Abstract
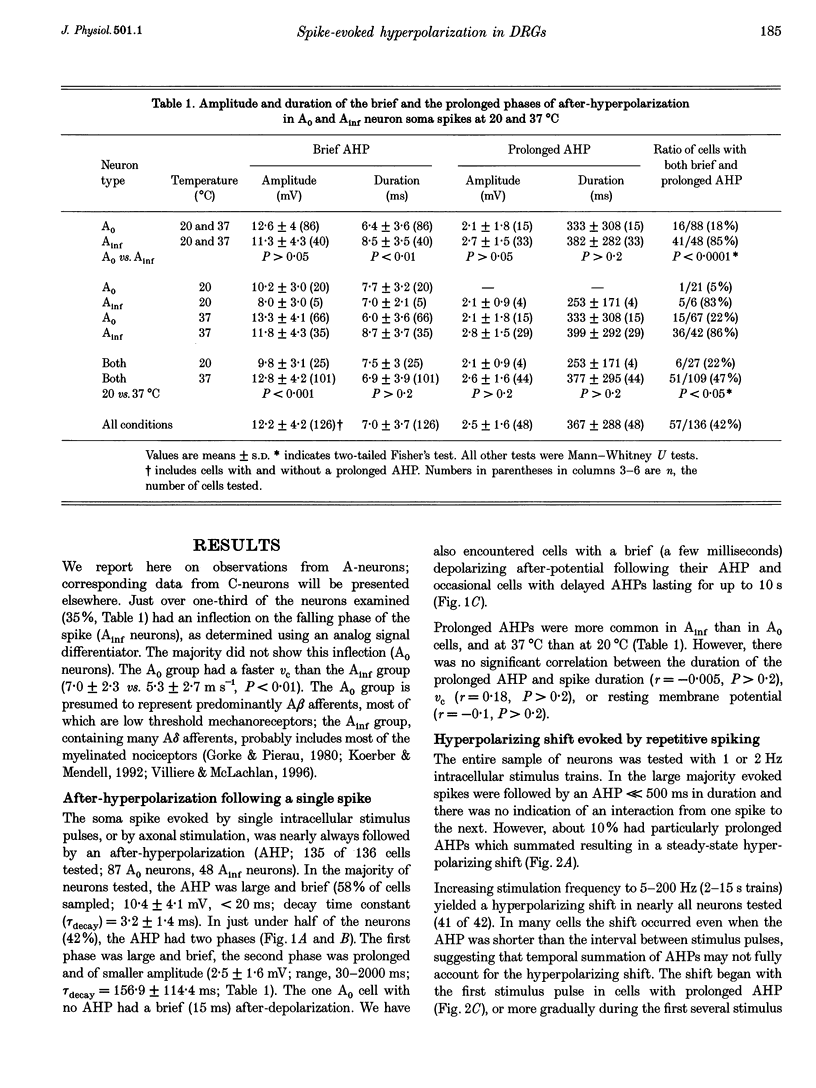
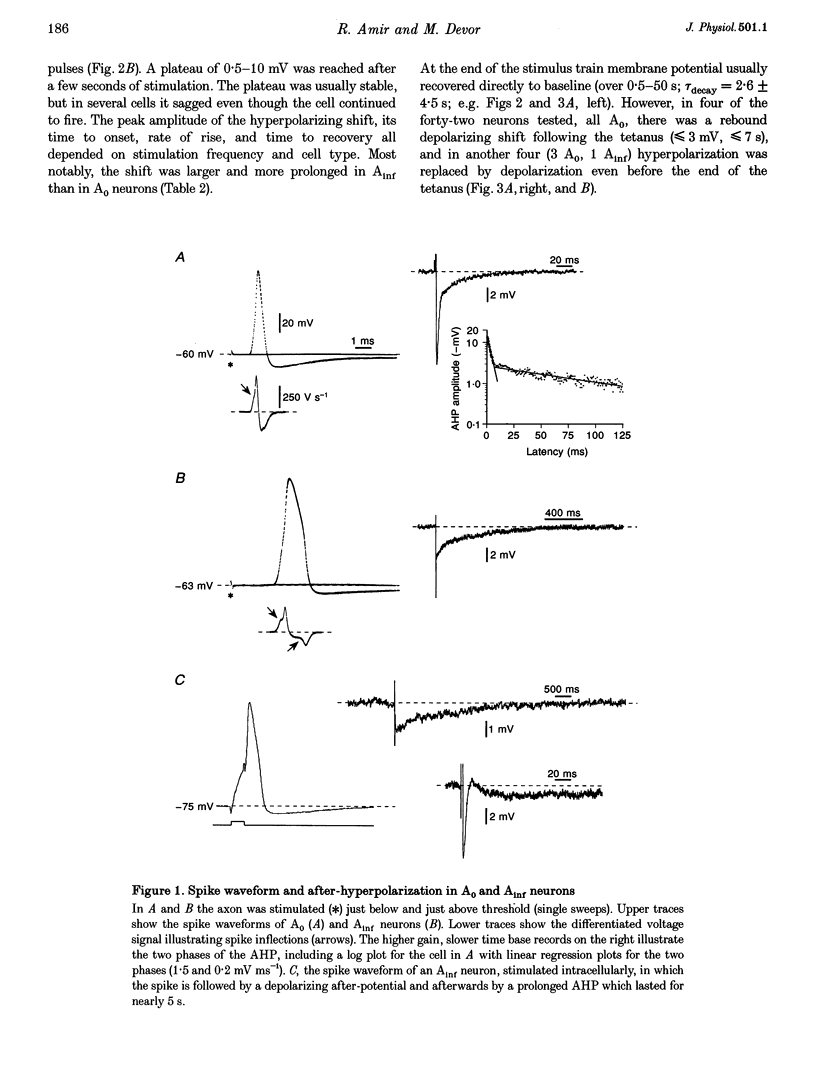
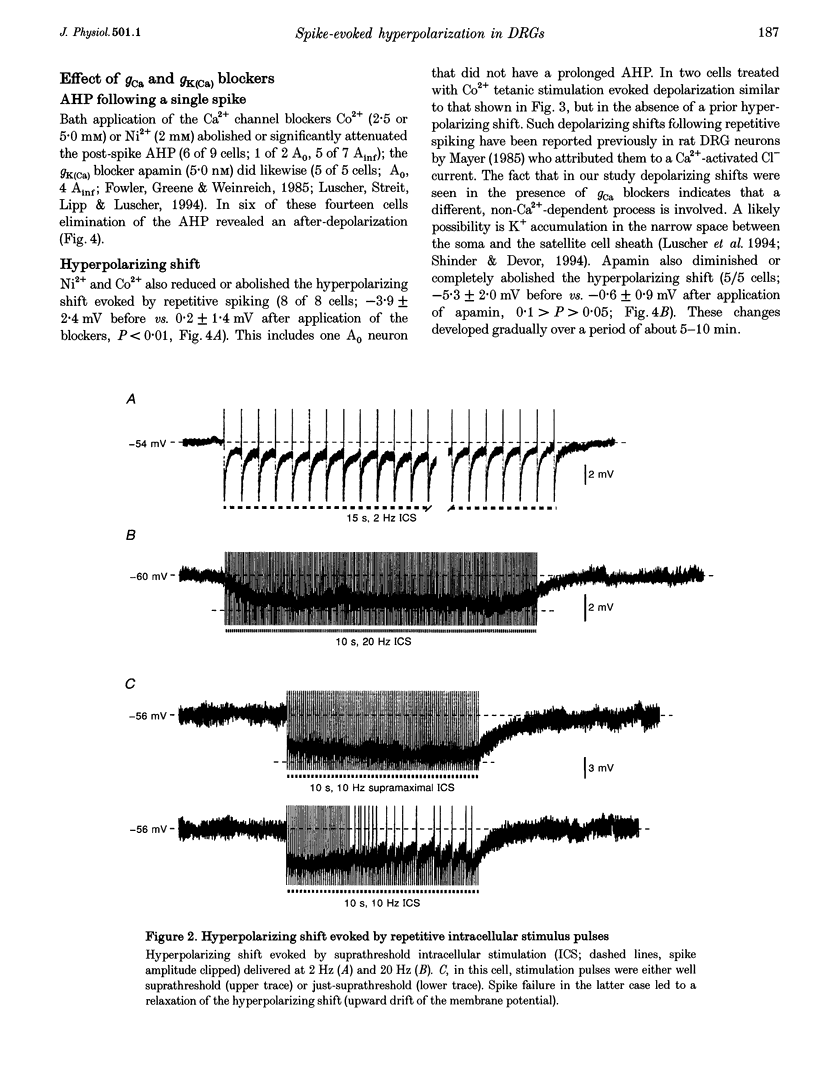
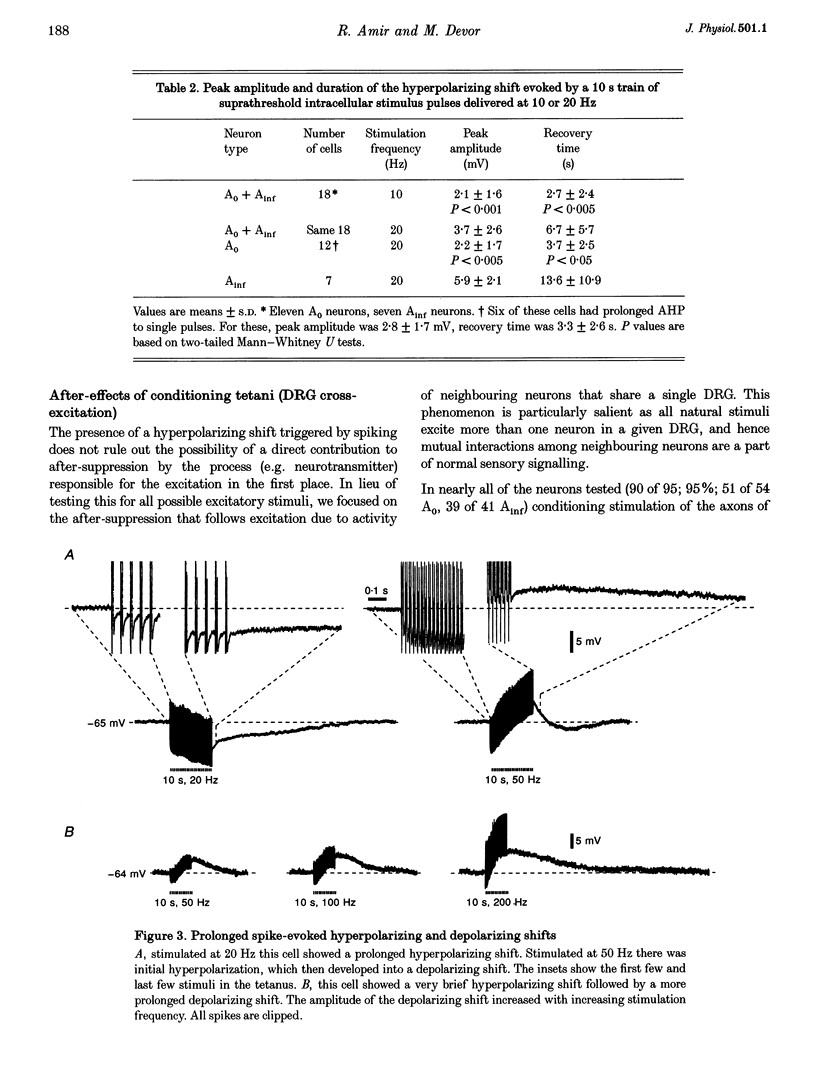
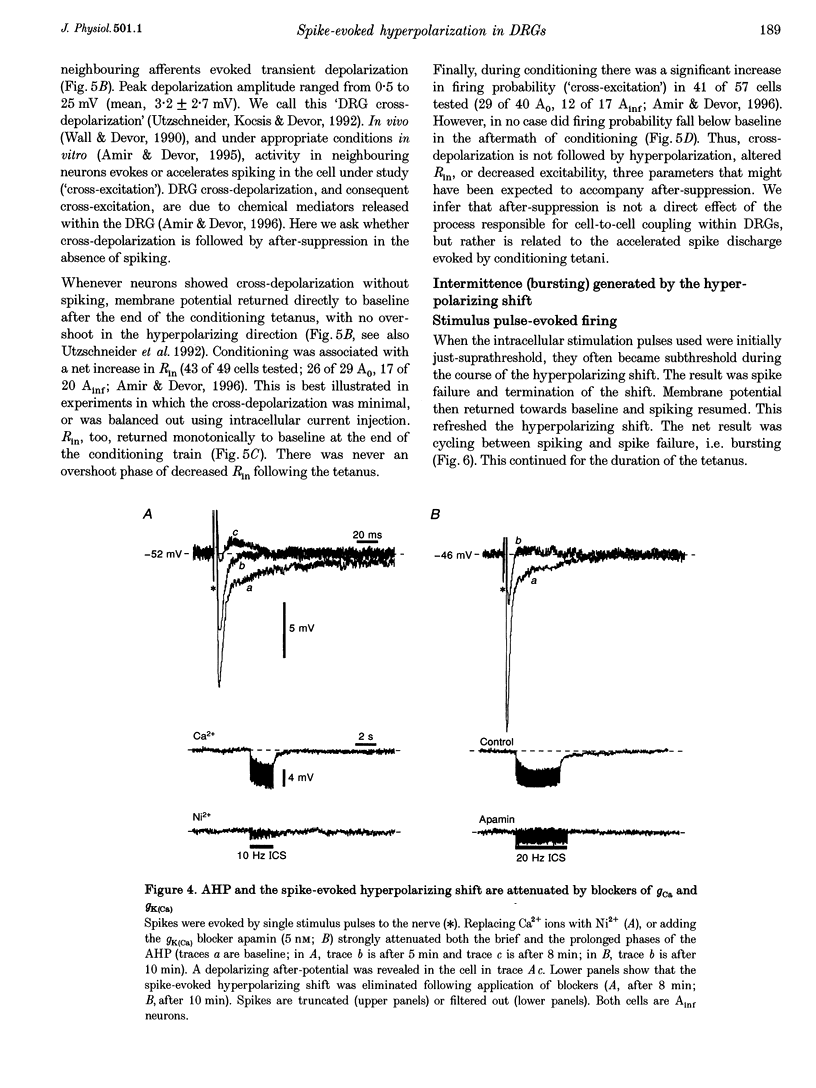
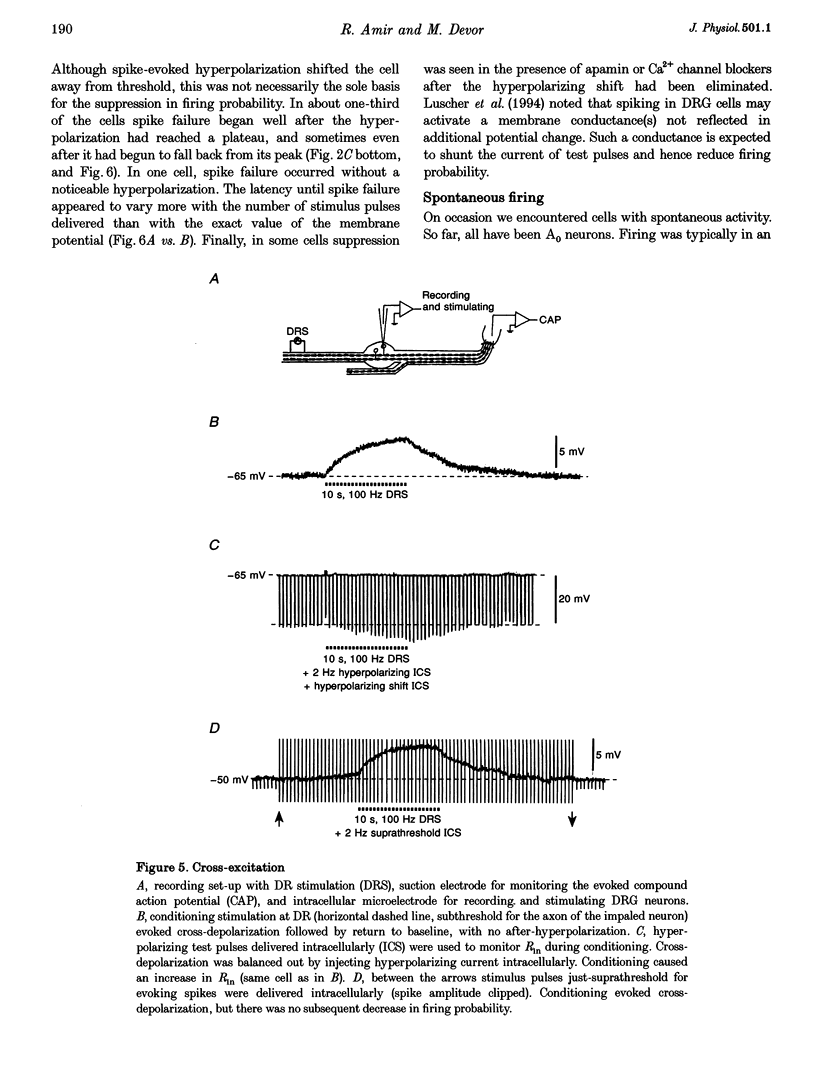
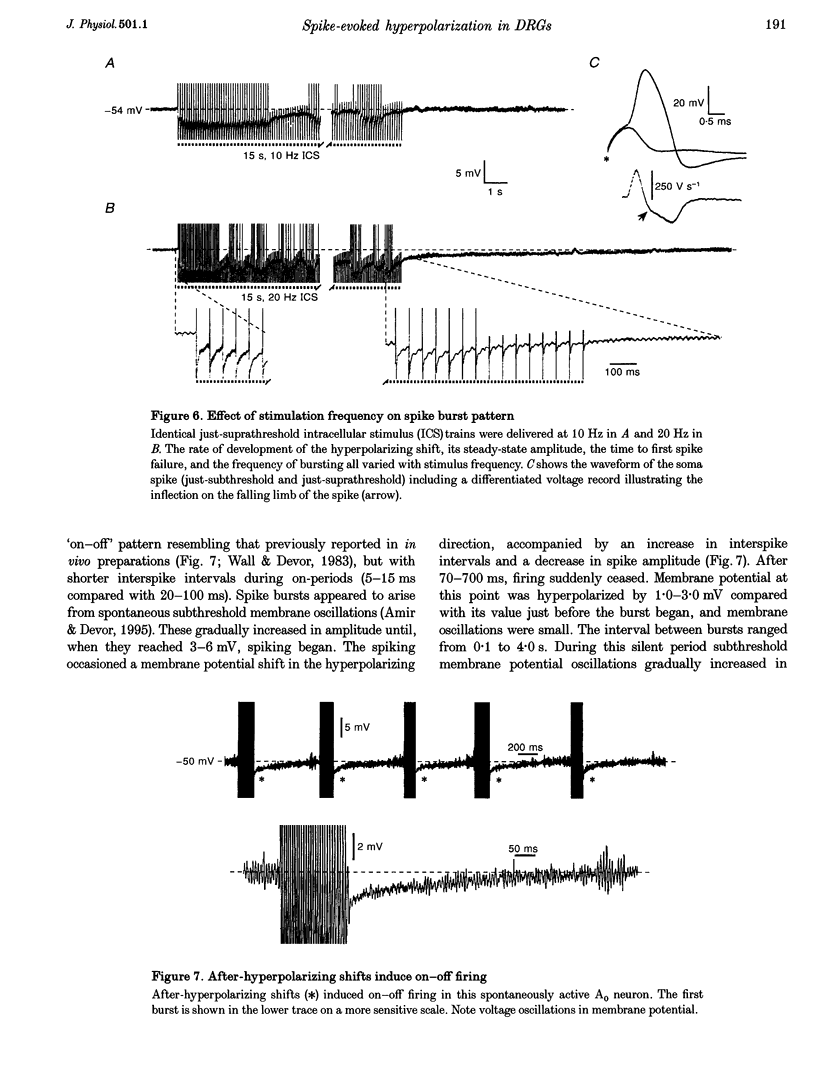
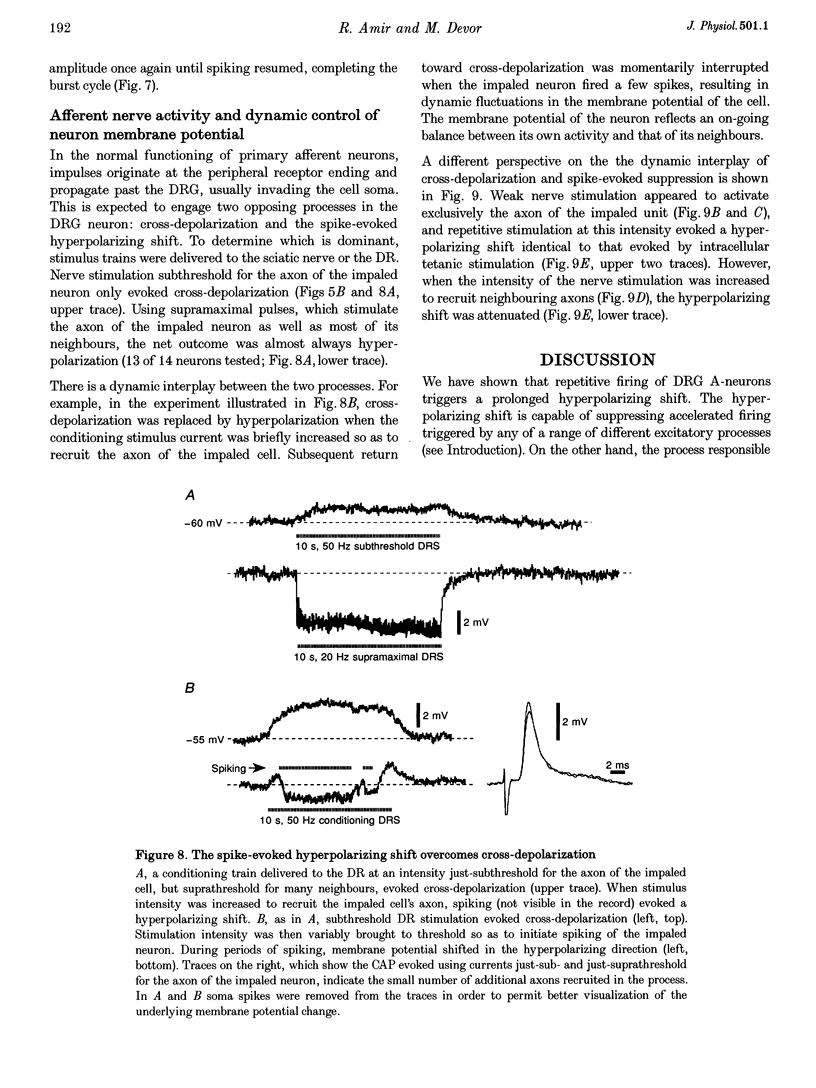
1. A low level of spontaneous impulse discharge is generated within dorsal root ganglia (DRGs) in intact animals, and this activity is enhanced following nerve injury. Many physiological stimuli present in vivo are capable of augmenting this ectopic discharge. Whatever their cause, episodes of sharply accelerated DRG firing tend to be followed by 'after-suppression' during which discharge falls below baseline rate. In this study we examined the process of postexcitation suppression of firing rate, and how it shapes spike patterning in primary sensory neurons. 2. We recorded intracellularly from sensory neurons in excised rat DRGs in vitro. Trains of spikes triggered by intracellular current pulses evoked a prolonged hyperpolarizing shift. This shift appeared to be due to activation of a Ca(2+)-dependent K+ conductance (9K(Ca)). Spikes evoked by just-suprathreshold pulses triggered a hyperpolarizing shift and