Abstract
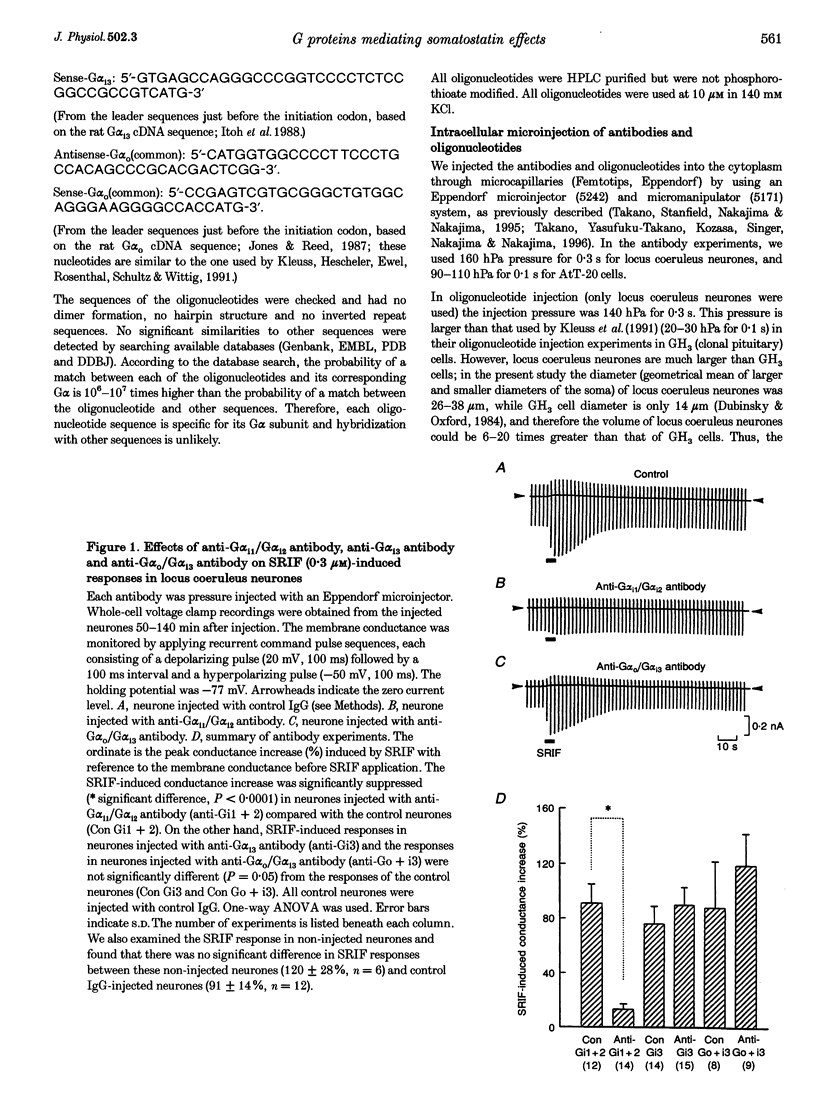
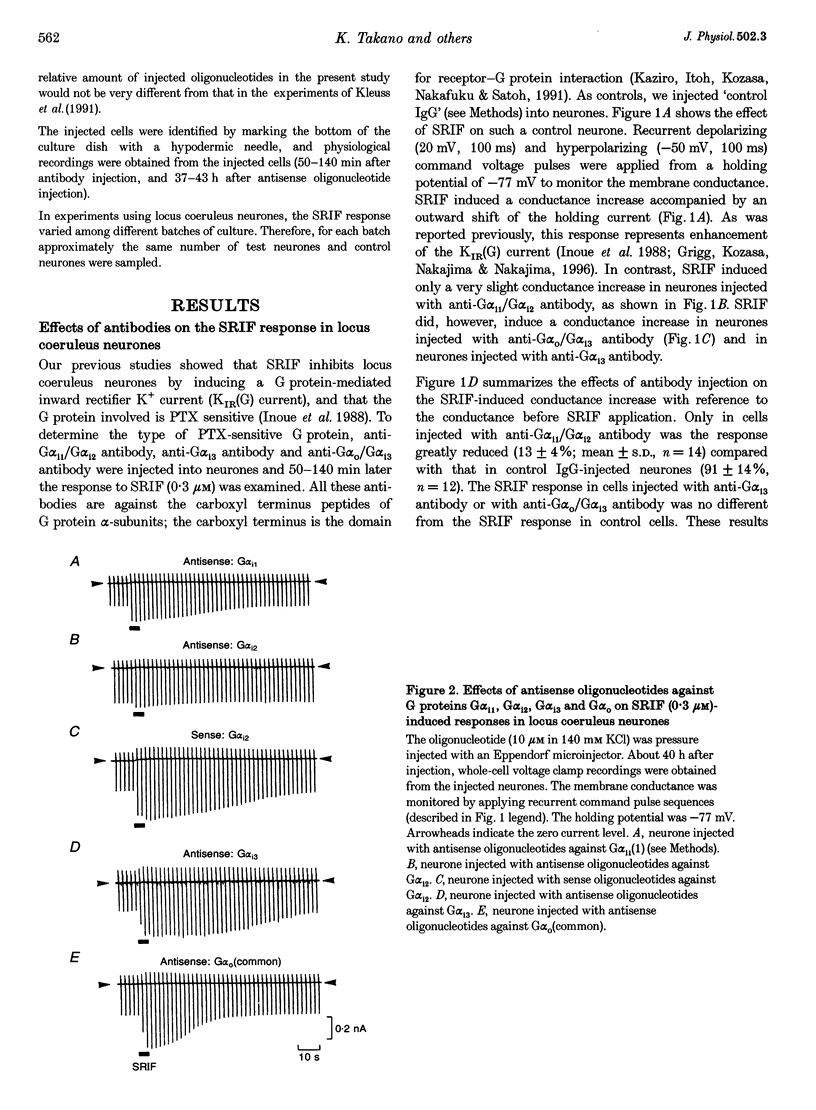
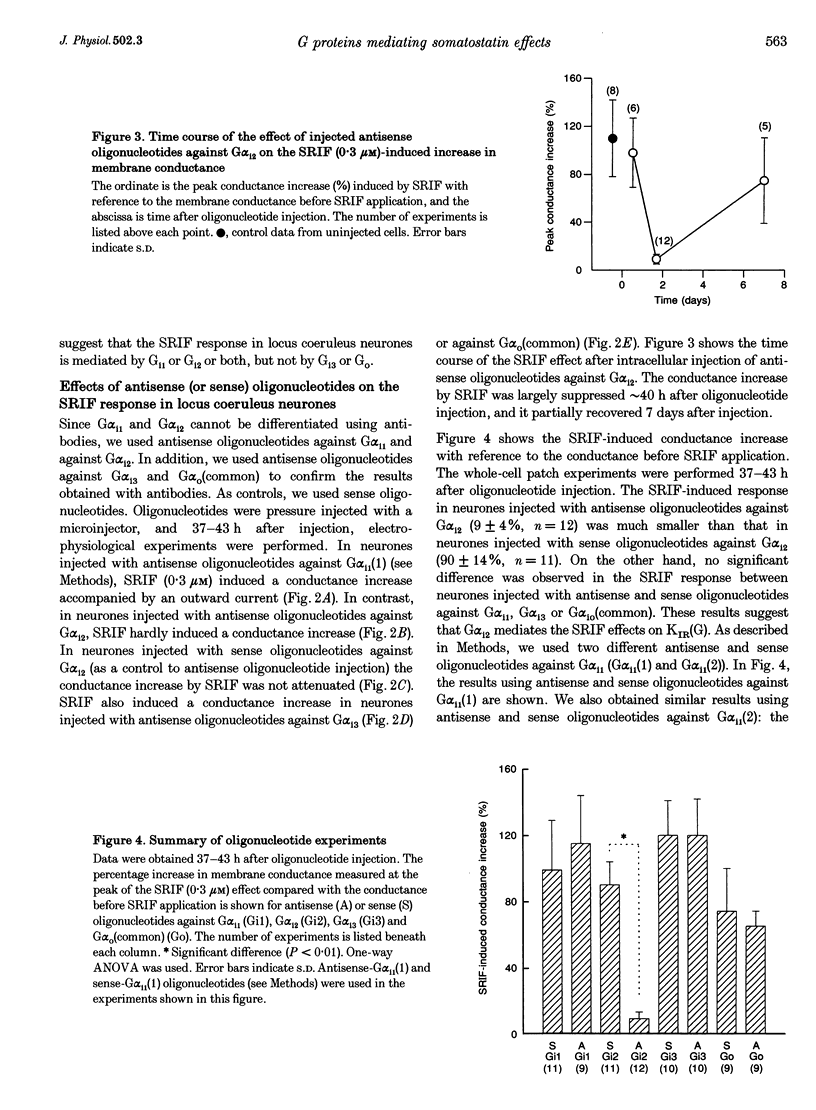
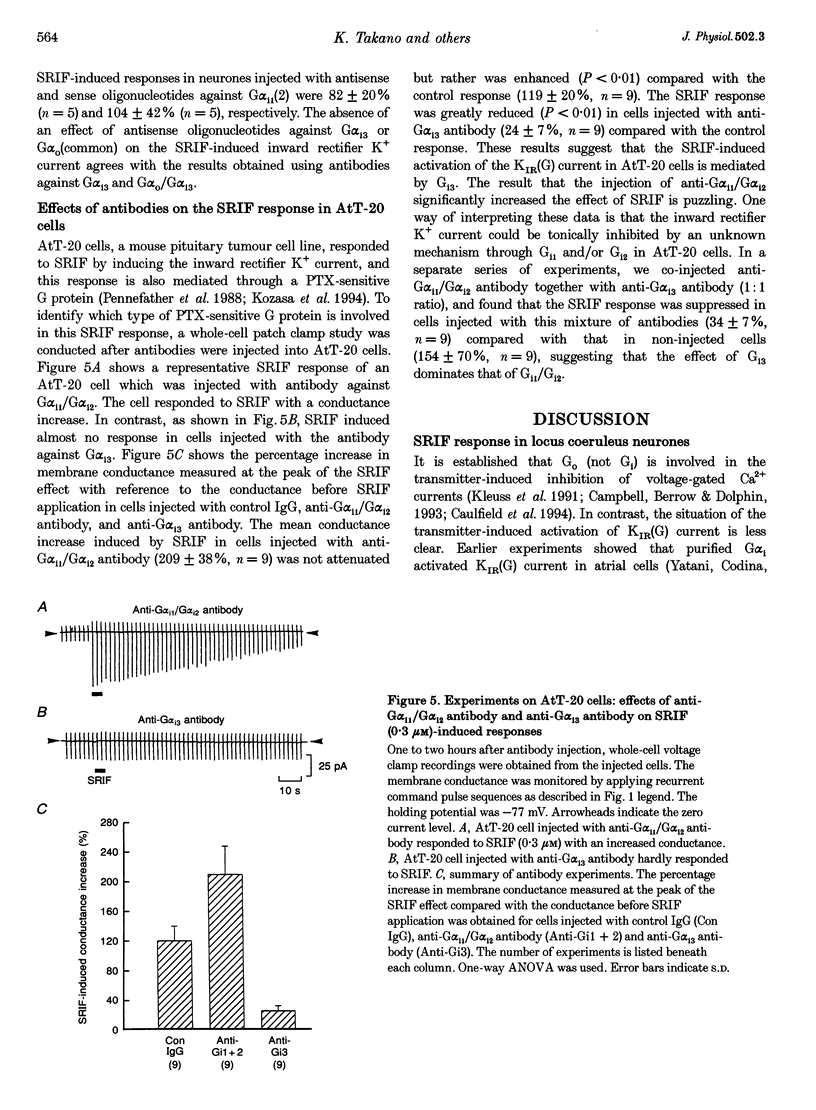
1. Types of G proteins (G protein alpha-subunit subtypes) which mediate the activation of inward rectifier K+ currents by somatostatin (somatotrophin release-inhibiting factor, SRIF) were determined in cultured locus coeruleus neurones from newborn rats and in AtT-20 cells (a mouse pituitary cell line). 2. The whole-cell patch clamp technique was used together with injection of antibodies against pertussis toxin (PTX)-sensitive G protein alpha-subunits or with injection of antisense (or sense) oligonucleotides against these G proteins. 3. In locus coeruleus neurones, the SRIF-induced activation of inward rectifier K+ currents was inhibited by anti-G alpha i1/G alpha i2 antibody injection, but not by anti-G alpha i3 or by anti-G alpha o/G alpha i3 antibody injection, suggesting that the SRIF response is mediated through G alpha i1 and/or G alpha i2. 4. The SRIF-induced activation of the inward rectifier was suppressed in locus coeruleus neurones after injection of antisense oligonucleotides against G alpha i2, but not by injection of sense oligonucleotides against G alpha i2. Injection of antisense (or sense) oligonucleotides against G alpha i1, G alpha i3 and G alpha O (common) had no effect. These results suggest that G alpha i2 is involved in this SRIF response. 5. In AtT-20 cells, the SRIF-induced activation of inward rectifier K+ currents was suppressed by injection of anti-G alpha i3 antibody, but not by injection of anti-G alpha i1/G alpha i2 antibody. 6. The above results indicate that Gi mediates the SRIF effects on inward rectifier K+ currents. However, different subtypes of Gi are involved in the brain neurones and in the endocrine cells: Gi2 in locus coeruleus neurones and Gi3 in AtT-20 cells.