Abstract
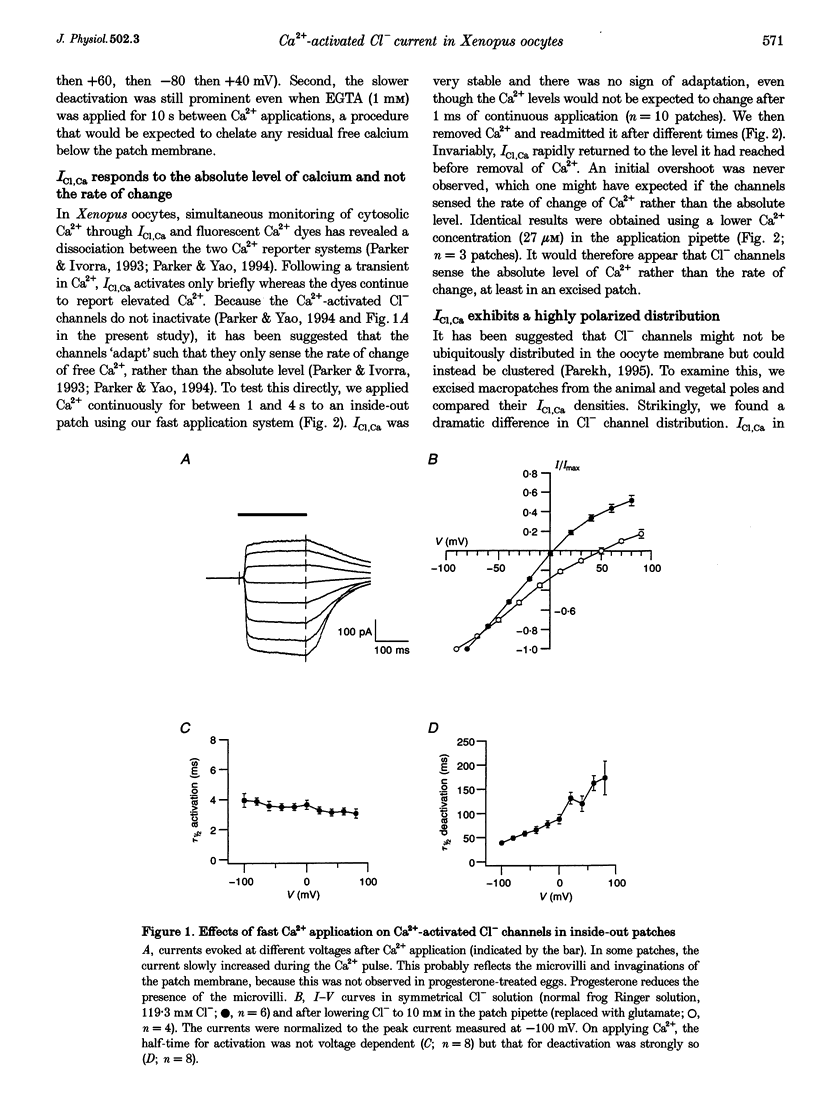
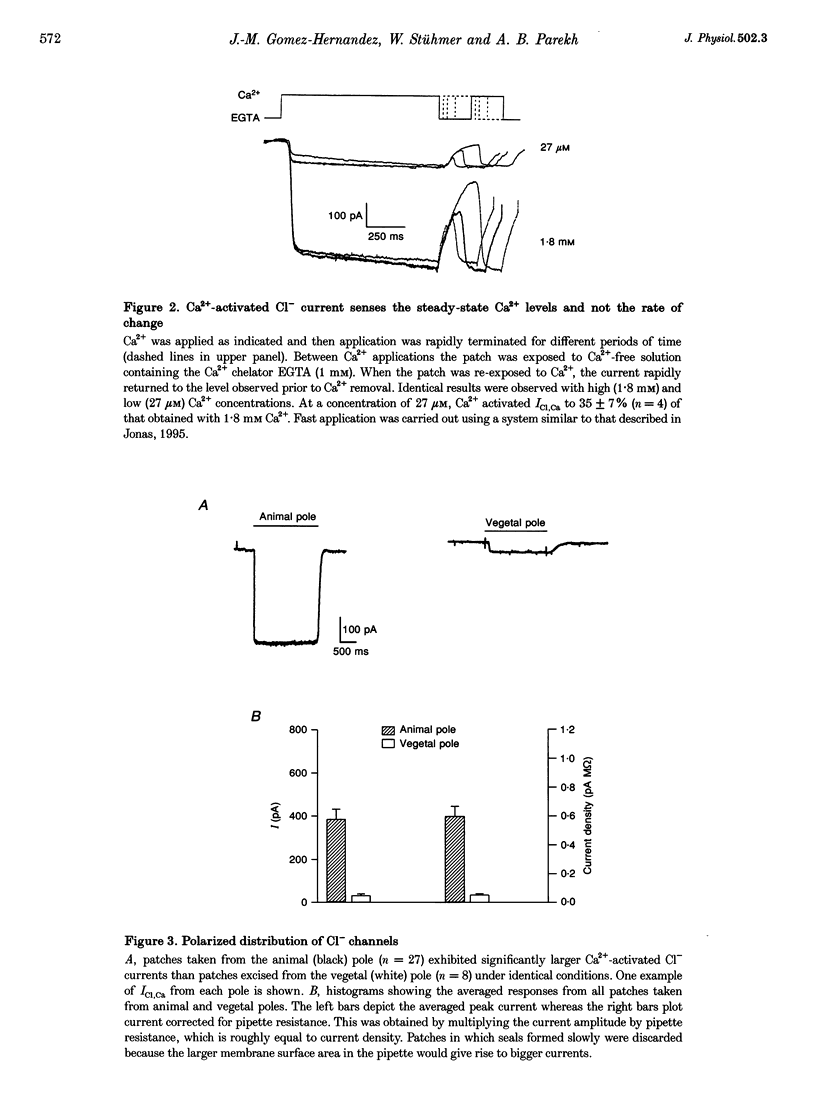
1. The Ca(2+)-dependent Cl- current (ICl,Ca), expressed in the plasma membrane of Xenopus oocytes, was examined in excised inside-out macropatches using a rapid perfusion system. 2. Application of Ca(2+)-containing Ringer solution resulted in the activation of a current whose reversal potential shifted to the right by 51 +/- 5.2 mV when Cl- in the pipette solution was lowered from 119.3 to 10 mM. No currents were generated when Ca2+ was omitted from the solution. The current is therefore a Ca(2+)-activated Cl- one. 3. Following exposure to Ca2+, the half-time for activation of ICl,Ca was not voltage dependent, whereas deactivation was strongly so. 4. ICl,Ca was stable in the continuous presence of Ca2+ and showed no sign of inactivation or adaptation. 5. Comparison of the size of the currents (normalized to pipette resistance) from the animal and vegetal poles revealed that ICl,Ca had a highly polarized distribution. The current density was almost 10 times higher in the animal pole. 6. The results suggest that Cl- channels provide a continuous and reliable indication of submembranous Ca2+, at least in an excised patch, and the clustering of the Cl- channels renders it necessary to exert caution in interpreting results involving the kinetics of Ca2+ signalling, when ICl,Ca is used as the sole monitor of calcium.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berridge M. J. Inositol trisphosphate and calcium signalling. Nature. 1993 Jan 28;361(6410):315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- Boton R., Dascal N., Gillo B., Lass Y. Two calcium-activated chloride conductances in Xenopus laevis oocytes permeabilized with the ionophore A23187. J Physiol. 1989 Jan;408:511–534. doi: 10.1113/jphysiol.1989.sp017473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dascal N. The use of Xenopus oocytes for the study of ion channels. CRC Crit Rev Biochem. 1987;22(4):317–387. doi: 10.3109/10409238709086960. [DOI] [PubMed] [Google Scholar]