spike cessation. As the shift decayed, spiking was restored. The net result was bursty (on-off) discharge, a previously unexplained peculiarity of ectopic discharge in some DRG neurons in vivo. 3. Conditioning nerve tetani delivered to axons of neurons which share the DRG with the impaled neuron evoked transient depolarization ('cross-depolarization'). However, when stimulus strength was increased so as to include the axon of the impaled neuron, the net result was a hyperpolarizing shift. Nerve stimulation that straddled the threshold of the axon of the impaled neuron drove it intermittently, but it always drove axons of at least some neighbouring neurons. The result was dynamic modulation of the membrane potential of the impaled neuron as cross-depolarization and spike-evoked hyperpolarizing shifts played off against one another. Membrane potential shifted in the hyperpolarizing direction whenever the axon was activated, and shifted in the depolarizing direction whenever it was silent. Dynamic modulation of this sort probably also occurs in vivo when stimuli are drawn over the surface of the skin.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amir R., Devor M. Chemically mediated cross-excitation in rat dorsal root ganglia. J Neurosci. 1996 Aug 1;16(15):4733–4741. doi: 10.1523/JNEUROSCI.16-15-04733.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burchiel K. J. Effects of electrical and mechanical stimulation on two foci of spontaneous activity which develop in primary afferent neurons after peripheral axotomy. Pain. 1984 Mar;18(3):249–265. doi: 10.1016/0304-3959(84)90820-0. [DOI] [PubMed] [Google Scholar]
- Burchiel K. J. Spontaneous impulse generation in normal and denervated dorsal root ganglia: sensitivity to alpha-adrenergic stimulation and hypoxia. Exp Neurol. 1984 Aug;85(2):257–272. doi: 10.1016/0014-4886(84)90139-0. [DOI] [PubMed] [Google Scholar]
- Devor M., Jänig W., Michaelis M. Modulation of activity in dorsal root ganglion neurons by sympathetic activation in nerve-injured rats. J Neurophysiol. 1994 Jan;71(1):38–47. doi: 10.1152/jn.1994.71.1.38. [DOI] [PubMed] [Google Scholar]
- Devor M., Wall P. D. Cross-excitation in dorsal root ganglia of nerve-injured and intact rats. J Neurophysiol. 1990 Dec;64(6):1733–1746. doi: 10.1152/jn.1990.64.6.1733. [DOI] [PubMed] [Google Scholar]