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brazeau P., Vale W., Burgus R., Ling N., Butcher M., Rivier J., Guillemin R. Hypothalamic polypeptide that inhibits the secretion of immunoreactive pituitary growth hormone. Science. 1973 Jan 5;179(4068):77–79. doi: 10.1126/science.179.4068.77. [DOI] [PubMed] [Google Scholar]
- Breder C. D., Yamada Y., Yasuda K., Seino S., Saper C. B., Bell G. I. Differential expression of somatostatin receptor subtypes in brain. J Neurosci. 1992 Oct;12(10):3920–3934. doi: 10.1523/JNEUROSCI.12-10-03920.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caulfield M. P., Jones S., Vallis Y., Buckley N. J., Kim G. D., Milligan G., Brown D. A. Muscarinic M-current inhibition via G alpha q/11 and alpha-adrenoceptor inhibition of Ca2+ current via G alpha o in rat sympathetic neurones. J Physiol. 1994 Jun 15;477(Pt 3):415–422. doi: 10.1113/jphysiol.1994.sp020203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Clarke I. J. G(o)-2 protein mediates the reduction in Ca2+ currents by somatostatin in cultured ovine somatotrophs. J Physiol. 1996 Feb 15;491(Pt 1):21–29. doi: 10.1113/jphysiol.1996.sp021193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubinsky J. M., Oxford G. S. Ionic currents in two strains of rat anterior pituitary tumor cells. J Gen Physiol. 1984 Mar;83(3):309–339. doi: 10.1085/jgp.83.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher T. L., Terhorst T., Cao X., Wagner R. W. Intracellular disposition and metabolism of fluorescently-labeled unmodified and modified oligonucleotides microinjected into mammalian cells. Nucleic Acids Res. 1993 Aug 11;21(16):3857–3865. doi: 10.1093/nar/21.16.3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith P., Gierschik P., Milligan G., Unson C. G., Vinitsky R., Malech H. L., Spiegel A. M. Antibodies directed against synthetic peptides distinguish between GTP-binding proteins in neutrophil and brain. J Biol Chem. 1987 Oct 25;262(30):14683–14688. [PubMed] [Google Scholar]
