Abstract
1. The sympathetic nerve terminals of the mouse vas deferens were loaded with the calcium indicator Oregon Green 488 BAPTA-1 by orthograde transport along the postganglionic nerves. Changes in the calcium concentration in the varicosity (delta [Ca2+]v) were determined following single impulses, and short (5-impulse) and long (200-impulse) trains at 5 Hz. 2. All varicosities showed a significant delta [Ca2+]v in response to every single impulse. The elevated delta [Ca2+]v declined in two phases with similar kinetics for all varicosities: a fast phase (time constant, 0.42 +/- 0.05 s) and a moderate phase (3.6 +/- 0.4 s). 3. Line scanning confocal microscopy revealed that the delta [Ca2+] of a single terminal following single impulses was smaller for the intervaricose regions than for the varicosities. 4. Blockade of the voltage-sensitive calcium channels with Cd2+ (in calcium-free solution) completely blocked the delta [Ca2+]v on stimulation. The addition of either nifedipine (10 microM), omega-conotoxin GVIA (100 nM) or omega-agatoxin TK (100 nM) showed that 47 +/- 6% of the evoked response was mediated by N-type calcium channels. 5. Ryanodine (10 microM) did not significantly change the amplitude of delta [Ca2+]v in response to short trains. 6. Spontaneous increases in delta [Ca2+]v were observed in individual varicosities, with coupling in the increase of delta [Ca2+]v between varicosities. 7. The presynaptic alpha 2-receptor antagonist yohimbine (10 microM) increased the amplitude of delta [Ca2+]v in response to five impulses (5 Hz) by 54 +/- 14%, while the alpha 2-receptor agonist clonidine (1 microM) decreased the delta [Ca2+]v by 55 +/- 4%. 8. These results are discussed in terms of the hypotheses that the increased probability for secretion at sympathetic nerve terminals which accompanies facilitation and augmentation is due to the residual delta [Ca2+]v remaining after the calcium influx following impulses and that noradrenaline acts presynaptically to decrease the probability of secretion by modifying calcium influx.
Full text
PDF














Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atluri P. P., Regehr W. G. Determinants of the time course of facilitation at the granule cell to Purkinje cell synapse. J Neurosci. 1996 Sep 15;16(18):5661–5671. doi: 10.1523/JNEUROSCI.16-18-05661.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURNSTOCK G., HOLMAN M. E., KURIYAMA H. FACILITATION OF TRANSMISSION FROM AUTONOMIC NERVE TO SMOOTH MUSCLE OF GUINEA-PIG VAS DEFERENS. J Physiol. 1964 Jul;172:31–49. doi: 10.1113/jphysiol.1964.sp007401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beattie D. T., Cunnane T. C., Muir T. C. Effects of calcium channel antagonists on action potential conduction and transmitter release in the guinea-pig vas deferens. Br J Pharmacol. 