Abstract
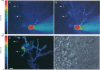
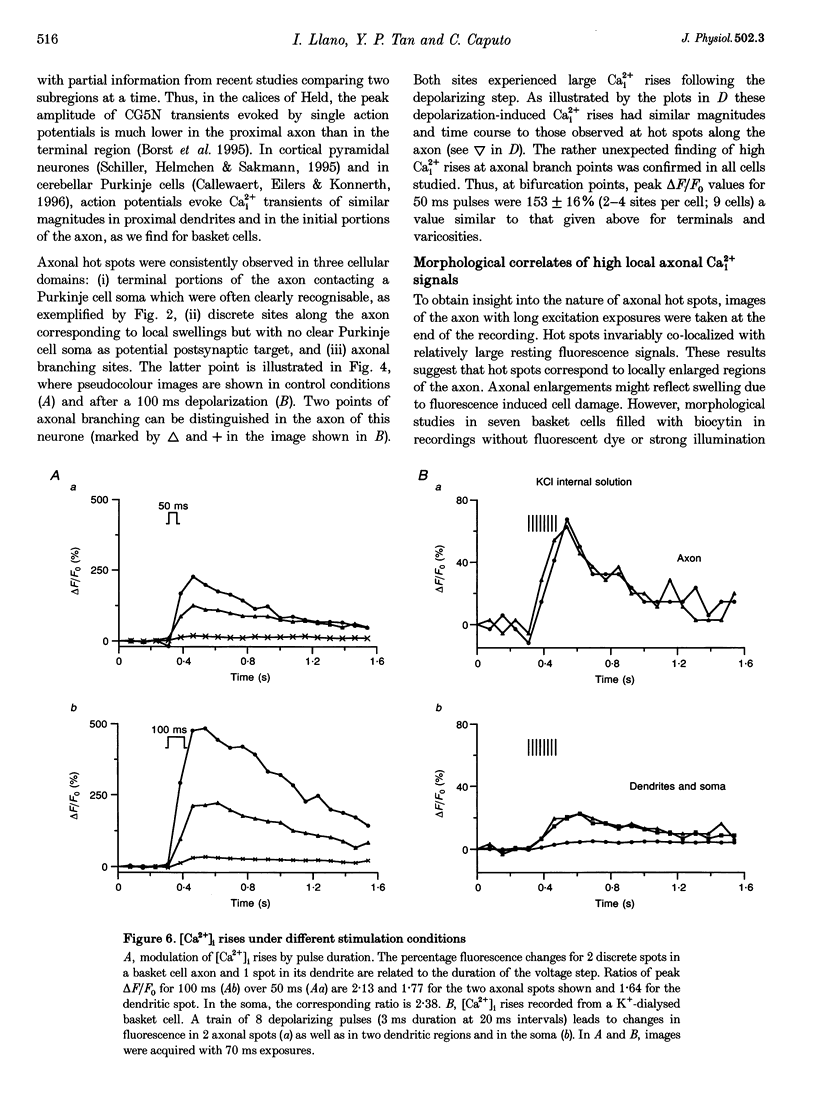
1. Using tight-seal whole-cell recording and digital fluorescence imaging, we studied intracellular calcium (Ca2+i) dynamics in cerebellar basket cells, whose dendrites, axon and presynaptic terminals are coplanar, an optimal configuration for simultaneous optical measurements of all functional domains. 2. In Cs(+)-loaded neurones, depolarizing pulses induced large Ca2+i transients in single axonal varicosities and synaptic terminals, contrasting with much weaker signals between varicosities or in the somato-dendritic domain. 3. Axonal branch points consistently displayed [Ca2+]i rises of similar magnitude and time course to those in axonal terminals and varicosities. 4. In biocytin-filled basket cells, varicosity-like swellings were present along the axon including its branch points. Thus, axonal enlargements are not due to fluorescence-induced cell damage. 5. The spatial heterogeneity of Ca2+i signals was also observed in K(+)-loaded cells upon depolarizing trains, suggesting that this behaviour is an intrinsic property of Ca2+i homeostasis in basket cells. 6. We conclude that depolarization of basket cell axons evokes high local Ca2+i signals in synaptic terminals, en passant varicosities and branch points. While high [Ca2+]i in presynaptic structures presumably triggers transmitter release, Ca2+i transients at branch points may control signal transmission in the axonal arborization.