- Grigg J. J., Kozasa T., Nakajima Y., Nakajima S. Single-channel properties of a G-protein-coupled inward rectifier potassium channel in brain neurons. J Neurophysiol. 1996 Jan;75(1):318–328. doi: 10.1152/jn.1996.75.1.318. [DOI] [PubMed] [Google Scholar]
- Inoue M., Nakajima S., Nakajima Y. Somatostatin induces an inward rectification in rat locus coeruleus neurones through a pertussis toxin-sensitive mechanism. J Physiol. 1988 Dec;407:177–198. doi: 10.1113/jphysiol.1988.sp017409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh H., Toyama R., Kozasa T., Tsukamoto T., Matsuoka M., Kaziro Y. Presence of three distinct molecular species of Gi protein alpha subunit. Structure of rat cDNAs and human genomic DNAs. J Biol Chem. 1988 May 15;263(14):6656–6664. [PubMed] [Google Scholar]
- Jones D. T., Reed R. R. Molecular cloning of five GTP-binding protein cDNA species from rat olfactory neuroepithelium. J Biol Chem. 1987 Oct 15;262(29):14241–14249. [PubMed] [Google Scholar]
- Kaziro Y., Itoh H., Kozasa T., Nakafuku M., Satoh T. Structure and function of signal-transducing GTP-binding proteins. Annu Rev Biochem. 1991;60:349–400. doi: 10.1146/annurev.bi.60.070191.002025. [DOI] [PubMed] [Google Scholar]
- Kleuss C., Hescheler J., Ewel C., Rosenthal W., Schultz G., Wittig B. Assignment of G-protein subtypes to specific receptors inducing inhibition of calcium currents. Nature. 1991 Sep 5;353(6339):43–48. doi: 10.1038/353043a0. [DOI] [PubMed] [Google Scholar]
- Kozasa T., Kaziro Y., Ohtsuka T., Grigg J. J., Nakajima S., Nakajima Y. G protein specificity of the muscarine-induced increase in an inward rectifier potassium current in AtT-20 cells. Neurosci Res. 1996 Nov;26(3):289–297. doi: 10.1016/s0168-0102(96)01111-x. [DOI] [PubMed] [Google Scholar]