- Fowler J. C., Greene R., Weinreich D. Two calcium-sensitive spike after-hyperpolarizations in visceral sensory neurones of the rabbit. J Physiol. 1985 Aug;365:59–75. doi: 10.1113/jphysiol.1985.sp015759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton B. P. Postnatal changes in conduction velocity and soma action potential parameters of rat dorsal root ganglion neurones. Neurosci Lett. 1987 Jan 14;73(2):125–130. doi: 10.1016/0304-3940(87)90005-x. [DOI] [PubMed] [Google Scholar]
- Gallego R. The ionic basis of action potentials in petrosal ganglion cells of the cat. J Physiol. 1983 Sep;342:591–602. doi: 10.1113/jphysiol.1983.sp014870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon T. R., Kocsis J. D., Waxman S. G. Electrogenic pump (Na+/K(+)-ATPase) activity in rat optic nerve. Neuroscience. 1990;37(3):829–837. doi: 10.1016/0306-4522(90)90112-h. [DOI] [PubMed] [Google Scholar]
- Görke K., Pierau F. K. Spike potentials and membrane properties of dorsal root ganglion cells in pigeons. Pflugers Arch. 1980 Jul;386(1):21–28. doi: 10.1007/BF00584182. [DOI] [PubMed] [Google Scholar]
- Haimann C., Bernheim L., Bertrand D., Bader C. R. Potassium current activated by intracellular sodium in quail trigeminal ganglion neurons. J Gen Physiol. 1990 May;95(5):961–979. doi: 10.1085/jgp.95.5.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper A. A., Lawson S. N. Electrical properties of rat dorsal root ganglion neurones with different peripheral nerve conduction velocities. J Physiol. 