Abstract
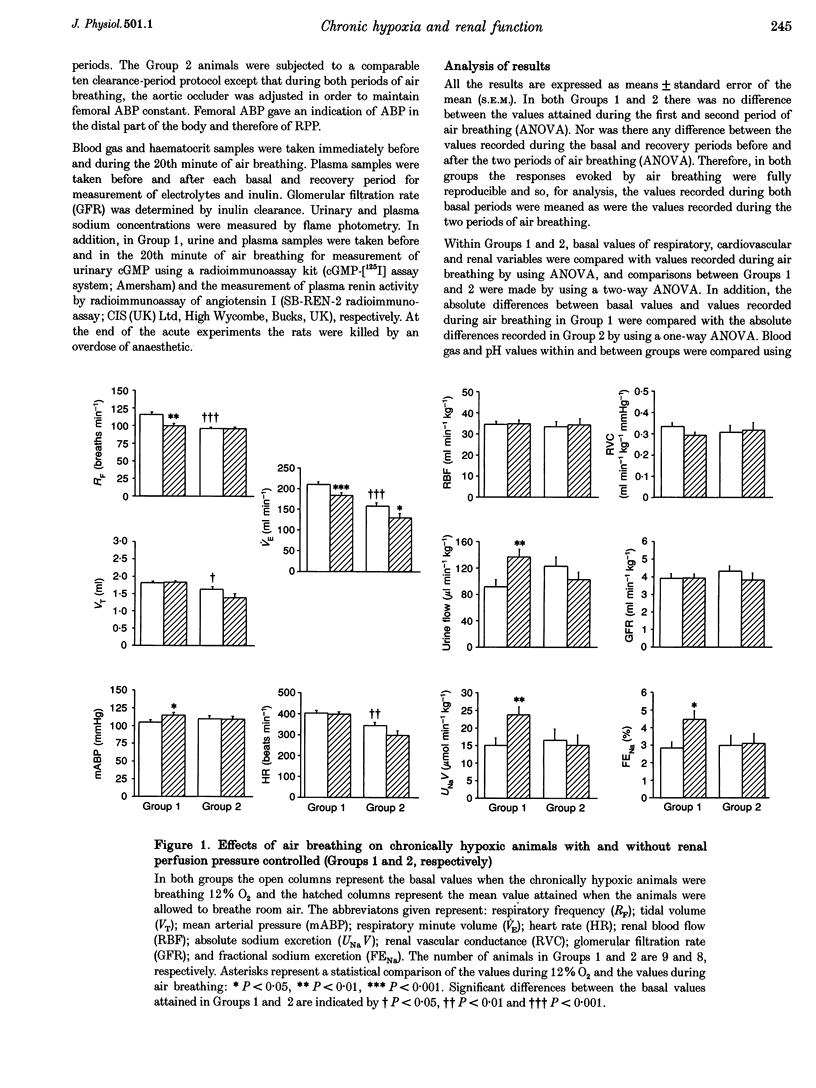
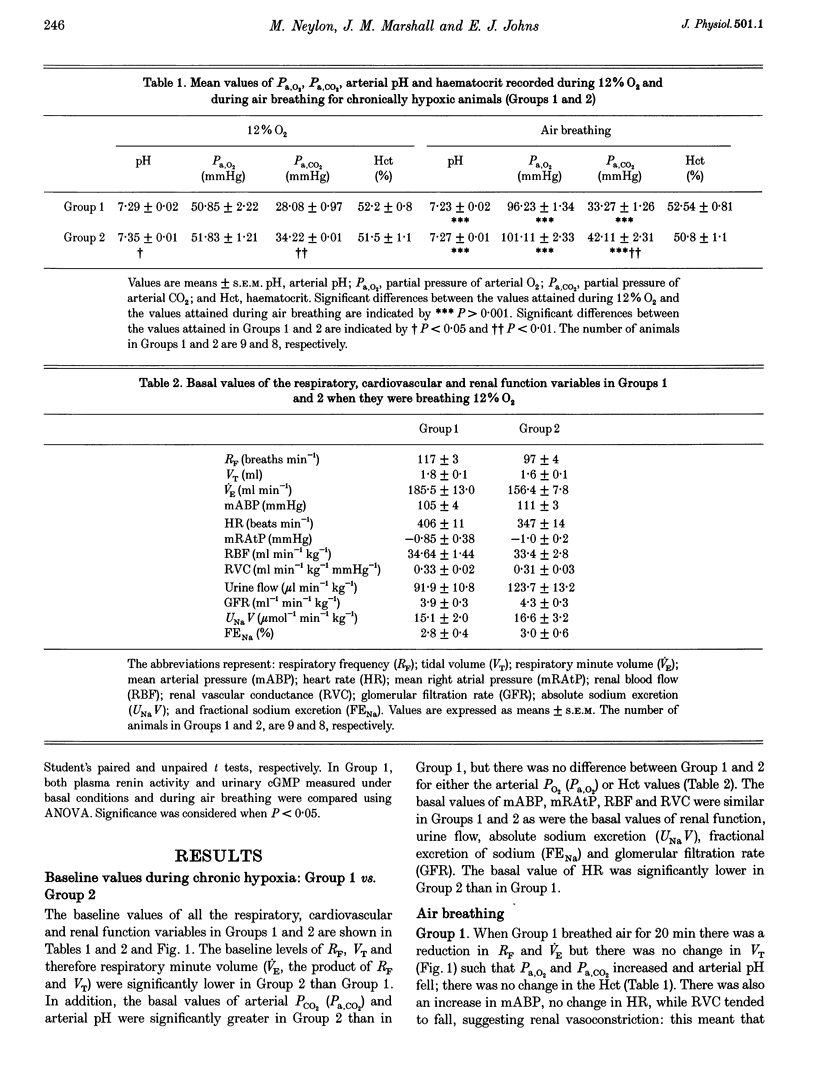
1. Studies were performed on rats that had been made chronically hypoxic (CH rats) in a normoxic chamber at 12% O2 for 3-5 weeks. Under Saffan anaesthesia, respiratory and cardiovascular variables, renal haemodynamics and renal function were recorded while the rats spontaneously breathed 12% O2 followed by a switch to air breathing for 20 min. Plasma renin activity was assessed by radioimmunoassay of angiotensin I. Plasma atrial natiruetic peptide (ANP) was indirectly assessed by measurement of cyclic GMP in urine. 2. When breathing 12% O2, CH rats showed hyperventilation and raised haematocrit (52%) relative to normoxic (N) rats. But arterial pressure (ABP), renal blood flow (RBF), renal vascular conductance (RVC), mean right atrial pressure (mRAtP), urine flow, glomerular filtration rate (GFR) and absolute sodium excretion (UNaV) were comparable to those recorded in N rats breathing air. Urinary cGMP was 40% greater than in N rats, but plasma renin activity was not significantly greater in CH than in N rats. 3. Air breathing in CH rats induced hypoventilation, a 12% increase in ABP, no change in mRAtP, RBF or GFR, but increases of 75 and 100% in urine flow and UNaV, respectively. Neither urinary cGMP nor plasma renin activity changed. Such increases in urine flow and UNaV were absent when renal perfusion pressure (RPP) was prevented from rising during air breathing by using an occluder on the dorsal aorta. 4. We propose that by 3-5 weeks of chronic hypoxia renal function was normalized, principally because arterial O2 content was normalized by the increase in haematocrit and because ABP and renal haemodynamics were normalized: acute hypoxia in N rats produces a fall in ABP. We suggest that plasma ANP was raised by the actions of hypoxia or erythropoietin on the atrium, rather than by atrial distension, but suggest that ANP had little direct influence on renal function and tended to limit the influence of the renin-angiotensin system. We further propose that the diuresis and natriuresis seen during air breathing were mediated by the increase in RPP; neither plasma ANP nor renin activity change in the immediate short term.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABER G. M., BAYLEY T. J., BISHOP J. M. INTER-RELATIONSHIPS BETWEEN RENAL AND CARDIAC FUNCTION AND RESPIRATORY GAS EXCHANGE IN OBSTRUCTIVE AIRWAYS DISEASE. Clin Sci. 1963 Oct;25:159–170. [PubMed] [Google Scholar]