1986 Sep;89(1):235–244. doi: 10.1111/j.1476-5381.1986.tb11140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R. An electrophysiological analysis of the storage and release of noradrenaline at sympathetic nerve terminals. J Physiol. 1973 Mar;229(2):515–531. doi: 10.1113/jphysiol.1973.sp010151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., Florin T. An electrophysiological analysis of the effect of Ca ions on neuromuscular transmission in the mouse vas deferens. Br J Pharmacol. 1975 Sep;55(1):97–104. doi: 10.1111/j.1476-5381.1975.tb07616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz W. J., Bewick G. S. Optical monitoring of transmitter release and synaptic vesicle recycling at the frog neuromuscular junction. J Physiol. 1993 Jan;460:287–309. doi: 10.1113/jphysiol.1993.sp019472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakeley A. G., Cunnane T. C., Maskell T., Mathie A., Petersen S. A. Alpha-adrenoceptors and facilitation at a sympathetic neuroeffector junction. J Auton Pharmacol. 1984 Mar;4(1):53–58. doi: 10.1111/j.1474-8673.1984.tb00433.x. [DOI] [PubMed] [Google Scholar]
- Boehm S., Huck S. Inhibition of N-type calcium channels: the only mechanism by which presynaptic alpha 2-autoreceptors control sympathetic transmitter release. Eur J Neurosci. 1996 Sep;8(9):1924–1931. doi: 10.1111/j.1460-9568.1996.tb01336.x. [DOI] [PubMed] [Google Scholar]
- Brain K. L., Bennett M. R. Calcium in the nerve terminals of chick ciliary ganglia during facilitation, augmentation and potentiation. J Physiol. 1995 Dec 15;489(Pt 3):637–648. doi: 10.1113/jphysiol.1995.sp021079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock J. A., Cunnane T. C. Effects of Ca2+ and K+ channel blockers on nerve impulses recorded from guinea-pig postganglionic sympathetic nerve terminals. J Physiol. 1995 Dec 1;489(Pt 2):389–402. doi: 10.1113/jphysiol.1995.sp021060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock J. A., Cunnane T. C., Evans R. J., Ziogas J. Inhibition of transmitter release from sympathetic nerve endings by omega-conotoxin. Clin Exp Pharmacol Physiol. 1989 Apr;16(4):333–339. doi: 10.1111/j.1440-1681.1989.tb01568.x. [DOI] [PubMed] [Google Scholar]
- Brock J. A., Cunnane T. C., Starke K., Wardell C. F. Alpha 2-adrenoceptor-mediated autoinhibition of sympathetic transmitter release in guinea-pig vas deferens studied by intracellular and focal extracellular recording of junction potentials and currents. Naunyn Schmiedebergs Arch Pharmacol. 1990 Jul;342(1):45–52. doi: 10.1007/BF00178971. [DOI] [PubMed] [Google Scholar]
- Illes P., Starke K. An electrophysiological study of presynaptic alpha-adrenoceptors in the vas deferens of the mouse. Br J Pharmacol. 1983 Feb;78(2):365–373. doi: 10.1111/j.1476-5381.1983.tb09402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavidis N. A., Bennett M. R. Probabilistic secretion of quanta from successive sets of visualized varicosities along single sympathetic nerve terminals. J Auton Nerv Syst. 1993 Apr;43(1):41–50. doi: 10.1016/0165-1838(93)90320-t. [DOI] [PubMed] [Google Scholar]
