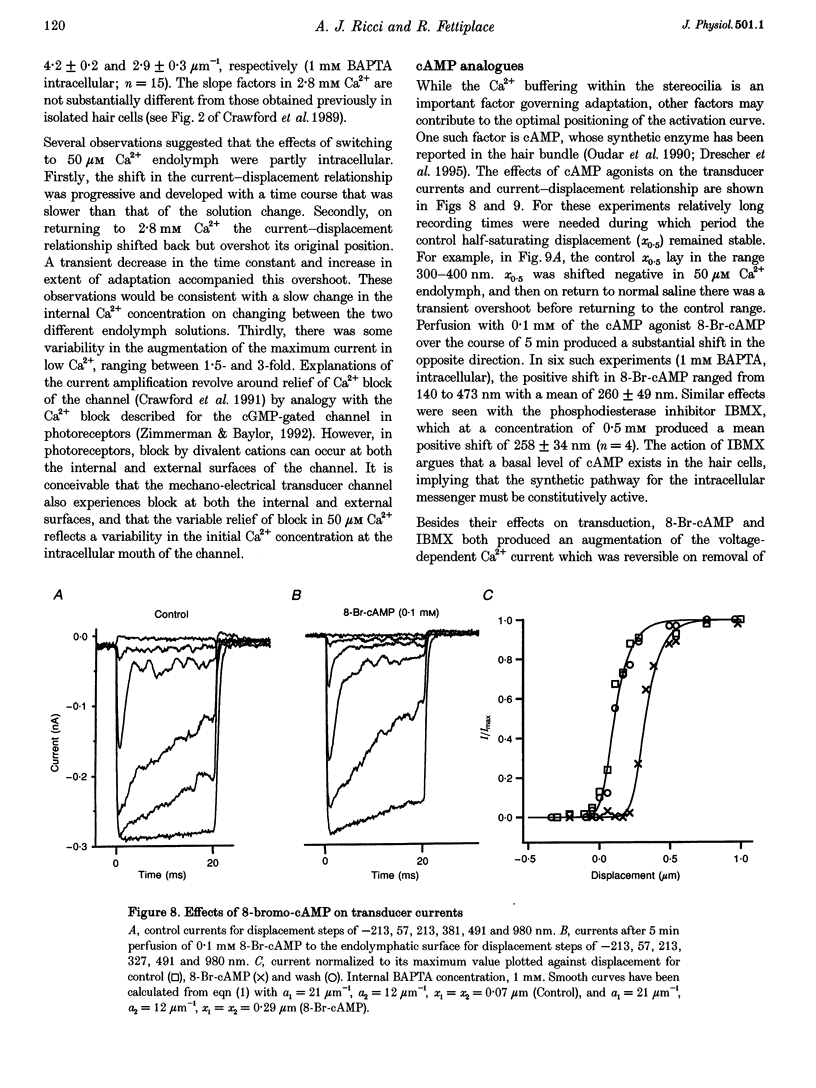
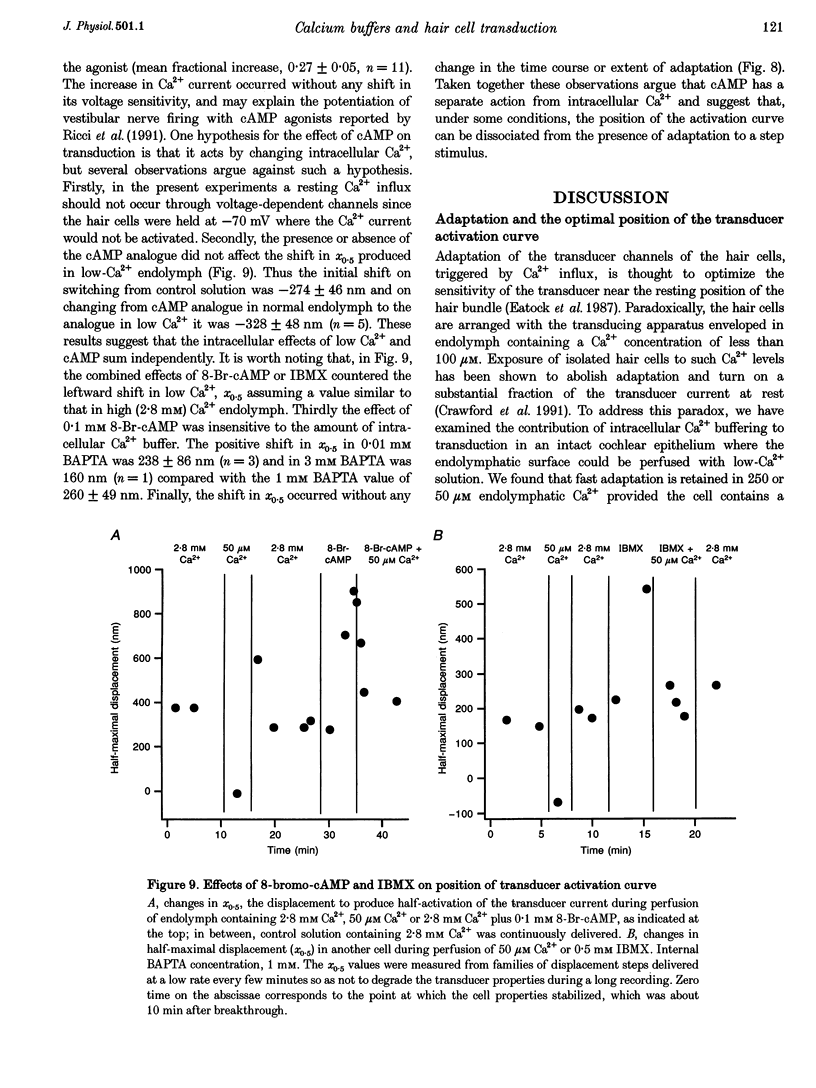
1. The effects of intracellular Ca2+ buffering on hair cell mechanotransduction were studied in an intact cochlear epithelium where the endolymphatic and perilymphatic surfaces could be separately perfused with different Ca2+ solutions. 2. The speed and extent of transducer adaptation increased as the concentration in the patch electrode of the Ca2+ buffer BAPTA was lowered. In 0.1 mM BAPTA or less, the transducer adapted almost completely, with a mean time constant of 0.8 ms. 3. For a fixed internal BAPTA concentration, the transducer conductance varied with hair cell location, increasing towards the high-frequency end of the cochlea, and the time constant of adaptation decreased proportionally. At a given cochlear location, hair cells with larger transducer conductances displayed faster adaptation. We suggest that transducer adaptation accounts for a variable high-pass filter observed in the acoustic tuning curve. 4. The effects of perfusion of 50 microM Ca2+ endolymph depended on the BAPTA concentration of the electrode: with 3 mM BAPTA, adaptation was abolished, but in most recordings with 0.01 or 0.1 mM BAPTA, rapid adaptation was retained. The current-displacement curve was also shifted less the lower the intracellular BAPTA concentration. Cells in the high-frequency half of the papilla retained adaptation at a higher BAPTA concentration. 5. Treatment with the cAMP agonist, 8-bromo-cAMP, or with the phosphodiesterase inhibitor 3-isobutyl-1-methylxanthine, caused a rightward shift in the current-displacement curve which was independent of the internal BAPTA concentration. 6. We conclude that the free Ca2+ and cyclic nucleotide concentrations of the hair bundle modulate the position of the activation curve of the transducer. The factors which may be important for the correct functioning of adaptation in vivo are discussed.