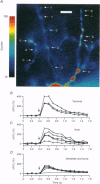
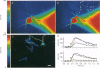
Full text
PDF

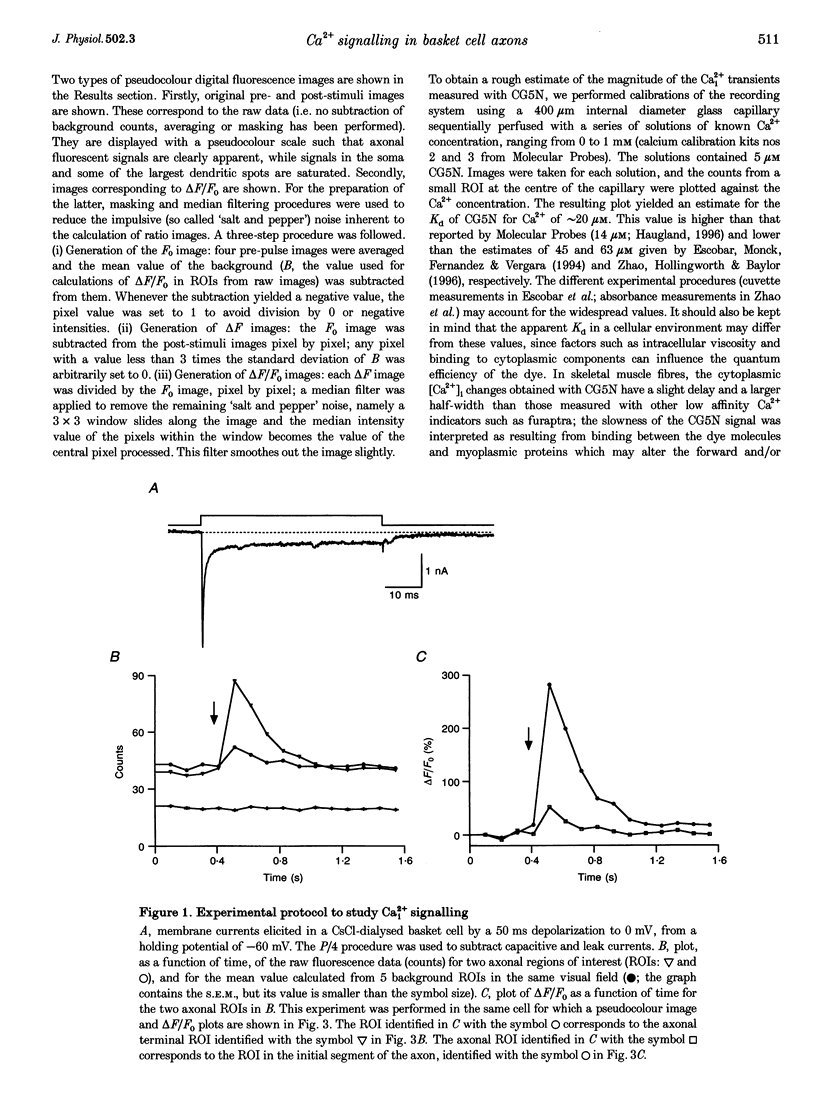
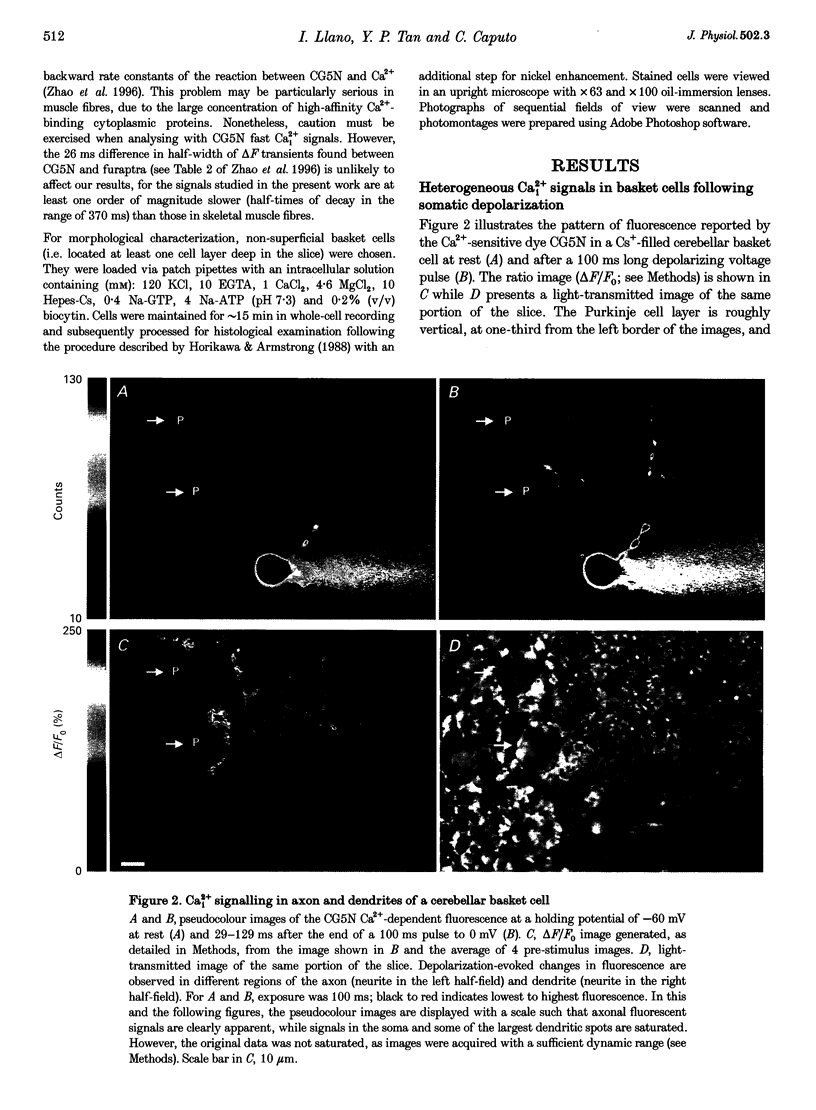
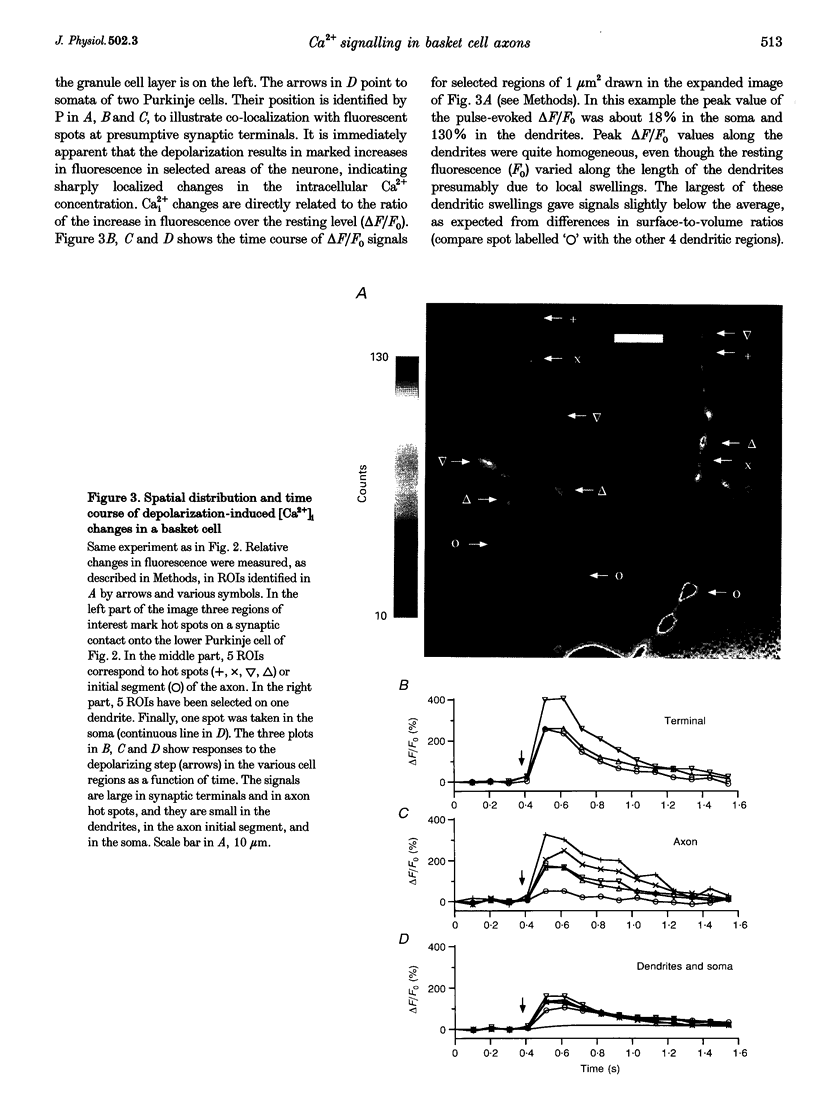
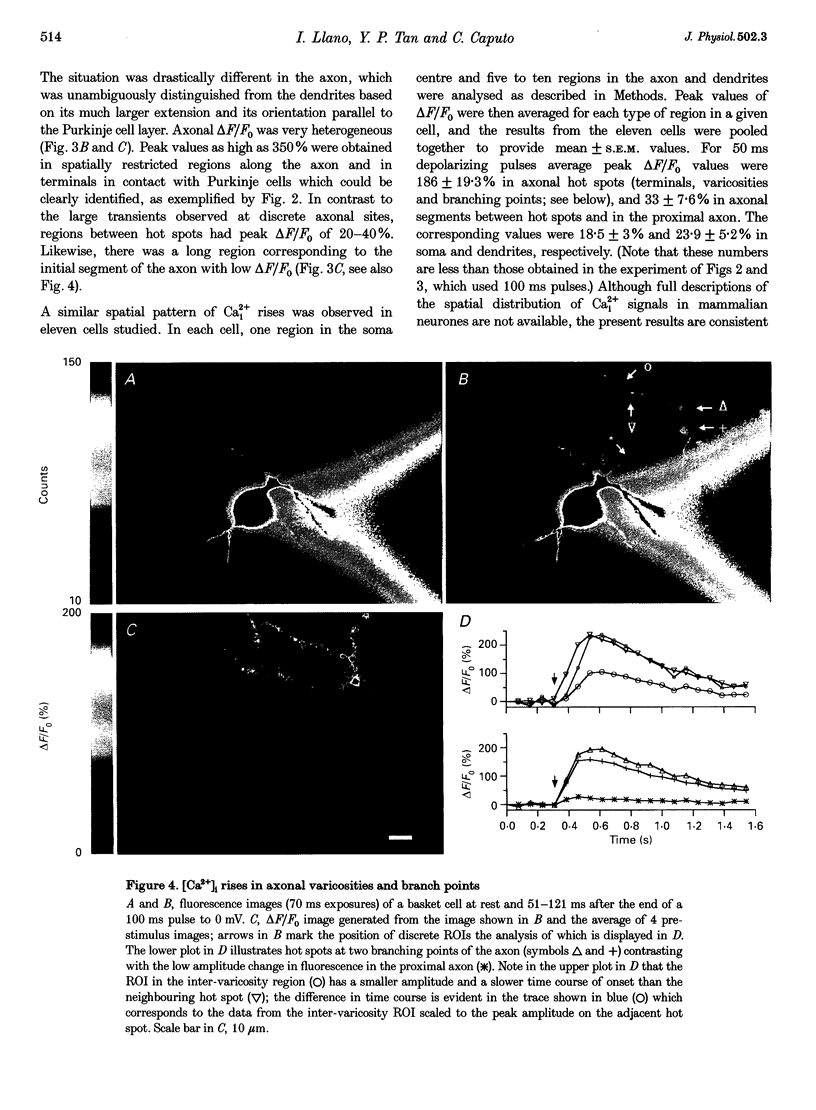




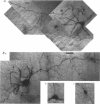
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong D. M., Rawson J. A. Activity patterns of cerebellar cortical neurones and climbing fibre afferents in the awake cat. J Physiol. 1979 Apr;289:425–448. doi: 10.1113/jphysiol.1979.sp012745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baimbridge K. G., Celio M. R., Rogers J. H. Calcium-binding proteins in the nervous system. Trends Neurosci. 1992 Aug;15(8):303–308. doi: 10.1016/0166-2236(92)90081-i. [DOI] [PubMed] [Google Scholar]
- Borst J. G., Helmchen F., Sakmann B. Pre- and postsynaptic whole-cell recordings in the medial nucleus of the trapezoid body of the rat. J Physiol. 1995 Dec 15;489(Pt 3):825–840. doi: 10.1113/jphysiol.1995.sp021095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callewaert G., Eilers J., Konnerth A. Axonal calcium entry during fast 'sodium' action potentials in rat cerebellar Purkinje neurones. J Physiol. 1996 Sep 15;495(Pt 3):641–647. doi: 10.1113/jphysiol.1996.sp021622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles J. C., Llinás R., Sasaki K. The inhibitory interneurones within the cerebellar cortex. Exp Brain Res. 1966;1(1):1–16. doi: 10.1007/BF00235206. [DOI] [PubMed] [Google Scholar]