- Lin Y. Q., Brain K. L., Nichol K. A., Morgan J. J., Bennett M. R. Vesicle-associated proteins and calcium in nerve terminals of chick ciliary ganglia during development of facilitation. J Physiol. 1996 Dec 15;497(Pt 3):639–656. doi: 10.1113/jphysiol.1996.sp021796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Gruner J. A., Sugimori M., McGuinness T. L., Greengard P. Regulation by synapsin I and Ca(2+)-calmodulin-dependent protein kinase II of the transmitter release in squid giant synapse. J Physiol. 1991 May;436:257–282. doi: 10.1113/jphysiol.1991.sp018549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN A. R., PILAR G. PRESYNAPTIC AND POST-SYNAPTIC EVENTS DURING POST-TETANIC POTENTIATION AND FACILITATION IN THE AVIAN CILIARY GANGLION. J Physiol. 1964 Dec;175:17–30. doi: 10.1113/jphysiol.1964.sp007500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macleod G. T., Lavidis N. A., Bennett M. R. Calcium dependence of quantal secretion from visualized sympathetic nerve varicosities on the mouse vas deferens. J Physiol. 1994 Oct 1;480(Pt 1):61–70. doi: 10.1113/jphysiol.1994.sp020340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L., Zengel J. E. A quantitative description of tetanic and post-tetanic potentiation of transmitter release at the frog neuromuscular junction. J Physiol. 1975 Feb;245(1):183–208. doi: 10.1113/jphysiol.1975.sp010840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L., Zengel J. E. Augmentation: A process that acts to increase transmitter release at the frog neuromuscular junction. J Physiol. 1976 May;257(2):449–470. doi: 10.1113/jphysiol.1976.sp011378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallart A., Martin A. R. Two components of facilitation at the neuromuscular junction of the frog. J Physiol. 1967 Jul;191(1):19P–20P. [PMC free article] [PubMed] [Google Scholar]
- Poage R. E., Zengel J. E. Kinetic and pharmacological examination of stimulation-induced increases in synaptic efficacy in the chick ciliary ganglion. Synapse. 1993 May;14(1):81–89. doi: 10.1002/syn.890140111. [DOI] [PubMed] [Google Scholar]
- Regehr W. G., Atluri P. P. Calcium transients in cerebellar granule cell presynaptic terminals. Biophys J. 1995 May;68(5):2156–2170. doi: 10.1016/S0006-3495(95)80398-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regehr W. G., Delaney K. R., Tank D. W. The role of presynaptic calcium in short-term enhancement at the hippocampal mossy fiber synapse. J Neurosci. 1994 Feb;14(2):523–537. doi: 10.1523/JNEUROSCI.14-02-00523.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter H. Measurements of exocytosis from single presynaptic nerve terminals reveal heterogeneous inhibition by Ca(2+)-channel blockers. Neuron. 1995 Apr;14(4):773–779. doi: 10.1016/0896-6273(95)90221-x. [DOI] [PubMed] [Google Scholar]