Full text
PDF

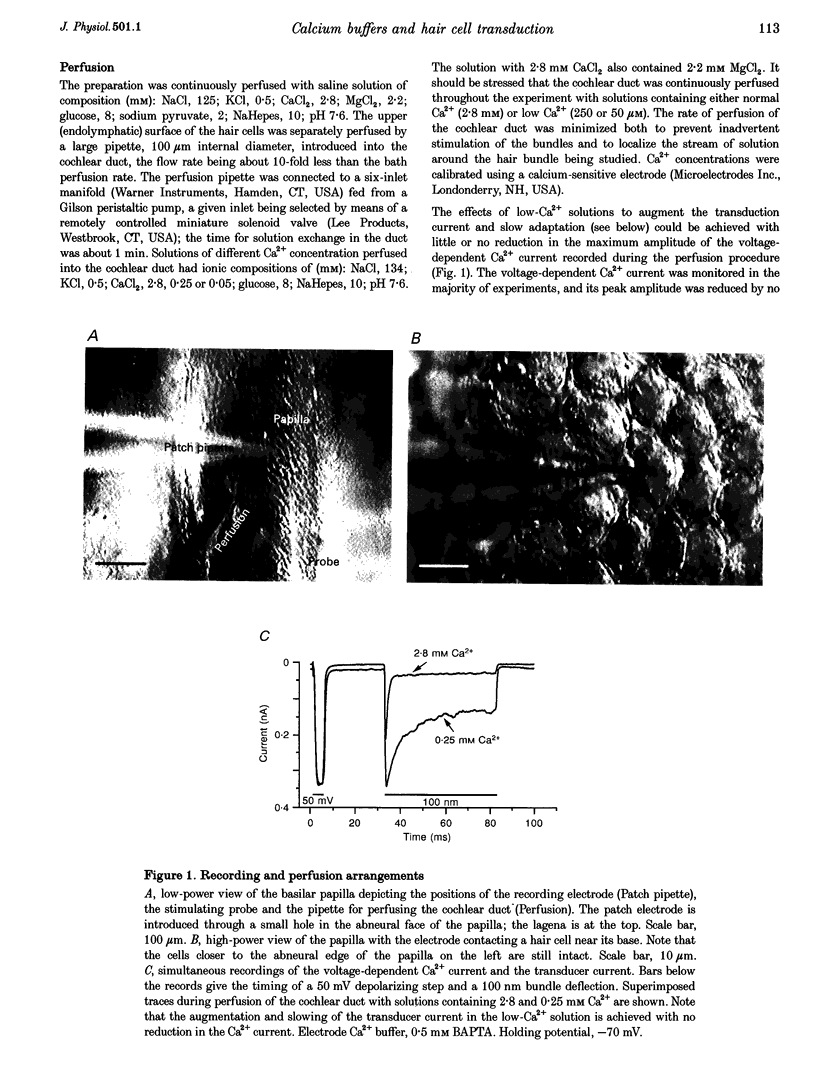
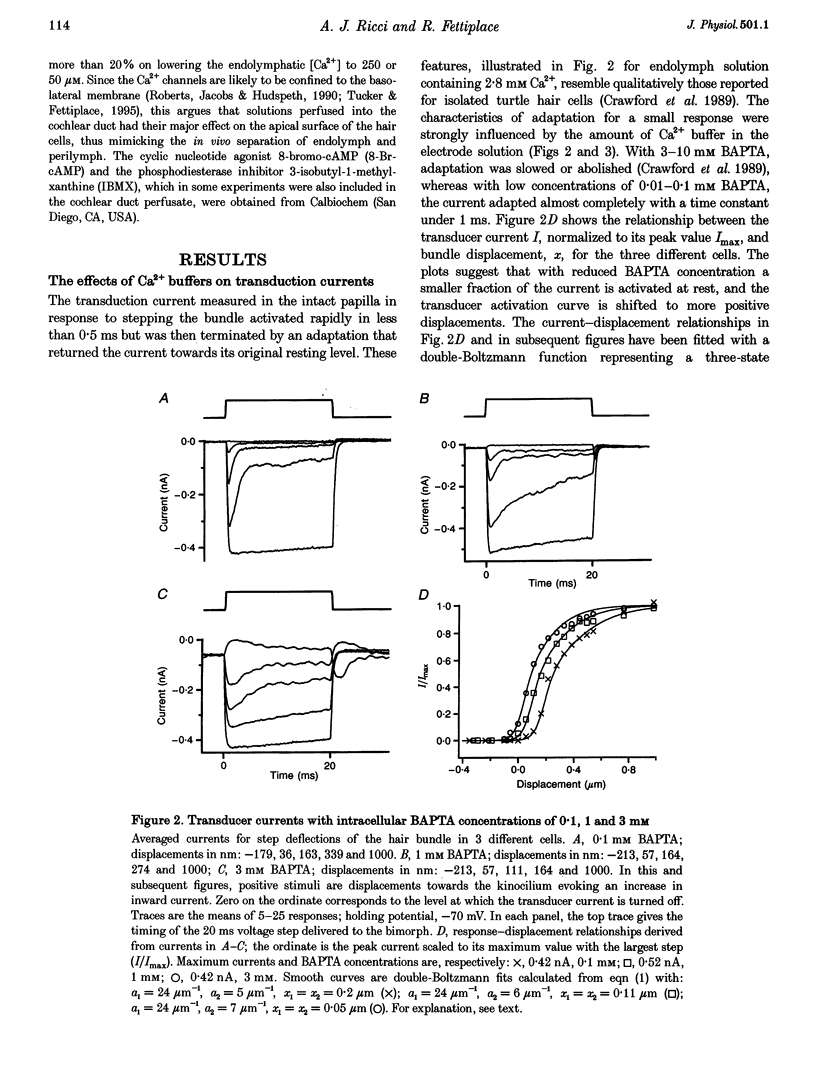
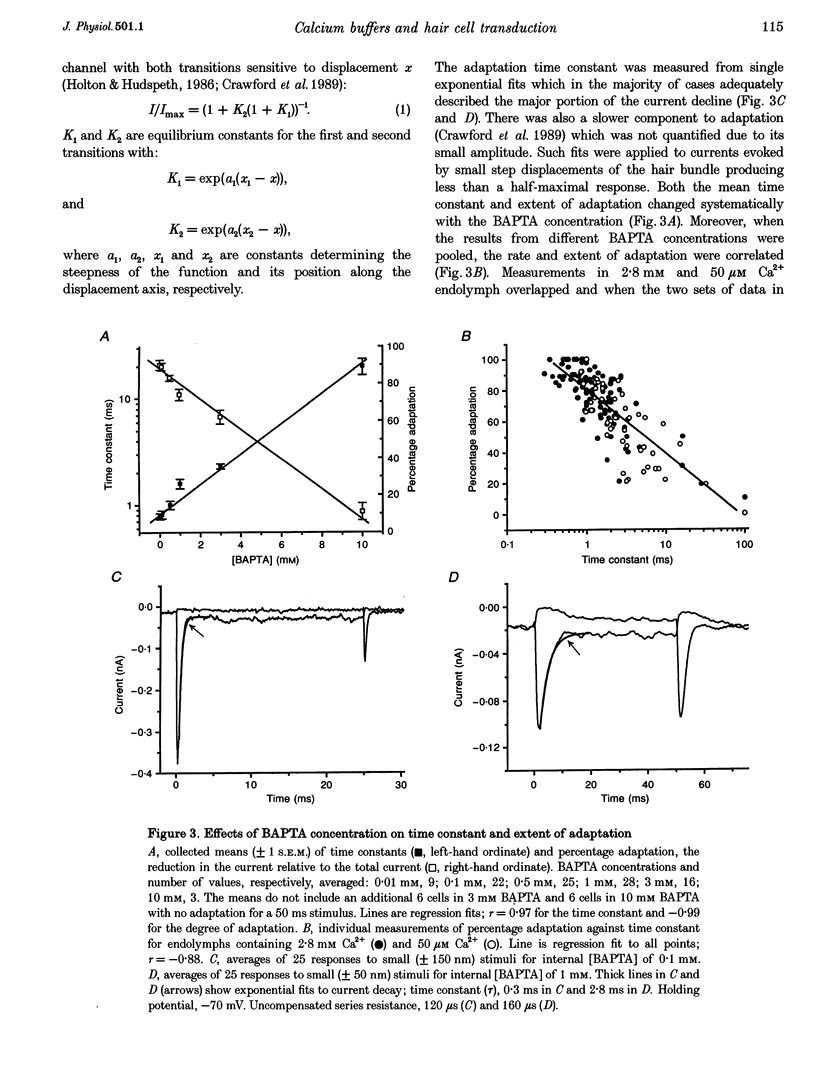
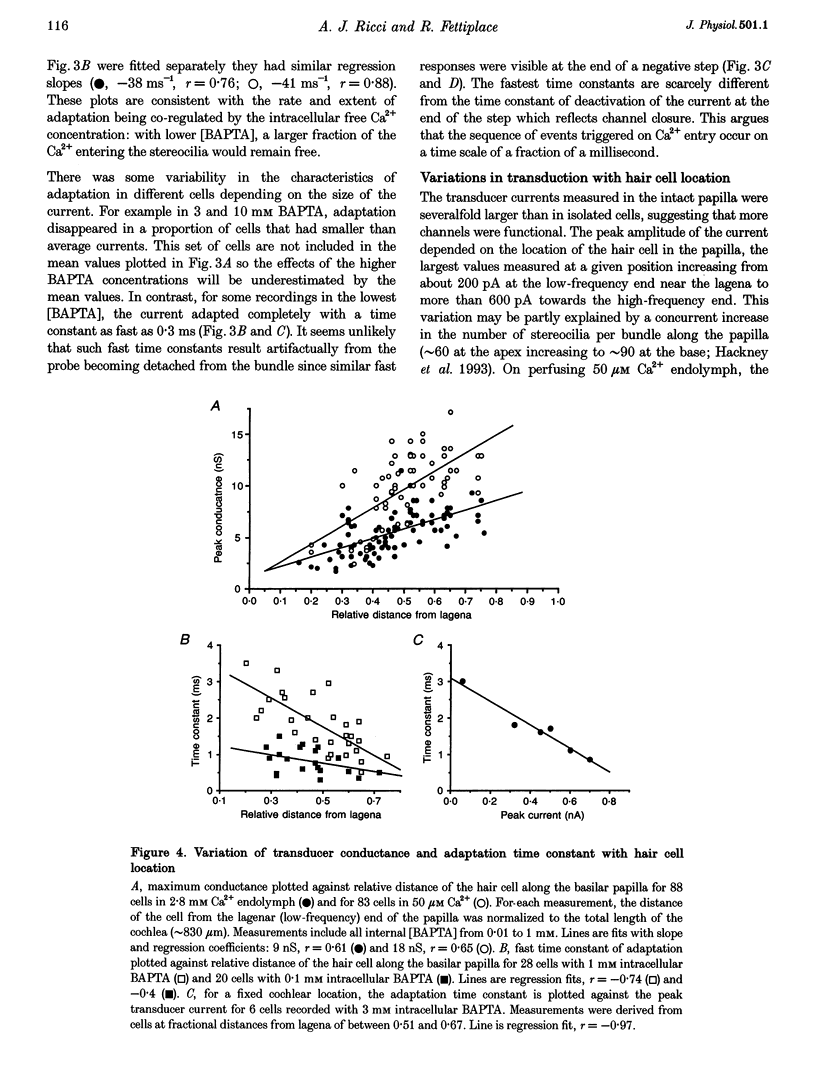



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Assad J. A., Hacohen N., Corey D. P. Voltage dependence of adaptation and active bundle movement in bullfrog saccular hair cells. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2918–2922. doi: 10.1073/pnas.86.8.2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosher S. K., Warren R. L. Very low calcium content of cochlear endolymph, an extracellular fluid. Nature. 1978 Jun 1;273(5661):377–378. doi: 10.1038/273377a0. [DOI] [PubMed] [Google Scholar]
- Crawford A. C., Evans M. G., Fettiplace R. Activation and adaptation of transducer currents in turtle hair cells. J Physiol. 1989 Dec;419:405–434. doi: 10.1113/jphysiol.1989.sp017878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford A. C., Evans M. G., Fettiplace R. The actions of calcium on the mechano-electrical transducer current of turtle hair cells. J Physiol. 1991 Mar;434:369–398. doi: 10.1113/jphysiol.1991.sp018475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford A. C., Fettiplace R. An electrical tuning mechanism in turtle cochlear hair cells. J Physiol. 1981 Mar;312:377–412. doi: 10.1113/jphysiol.1981.sp013634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford A. C., Fettiplace R. Non-linearities in the responses of turtle hair cells. J Physiol. 