- Adnot S., Andrivet P., Chabrier P. E., Piquet J., Plas P., Braquet P., Roudot-Thoraval F., Brun-Buisson C. Atrial natriuretic factor in chronic obstructive lung disease with pulmonary hypertension. Physiological correlates and response to peptide infusion. J Clin Invest. 1989 Mar;83(3):986–993. doi: 10.1172/JCI113985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baertschi A. J., Hausmaninger C., Walsh R. S., Mentzer R. M., Jr, Wyatt D. A., Pence R. A. Hypoxia-induced release of atrial natriuretic factor (ANF) from the isolated rat and rabbit heart. Biochem Biophys Res Commun. 1986 Oct 15;140(1):427–433. doi: 10.1016/0006-291x(86)91108-3. [DOI] [PubMed] [Google Scholar]
- Behm R., Mewes H., DeMuinck Keizer W. H., Unger T., Rettig R. Cardiovascular and renal effects of hypoxia in conscious carotid body-denervated rats. J Appl Physiol (1985) 1993 Jun;74(6):2795–2800. doi: 10.1152/jappl.1993.74.6.2795. [DOI] [PubMed] [Google Scholar]
- Brenner B. M., Ballermann B. J., Gunning M. E., Zeidel M. L. Diverse biological actions of atrial natriuretic peptide. Physiol Rev. 1990 Jul;70(3):665–699. doi: 10.1152/physrev.1990.70.3.665. [DOI] [PubMed] [Google Scholar]
- Cowley A. W., Jr, Roman R. J., Krieger J. E. Pathways linking renal excretion and arterial pressure with vascular structure and function. Clin Exp Pharmacol Physiol. 1991 Jan;18(1):21–27. doi: 10.1111/j.1440-1681.1991.tb01371.x. [DOI] [PubMed] [Google Scholar]
- Dempsey J. A., Forster H. V. Mediation of Ventilatory Adaptations. Physiol Rev. 1982 Jan;62(1):262–346. doi: 10.1152/physrev.1982.62.1.262. [DOI] [PubMed] [Google Scholar]
- Farber M. O., Kiblawi S. S., Strawbridge R. A., Robertson G. L., Weinberger M. H., Manfredi F. Studies on plasma vasopressin and the renin-angiotensin-aldosterone system in chronic obstructive lung disease. J Lab Clin Med. 1977 Aug;90(2):373–380. [PubMed] [Google Scholar]
- Kuwahira I., Heisler N., Piiper J., Gonzalez N. C. Effect of chronic hypoxia on hemodynamics, organ blood flow and O2 supply in rats. Respir Physiol. 1993 May;92(2):227–238. doi: 10.1016/0034-5687(93)90041-8. [DOI] [PubMed] [Google Scholar]
- Marshall J. M. Peripheral chemoreceptors and cardiovascular regulation. Physiol Rev. 1994 Jul;74(3):543–594. doi: 10.1152/physrev.1994.74.3.543. [DOI] [PubMed] [Google Scholar]
- Mian R., Marshall J. M. The behaviour of muscle microcirculation in chronically hypoxic rats: the role of adenosine. J Physiol. 1996 Mar 1;491(Pt 2):489–498. doi: 10.1113/jphysiol.1996.sp021233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milledge J. S., Beeley J. M., McArthur S., Morice A. H. Atrial natriuretic peptide, altitude and acute mountain sickness. Clin Sci (Lond) 1989 Nov;77(5):509–514. doi: 10.1042/cs0770509. [DOI] [PubMed] [Google Scholar]