- Smith A. B., Cunnane T. C. Omega-conotoxin GVIA-resistant neurotransmitter release in postganglionic sympathetic nerve terminals. Neuroscience. 1996 Feb;70(3):817–824. doi: 10.1016/s0306-4522(96)83018-1. [DOI] [PubMed] [Google Scholar]
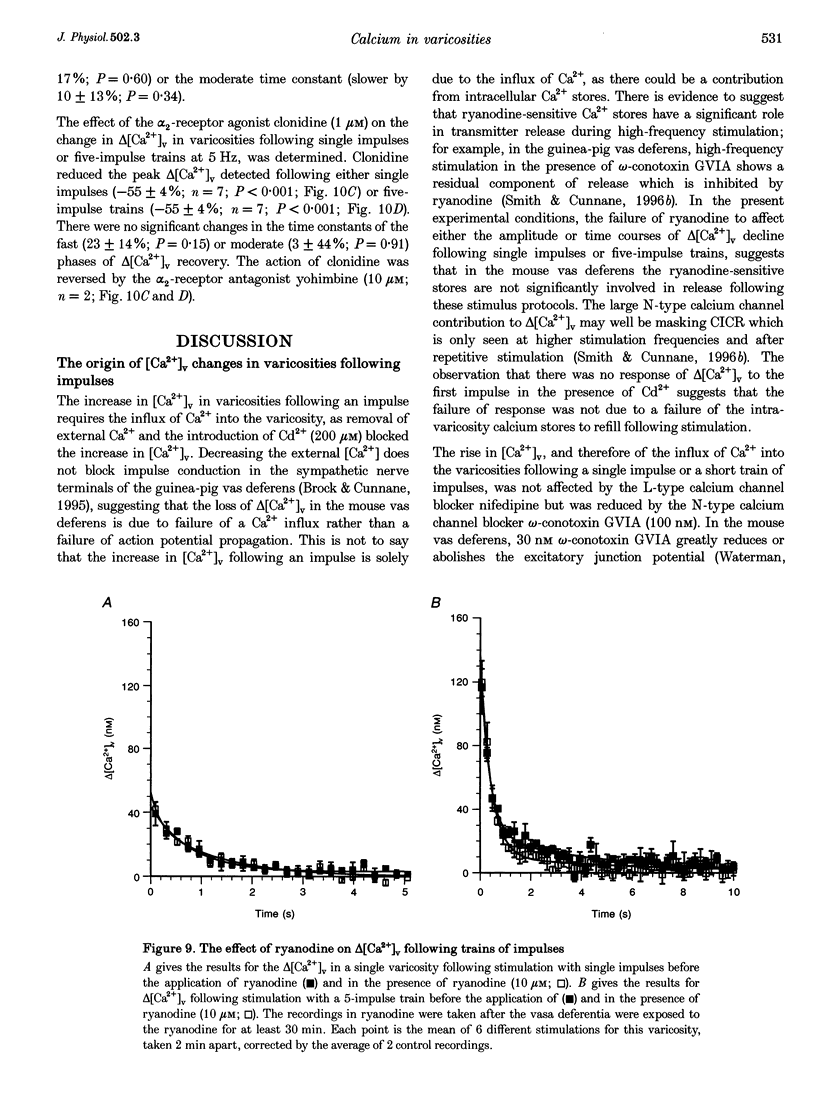
- Smith A. B., Cunnane T. C. Ryanodine-sensitive calcium stores involved in neurotransmitter release from sympathetic nerve terminals of the guinea-pig. J Physiol. 1996 Dec 15;497(Pt 3):657–664. doi: 10.1113/jphysiol.1996.sp021797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneddon P., Burnstock G. ATP as a co-transmitter in rat tail artery. Eur J Pharmacol. 1984 Oct 30;106(1):149–152. doi: 10.1016/0014-2999(84)90688-5. [DOI] [PubMed] [Google Scholar]
- Stjärne L., Stjärne E., Msghina M., Bao J. X. K+ and Ca2+ channel blockers may enhance or depress sympathetic transmitter release via a Ca(2+)-dependent mechanism "upstream" of the release site. Neuroscience. 1991;44(3):673–692. doi: 10.1016/0306-4522(91)90087-5. [DOI] [PubMed] [Google Scholar]
- Surprenant A., Neild T. O., Holman M. E. Effects of nifedipine on nerve-evoked action potentials and consequent contractions in rat tail artery. Pflugers Arch. 1983 Mar;396(4):342–349. doi: 10.1007/BF01063940. [DOI] [PubMed] [Google Scholar]
- Turner T. J., Lampe R. A., Dunlap K. Characterization of presynaptic calcium channels with omega-conotoxin MVIIC and omega-grammotoxin SIA: role for a resistant calcium channel type in neurosecretion. Mol Pharmacol. 1995 Feb;47(2):348–353. [PubMed] [Google Scholar]
- Waterman S. A. Role of N-, P- and Q-type voltage-gated calcium channels in transmitter release from sympathetic neurones in the mouse isolated vas deferens. Br J Pharmacol. 1997 Feb;120(3):393–398. doi: 10.1038/sj.bjp.0700948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright C. E., Angus J. A. Effects of N-, P- and Q-type neuronal calcium channel antagonists on mammalian peripheral neurotransmission. Br J Pharmacol. 1996 Sep;119(1):49–56. doi: 10.1111/j.1476-5381.1996.tb15676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada W. M., Zucker R. S. Time course of transmitter release calculated from simulations of a calcium diffusion model. Biophys J. 1992 Mar;61(3):671–682. doi: 10.1016/S0006-3495(92)81872-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yawo H., Chuhma N. Omega-conotoxin-sensitive and -resistant transmitter release from the chick ciliary presynaptic terminal. J Physiol. 1994 Jun 15;477(Pt 3):437–448. doi: 10.1113/jphysiol.1994.sp020205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zengel J. E., Magleby K. L. Augmentation and facilitation of transmitter release. A quantitative description at the frog neuromuscular junction. J Gen Physiol. 1982 Oct;80(4):583–611. doi: 10.1085/jgp.80.4.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker R. S. Short-term synaptic plasticity. Annu Rev Neurosci. 1989;12:13–31. doi: 10.1146/annurev.ne.12.030189.000305. [DOI] [PubMed] [Google Scholar]