- Escobar A. L., Monck J. R., Fernandez J. M., Vergara J. L. Localization of the site of Ca2+ release at the level of a single sarcomere in skeletal muscle fibres. Nature. 1994 Feb 24;367(6465):739–741. doi: 10.1038/367739a0. [DOI] [PubMed] [Google Scholar]
- Heidelberger R., Heinemann C., Neher E., Matthews G. Calcium dependence of the rate of exocytosis in a synaptic terminal. Nature. 1994 Oct 6;371(6497):513–515. doi: 10.1038/371513a0. [DOI] [PubMed] [Google Scholar]
- Horikawa K., Armstrong W. E. A versatile means of intracellular labeling: injection of biocytin and its detection with avidin conjugates. J Neurosci Methods. 1988 Aug;25(1):1–11. doi: 10.1016/0165-0270(88)90114-8. [DOI] [PubMed] [Google Scholar]
- Kano M., Rexhausen U., Dreessen J., Konnerth A. Synaptic excitation produces a long-lasting rebound potentiation of inhibitory synaptic signals in cerebellar Purkinje cells. Nature. 1992 Apr 16;356(6370):601–604. doi: 10.1038/356601a0. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. A study of synaptic transmission in the absence of nerve impulses. J Physiol. 1967 Sep;192(2):407–436. doi: 10.1113/jphysiol.1967.sp008307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosaka T., Kosaka K., Nakayama T., Hunziker W., Heizmann C. W. Axons and axon terminals of cerebellar Purkinje cells and basket cells have higher levels of parvalbumin immunoreactivity than somata and dendrites: quantitative analysis by immunogold labeling. Exp Brain Res. 1993;93(3):483–491. doi: 10.1007/BF00229363. [DOI] [PubMed] [Google Scholar]