- Law S. F., Manning D., Reisine T. Identification of the subunits of GTP-binding proteins coupled to somatostatin receptors. J Biol Chem. 1991 Sep 25;266(27):17885–17897. [PubMed] [Google Scholar]
- Law S. F., Reisine T. Agonist binding to rat brain somatostatin receptors alters the interaction of the receptors with guanine nucleotide-binding regulatory proteins. Mol Pharmacol. 1992 Sep;42(3):398–402. [PubMed] [Google Scholar]
- Law S. F., Woulfe D., Reisine T. Somatostatin receptor activation of cellular effector systems. Cell Signal. 1995 Jan;7(1):1–8. doi: 10.1016/0898-6568(94)00064-i. [DOI] [PubMed] [Google Scholar]
- Lewis D. L., Weight F. F., Luini A. A guanine nucleotide-binding protein mediates the inhibition of voltage-dependent calcium current by somatostatin in a pituitary cell line. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9035–9039. doi: 10.1073/pnas.83.23.9035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luthin D. R., Eppler C. M., Linden J. Identification and quantification of Gi-type GTP-binding proteins that copurify with a pituitary somatostatin receptor. J Biol Chem. 1993 Mar 15;268(8):5990–5996. [PubMed] [Google Scholar]
- Masuko S., Nakajima Y., Nakajima S., Yamaguchi K. Noradrenergic neurons from the locus ceruleus in dissociated cell culture: culture methods, morphology, and electrophysiology. J Neurosci. 1986 Nov;6(11):3229–3241. doi: 10.1523/JNEUROSCI.06-11-03229.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumby S. M., Gilman A. G. Synthetic peptide antisera with determined specificity for G protein alpha or beta subunits. Methods Enzymol. 1991;195:215–233. doi: 10.1016/0076-6879(91)95168-j. [DOI] [PubMed] [Google Scholar]