- Fasolato C., Innocenti B., Pozzan T. Receptor-activated Ca2+ influx: how many mechanisms for how many channels? Trends Pharmacol Sci. 1994 Mar;15(3):77–83. doi: 10.1016/0165-6147(94)90282-8. [DOI] [PubMed] [Google Scholar]
- Györke S., Fill M. Ryanodine receptor adaptation: control mechanism of Ca(2+)-induced Ca2+ release in heart. Science. 1993 May 7;260(5109):807–809. doi: 10.1126/science.8387229. [DOI] [PubMed] [Google Scholar]
- Hartzell H. C. Activation of different Cl currents in Xenopus oocytes by Ca liberated from stores and by capacitative Ca influx. J Gen Physiol. 1996 Sep;108(3):157–175. doi: 10.1085/jgp.108.3.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechleiter J. D., Clapham D. E. Molecular mechanisms of intracellular calcium excitability in X. laevis oocytes. Cell. 1992 Apr 17;69(2):283–294. doi: 10.1016/0092-8674(92)90409-6. [DOI] [PubMed] [Google Scholar]
- Lechleiter J., Girard S., Peralta E., Clapham D. Spiral calcium wave propagation and annihilation in Xenopus laevis oocytes. Science. 1991 Apr 5;252(5002):123–126. doi: 10.1126/science.2011747. [DOI] [PubMed] [Google Scholar]
- Lupu-Meiri M., Shapira H., Oron Y. Hemispheric asymmetry of rapid chloride responses to inositol trisphosphate and calcium in Xenopus oocytes. FEBS Lett. 1988 Nov 21;240(1-2):83–87. doi: 10.1016/0014-5793(88)80344-2. [DOI] [PubMed] [Google Scholar]
- Miledi R., Parker I. Chloride current induced by injection of calcium into Xenopus oocytes. J Physiol. 1984 Dec;357:173–183. doi: 10.1113/jphysiol.1984.sp015495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neely A., Olcese R., Wei X., Birnbaumer L., Stefani E. Ca(2+)-dependent inactivation of a cloned cardiac Ca2+ channel alpha 1 subunit (alpha 1C) expressed in Xenopus oocytes. Biophys J. 1994 Jun;66(6):1895–1903. doi: 10.1016/S0006-3495(94)80983-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekh A. B., Foguet M., Lübbert H., Stühmer W. Ca2+ oscillations and Ca2+ influx in Xenopus oocytes expressing a novel 5-hydroxytryptamine receptor. J Physiol. 