- Llano I., Gerschenfeld H. M. Beta-adrenergic enhancement of inhibitory synaptic activity in rat cerebellar stellate and Purkinje cells. J Physiol. 1993 Aug;468:201–224. doi: 10.1113/jphysiol.1993.sp019767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llano I., Leresche N., Marty A. Calcium entry increases the sensitivity of cerebellar Purkinje cells to applied GABA and decreases inhibitory synaptic currents. Neuron. 1991 Apr;6(4):565–574. doi: 10.1016/0896-6273(91)90059-9. [DOI] [PubMed] [Google Scholar]
- Llano I., Marty A., Armstrong C. M., Konnerth A. Synaptic- and agonist-induced excitatory currents of Purkinje cells in rat cerebellar slices. J Physiol. 1991 Mar;434:183–213. doi: 10.1113/jphysiol.1991.sp018465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Sugimori M., Silver R. B. Microdomains of high calcium concentration in a presynaptic terminal. Science. 1992 May 1;256(5057):677–679. doi: 10.1126/science.1350109. [DOI] [PubMed] [Google Scholar]
- Lüscher C., Lipp P., Lüscher H. R., Niggli E. Control of action potential propagation by intracellular Ca2+ in cultured rat dorsal root ganglion cells. J Physiol. 1996 Jan 15;490(Pt 2):319–324. doi: 10.1113/jphysiol.1996.sp021146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E., Augustine G. J. Calcium gradients and buffers in bovine chromaffin cells. J Physiol. 1992 May;450:273–301. doi: 10.1113/jphysiol.1992.sp019127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donoghue D. L., King J. S., Bishop G. A. Physiological and anatomical studies of the interactions between Purkinje cells and basket cells in the cat's cerebellar cortex: evidence for a unitary relationship. J Neurosci. 