- Murray-Whelan R., Schlegel W. Brain somatostatin receptor-G protein interaction. G alpha C-terminal antibodies demonstrate coupling of the soluble receptor with Gi(1-3) but not with Go. J Biol Chem. 1992 Feb 15;267(5):2960–2965. [PubMed] [Google Scholar]
- Nakajima Y., Nakajima S., Kozasa T. Activation of G protein-coupled inward rectifier K+ channels in brain neurons requires association of G protein beta gamma subunits with cell membrane. FEBS Lett. 1996 Jul 22;390(2):217–220. doi: 10.1016/0014-5793(96)00661-8. [DOI] [PubMed] [Google Scholar]
- Patel Y. C., Panetta R., Escher E., Greenwood M., Srikant C. B. Expression of multiple somatostatin receptor genes in AtT-20 cells. Evidence for a novel somatostatin-28 selective receptor subtype. J Biol Chem. 1994 Jan 14;269(2):1506–1509. [PubMed] [Google Scholar]
- Pennefather P. S., Heisler S., MacDonald J. F. A potassium conductance contributes to the action of somatostatin-14 to suppress ACTH secretion. Brain Res. 1988 Mar 22;444(2):346–350. doi: 10.1016/0006-8993(88)90944-4. [DOI] [PubMed] [Google Scholar]
- Pérez J., Rigo M., Kaupmann K., Bruns C., Yasuda K., Bell G. I., Lübbert H., Hoyer D. Localization of somatostatin (SRIF) SSTR-1, SSTR-2 and SSTR-3 receptor mRNA in rat brain by in situ hybridization. Naunyn Schmiedebergs Arch Pharmacol. 1994 Feb;349(2):145–160. doi: 10.1007/BF00169831. [DOI] [PubMed] [Google Scholar]
- Reisine T., Bell G. I. Molecular properties of somatostatin receptors. Neuroscience. 1995 Aug;67(4):777–790. doi: 10.1016/0306-4522(95)00072-q. [DOI] [PubMed] [Google Scholar]
- Slesinger P. A., Reuveny E., Jan Y. N., Jan L. Y. Identification of structural elements involved in G protein gating of the GIRK1 potassium channel. Neuron. 1995 Nov;15(5):1145–1156. doi: 10.1016/0896-6273(95)90102-7. [DOI] [PubMed] [Google Scholar]