1985 Feb;359:47–63. doi: 10.1113/jphysiol.1985.sp015574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe J. F., Loeser J. D., Calvin W. H. Mechanosensitivity of dorsal root ganglia and chronically injured axons: a physiological basis for the radicular pain of nerve root compression. Pain. 1977 Feb;3(1):25–41. doi: 10.1016/0304-3959(77)90033-1. [DOI] [PubMed] [Google Scholar]
- KUGELBERG E., LINDBLOM U. The mechanism of the pain in trigeminal neuralgia. J Neurol Neurosurg Psychiatry. 1959 Feb;22(1):36–43. doi: 10.1136/jnnp.22.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajander K. C., Wakisaka S., Bennett G. J. Spontaneous discharge originates in the dorsal root ganglion at the onset of a painful peripheral neuropathy in the rat. Neurosci Lett. 1992 Apr 27;138(2):225–228. doi: 10.1016/0304-3940(92)90920-3. [DOI] [PubMed] [Google Scholar]
- Leonard J. P., Wickelgren W. O. Calcium spike and calcium-dependent potassium conductance in mechanosensory neurons of the lamprey. J Neurophysiol. 1985 Jan;53(1):171–182. doi: 10.1152/jn.1985.53.1.171. [DOI] [PubMed] [Google Scholar]
- Lutsky I., Aizer F., Mor N. The Sabra rat: definition of a laboratory animal. Isr J Med Sci. 1984 Jul;20(7):603–612. [PubMed] [Google Scholar]