1981 Jun;315:317–338. doi: 10.1113/jphysiol.1981.sp013750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouch J. J., Schulte B. A. Expression of plasma membrane Ca-ATPase in the adult and developing gerbil cochlea. Hear Res. 1995 Dec;92(1-2):112–119. doi: 10.1016/0378-5955(95)00201-4. [DOI] [PubMed] [Google Scholar]
- Denk W., Holt J. R., Shepherd G. M., Corey D. P. Calcium imaging of single stereocilia in hair cells: localization of transduction channels at both ends of tip links. Neuron. 1995 Dec;15(6):1311–1321. doi: 10.1016/0896-6273(95)90010-1. [DOI] [PubMed] [Google Scholar]
- Drescher M. J., Kern R. C., Hatfield J. S., Drescher D. G. Cytochemical localization of adenylyl cyclase activity within the sensory epithelium of the trout saccule. Neurosci Lett. 1995 Aug 25;196(3):145–148. doi: 10.1016/0304-3940(95)11764-n. [DOI] [PubMed] [Google Scholar]
- Eatock R. A., Corey D. P., Hudspeth A. J. Adaptation of mechanoelectrical transduction in hair cells of the bullfrog's sacculus. J Neurosci. 1987 Sep;7(9):2821–2836. doi: 10.1523/JNEUROSCI.07-09-02821.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackney C. M., Fettiplace R., Furness D. N. The functional morphology of stereociliary bundles on turtle cochlear hair cells. Hear Res. 1993 Sep;69(1-2):163–175. doi: 10.1016/0378-5955(93)90104-9. [DOI] [PubMed] [Google Scholar]