- Takano K., Stanfield P. R., Nakajima S., Nakajima Y. Protein kinase C-mediated inhibition of an inward rectifier potassium channel by substance P in nucleus basalis neurons. Neuron. 1995 May;14(5):999–1008. doi: 10.1016/0896-6273(95)90338-0. [DOI] [PubMed] [Google Scholar]
- Takano K., Yasufuku-Takano J., Kozasa T., Singer W. D., Nakajima S., Nakajima Y. Gq/11 and PLC-beta 1 mediate the substance P-induced inhibition of an inward rectifier K+ channel in brain neurons. J Neurophysiol. 1996 Sep;76(3):2131–2136. doi: 10.1152/jn.1996.76.3.2131. [DOI] [PubMed] [Google Scholar]
- Tallent M., Liapakis G., O'Carroll A. M., Lolait S. J., Dichter M., Reisine T. Somatostatin receptor subtypes SSTR2 and SSTR5 couple negatively to an L-type Ca2+ current in the pituitary cell line AtT-20. Neuroscience. 1996 Apr;71(4):1073–1081. doi: 10.1016/0306-4522(95)00510-2. [DOI] [PubMed] [Google Scholar]
- Tallent M., Reisine T. Gi alpha 1 selectively couples somatostatin receptors to adenylyl cyclase in pituitary-derived AtT-20 cells. Mol Pharmacol. 1992 Mar;41(3):452–455. [PubMed] [Google Scholar]
- Wickman K. D., Iñiguez-Lluhl J. A., Davenport P. A., Taussig R., Krapivinsky G. B., Linder M. E., Gilman A. G., Clapham D. E. Recombinant G-protein beta gamma-subunits activate the muscarinic-gated atrial potassium channel. Nature. 1994 Mar 17;368(6468):255–257. doi: 10.1038/368255a0. [DOI] [PubMed] [Google Scholar]
- Yamashita N., Shibuya N., Ogata E. Requirement of GTP on somatostatin-induced K+ current in human pituitary tumor cells. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4924–4928. doi: 10.1073/pnas.85.13.4924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatani A., Codina J., Brown A. M., Birnbaumer L. Direct activation of mammalian atrial muscarinic potassium channels by GTP regulatory protein Gk. Science. 1987 Jan 9;235(4785):207–211. doi: 10.1126/science.2432660. [DOI] [PubMed] [Google Scholar]