- Lüscher C., Streit J., Lipp P., Lüscher H. R. Action potential propagation through embryonic dorsal root ganglion cells in culture. II. Decrease of conduction reliability during repetitive stimulation. J Neurophysiol. 1994 Aug;72(2):634–643. doi: 10.1152/jn.1994.72.2.634. [DOI] [PubMed] [Google Scholar]
- Matzner O., Devor M. Na+ conductance and the threshold for repetitive neuronal firing. Brain Res. 1992 Nov 27;597(1):92–98. doi: 10.1016/0006-8993(92)91509-d. [DOI] [PubMed] [Google Scholar]
- Mayer M. L. A calcium-activated chloride current generates the after-depolarization of rat sensory neurones in culture. J Physiol. 1985 Jul;364:217–239. doi: 10.1113/jphysiol.1985.sp015740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzack R., Bromage P. R. Experimental phantom limbs. Exp Neurol. 1973 Mar-Apr;39(2):261–269. doi: 10.1016/0014-4886(73)90228-8. [DOI] [PubMed] [Google Scholar]
- Rang H. P., Ritchie J. M. On the electrogenic sodium pump in mammalian non-myelinated nerve fibres and its activation by various external cations. J Physiol. 1968 May;196(1):183–221. doi: 10.1113/jphysiol.1968.sp008502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappaport Z. H., Devor M. Trigeminal neuralgia: the role of self-sustaining discharge in the trigeminal ganglion. Pain. 1994 Feb;56(2):127–138. doi: 10.1016/0304-3959(94)90086-8. [DOI] [PubMed] [Google Scholar]