- Holton T., Hudspeth A. J. The transduction channel of hair cells from the bull-frog characterized by noise analysis. J Physiol. 1986 Jun;375:195–227. doi: 10.1113/jphysiol.1986.sp016113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaramillo F., Hudspeth A. J. Localization of the hair cell's transduction channels at the hair bundle's top by iontophoretic application of a channel blocker. Neuron. 1991 Sep;7(3):409–420. doi: 10.1016/0896-6273(91)90293-9. [DOI] [PubMed] [Google Scholar]
- Kimitsuki T., Ohmori H. The effect of caged calcium release on the adaptation of the transduction current in chick hair cells. J Physiol. 1992 Dec;458:27–40. doi: 10.1113/jphysiol.1992.sp019404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroese A. B., Das A., Hudspeth A. J. Blockage of the transduction channels of hair cells in the bullfrog's sacculus by aminoglycoside antibiotics. Hear Res. 1989 Feb;37(3):203–217. doi: 10.1016/0378-5955(89)90023-3. [DOI] [PubMed] [Google Scholar]
- Kros C. J., Rüsch A., Richardson G. P. Mechano-electrical transducer currents in hair cells of the cultured neonatal mouse cochlea. Proc Biol Sci. 1992 Aug 22;249(1325):185–193. doi: 10.1098/rspb.1992.0102. [DOI] [PubMed] [Google Scholar]
- Lumpkin E. A., Hudspeth A. J. Detection of Ca2+ entry through mechanosensitive channels localizes the site of mechanoelectrical transduction in hair cells. Proc Natl Acad Sci U S A. 1995 Oct 24;92(22):10297–10301. doi: 10.1073/pnas.92.22.10297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noben-Trauth K., Naggert J. K., North M. A., Nishina P. M. A candidate gene for the mouse mutation tubby. Nature. 1996 Apr 11;380(6574):534–538. doi: 10.1038/380534a0. [DOI] [PubMed] [Google Scholar]