1989 Jun;9(6):2141–2150. doi: 10.1523/JNEUROSCI.09-06-02141.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regehr W. G., Atluri P. P. Calcium transients in cerebellar granule cell presynaptic terminals. Biophys J. 1995 May;68(5):2156–2170. doi: 10.1016/S0006-3495(95)80398-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regehr W. G., Delaney K. R., Tank D. W. The role of presynaptic calcium in short-term enhancement at the hippocampal mossy fiber synapse. J Neurosci. 1994 Feb;14(2):523–537. doi: 10.1523/JNEUROSCI.14-02-00523.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regehr W. G., Tank D. W. Dendritic calcium dynamics. Curr Opin Neurobiol. 1994 Jun;4(3):373–382. doi: 10.1016/0959-4388(94)90099-x. [DOI] [PubMed] [Google Scholar]
- Regehr W. G., Tank D. W. The maintenance of LTP at hippocampal mossy fiber synapses is independent of sustained presynaptic calcium. Neuron. 1991 Sep;7(3):451–459. doi: 10.1016/0896-6273(91)90297-d. [DOI] [PubMed] [Google Scholar]
- Roberts W. M. Spatial calcium buffering in saccular hair cells. Nature. 1993 May 6;363(6424):74–76. doi: 10.1038/363074a0. [DOI] [PubMed] [Google Scholar]
- Simon S. M., Llinás R. R. Compartmentalization of the submembrane calcium activity during calcium influx and its significance in transmitter release. Biophys J. 1985 Sep;48(3):485–498. doi: 10.1016/S0006-3495(85)83804-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent P., Armstrong C. M., Marty A. Inhibitory synaptic currents in rat cerebellar Purkinje cells: modulation by postsynaptic depolarization. J Physiol. 