- Schwindt P. C., Spain W. J., Crill W. E. Long-lasting reduction of excitability by a sodium-dependent potassium current in cat neocortical neurons. J Neurophysiol. 1989 Feb;61(2):233–244. doi: 10.1152/jn.1989.61.2.233. [DOI] [PubMed] [Google Scholar]
- Sheen K., Chung J. M. Signs of neuropathic pain depend on signals from injured nerve fibers in a rat model. Brain Res. 1993 Apr 30;610(1):62–68. doi: 10.1016/0006-8993(93)91217-g. [DOI] [PubMed] [Google Scholar]
- Shinder V., Devor M. Structural basis of neuron-to-neuron cross-excitation in dorsal root ganglia. J Neurocytol. 1994 Sep;23(9):515–531. doi: 10.1007/BF01262054. [DOI] [PubMed] [Google Scholar]
- Study R. E., Kral M. G. Spontaneous action potential activity in isolated dorsal root ganglion neurons from rats with a painful neuropathy. Pain. 1996 May-Jun;65(2-3):235–242. doi: 10.1016/0304-3959(95)00216-2. [DOI] [PubMed] [Google Scholar]
- Tal M., Eliav E. Abnormal discharge originates at the site of nerve injury in experimental constriction neuropathy (CCI) in the rat. Pain. 1996 Mar;64(3):511–518. doi: 10.1016/0304-3959(95)00175-1. [DOI] [PubMed] [Google Scholar]