- Ohmori H. Mechano-electrical transduction currents in isolated vestibular hair cells of the chick. J Physiol. 1985 Feb;359:189–217. doi: 10.1113/jphysiol.1985.sp015581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliva C., Cohen I. S., Mathias R. T. Calculation of time constants for intracellular diffusion in whole cell patch clamp configuration. Biophys J. 1988 Nov;54(5):791–799. doi: 10.1016/S0006-3495(88)83017-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oudar O., Ferrary E., Feldmann G. Adenylate cyclase and carbonic anhydrase in the semicircular canal epithelium of the frog Rana esculenta. An ultrastructural cytochemical localization. Cell Tissue Res. 1990 Dec;262(3):579–585. doi: 10.1007/BF00305255. [DOI] [PubMed] [Google Scholar]
- Pickles J. O., Comis S. D., Osborne M. P. Cross-links between stereocilia in the guinea pig organ of Corti, and their possible relation to sensory transduction. Hear Res. 1984 Aug;15(2):103–112. doi: 10.1016/0378-5955(84)90041-8. [DOI] [PubMed] [Google Scholar]
- Ricci A., Norris C., Guth P. Cyclic AMP modulates sensory-neural communication at the vestibular end organ. Brain Res. 1991 Nov 22;565(1):78–84. doi: 10.1016/0006-8993(91)91738-m. [DOI] [PubMed] [Google Scholar]