1992 Oct;456:453–471. doi: 10.1113/jphysiol.1992.sp019346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent P., Marty A. Fluctuations of inhibitory postsynaptic currents in Purkinje cells from rat cerebellar slices. J Physiol. 1996 Jul 1;494(Pt 1):183–199. doi: 10.1113/jphysiol.1996.sp021484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent P., Marty A. Neighboring cerebellar Purkinje cells communicate via retrograde inhibition of common presynaptic interneurons. Neuron. 1993 Nov;11(5):885–893. doi: 10.1016/0896-6273(93)90118-b. [DOI] [PubMed] [Google Scholar]
- Wu L. G., Saggau P. Adenosine inhibits evoked synaptic transmission primarily by reducing presynaptic calcium influx in area CA1 of hippocampus. Neuron. 1994 May;12(5):1139–1148. doi: 10.1016/0896-6273(94)90321-2. [DOI] [PubMed] [Google Scholar]
- Wu L. G., Saggau P. Presynaptic calcium is increased during normal synaptic transmission and paired-pulse facilitation, but not in long-term potentiation in area CA1 of hippocampus. J Neurosci. 1994 Feb;14(2):645–654. doi: 10.1523/JNEUROSCI.14-02-00645.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M., Hollingworth S., Baylor S. M. Properties of tri- and tetracarboxylate Ca2+ indicators in frog skeletal muscle fibers. Biophys J. 1996 Feb;70(2):896–916. doi: 10.1016/S0006-3495(96)79633-9. [DOI] [PMC free article] [PubMed] [Google Scholar]