- Utzschneider D., Kocsis J., Devor M. Mutual excitation among dorsal root ganglion neurons in the rat. Neurosci Lett. 1992 Oct 26;146(1):53–56. doi: 10.1016/0304-3940(92)90170-c. [DOI] [PubMed] [Google Scholar]
- Villière V., McLachlan E. M. Electrophysiological properties of neurons in intact rat dorsal root ganglia classified by conduction velocity and action potential duration. J Neurophysiol. 1996 Sep;76(3):1924–1941. doi: 10.1152/jn.1996.76.3.1924. [DOI] [PubMed] [Google Scholar]
- Wall P. D., Devor M. Sensory afferent impulses originate from dorsal root ganglia as well as from the periphery in normal and nerve injured rats. Pain. 1983 Dec;17(4):321–339. doi: 10.1016/0304-3959(83)90164-1. [DOI] [PubMed] [Google Scholar]
- Xie Y., Zhang J., Petersen M., LaMotte R. H. Functional changes in dorsal root ganglion cells after chronic nerve constriction in the rat. J Neurophysiol. 1995 May;73(5):1811–1820. doi: 10.1152/jn.1995.73.5.1811. [DOI] [PubMed] [Google Scholar]
- Yoon Y. W., Na H. S., Chung J. M. Contributions of injured and intact afferents to neuropathic pain in an experimental rat model. Pain. 1996 Jan;64(1):27–36. doi: 10.1016/0304-3959(95)00096-8. [DOI] [PubMed] [Google Scholar]