- Roberts W. M., Jacobs R. A., Hudspeth A. J. Colocalization of ion channels involved in frequency selectivity and synaptic transmission at presynaptic active zones of hair cells. J Neurosci. 1990 Nov;10(11):3664–3684. doi: 10.1523/JNEUROSCI.10-11-03664.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts W. M. Spatial calcium buffering in saccular hair cells. Nature. 1993 May 6;363(6424):74–76. doi: 10.1038/363074a0. [DOI] [PubMed] [Google Scholar]
- Shepherd G. M., Corey D. P. The extent of adaptation in bullfrog saccular hair cells. J Neurosci. 1994 Oct;14(10):6217–6229. doi: 10.1523/JNEUROSCI.14-10-06217.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torre V., Straforini M., Sesti F., Lamb T. D. Different channel-gating properties of two classes of cyclic GMP-activated channel in vertebrate photoreceptors. Proc Biol Sci. 1992 Dec 22;250(1329):209–215. doi: 10.1098/rspb.1992.0151. [DOI] [PubMed] [Google Scholar]
- Tucker T. R., Fettiplace R. Monitoring calcium in turtle hair cells with a calcium-activated potassium channel. J Physiol. 1996 Aug 1;494(Pt 3):613–626. doi: 10.1113/jphysiol.1996.sp021519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker T., Fettiplace R. Confocal imaging of calcium microdomains and calcium extrusion in turtle hair cells. Neuron. 1995 Dec;15(6):1323–1335. doi: 10.1016/0896-6273(95)90011-x. [DOI] [PubMed] [Google Scholar]
- Zimmerman A. L., Baylor D. A. Cation interactions within the cyclic GMP-activated channel of retinal rods from the tiger salamander. J Physiol. 1992 Apr;449:759–783. doi: 10.1113/jphysiol.1992.sp019112. [DOI] [PMC free article] [PubMed] [Google Scholar]