- Milledge J. S., Catley D. M. Angiotensin converting enzyme response to hypoxia in man: its role in altitude acclimatization. Clin Sci (Lond) 1984 Oct;67(4):453–456. doi: 10.1042/cs0670453. [DOI] [PubMed] [Google Scholar]
- Milledge J. S., Catley D. M., Ward M. P., Williams E. S., Clarke C. R. Renin-aldosterone and angiotensin-converting enzyme during prolonged altitude exposure. J Appl Physiol Respir Environ Exerc Physiol. 1983 Sep;55(3):699–702. doi: 10.1152/jappl.1983.55.3.699. [DOI] [PubMed] [Google Scholar]
- Neylon M., Marshall J. M. The role of adenosine in the respiratory and cardiovascular response to systemic hypoxia in the rat. J Physiol. 1991;440:529–545. doi: 10.1113/jphysiol.1991.sp018723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neylon M., Marshall J., Johns E. J. The role of the renin-angiotensin system in the renal response to moderate hypoxia in the rat. J Physiol. 1996 Mar 1;491(Pt 2):479–488. doi: 10.1113/jphysiol.1996.sp021232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou L. C., Chen J., Fiore E., Leiter J. C., Brinck-Johnsen T., Birchard G. F., Clemons G., Smith R. P. Ventilatory and hematopoietic responses to chronic hypoxia in two rat strains. J Appl Physiol (1985) 1992 Jun;72(6):2354–2363. doi: 10.1152/jappl.1992.72.6.2354. [DOI] [PubMed] [Google Scholar]
- Porat O., Neumann D., Zamir O., Nachshon S., Feigin E., Cohen J., Zamir N. Erythropoietin stimulates atrial natriuretic peptide secretion from adult rat cardiac atrium. J Pharmacol Exp Ther. 1996 Mar;276(3):1162–1168. [PubMed] [Google Scholar]
- Shigeoka J. W., Colice G. L., Ramirez G. Effect of normoxemic and hypoxemic exercise on renin and aldosterone. J Appl Physiol (1985) 1985 Jul;59(1):142–148. doi: 10.1152/jappl.1985.59.1.142. [DOI] [PubMed] [Google Scholar]
- Thomas T., Marshall J. M. A study on rats of the effects of chronic hypoxia from birth on respiratory and cardiovascular responses evoked by acute hypoxia. J Physiol. 1995 Sep 1;487(Pt 2):513–525. doi: 10.1113/jphysiol.1995.sp020896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter R. J., Meleagros L., Pervez S., Jamal H., Krausz T., Polak J. M., Bloom S. R. Atrial natriuretic peptide levels in plasma and in cardiac tissues after chronic hypoxia in rats. Clin Sci (Lond) 1989 Jan;76(1):95–101. doi: 10.1042/cs0760095. [DOI] [PubMed] [Google Scholar]
- Zhao L., Winter R. J., Krausz T., Hughes J. M. Effects of continuous infusion of atrial natriuretic peptide on the pulmonary hypertension induced by chronic hypoxia in rats. Clin Sci (Lond) 1991 Sep;81(3):379–385. doi: 10.1042/cs0810379. [DOI] [PubMed] [Google Scholar]