1993 Sep;469:653–671. doi: 10.1113/jphysiol.1993.sp019836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekh A. B. Interaction between capacitative Ca2+ influx and Ca2+-dependent Cl- currents in Xenopus oocytes. Pflugers Arch. 1995 Oct;430(6):954–963. doi: 10.1007/BF01837409. [DOI] [PubMed] [Google Scholar]
- Parekh A. B., Terlau H., Stühmer W. Depletion of InsP3 stores activates a Ca2+ and K+ current by means of a phosphatase and a diffusible messenger. Nature. 1993 Aug 26;364(6440):814–818. doi: 10.1038/364814a0. [DOI] [PubMed] [Google Scholar]
- Parker I., Ivorra I. Confocal microfluorimetry of Ca2+ signals evoked in Xenopus oocytes by photoreleased inositol trisphosphate. J Physiol. 1993 Feb;461:133–165. doi: 10.1113/jphysiol.1993.sp019506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker I., Ivorra I. Inhibition by Ca2+ of inositol trisphosphate-mediated Ca2+ liberation: a possible mechanism for oscillatory release of Ca2+. Proc Natl Acad Sci U S A. 1990 Jan;87(1):260–264. doi: 10.1073/pnas.87.1.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker I., Yao Y. Relation between intracellular Ca2+ signals and Ca(2+)-activated Cl- current in Xenopus oocytes. Cell Calcium. 1994 Apr;15(4):276–288. doi: 10.1016/0143-4160(94)90067-1. [DOI] [PubMed] [Google Scholar]
- Peter A. B., Schittny J. C., Niggli V., Reuter H., Sigel E. The polarized distribution of poly(A+)-mRNA-induced functional ion channels in the Xenopus oocyte plasma membrane is prevented by anticytoskeletal drugs. J Cell Biol. 1991 Aug;114(3):455–464. doi: 10.1083/jcb.114.3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen C. C., Berridge M. J. The regulation of capacitative calcium entry by calcium and protein kinase C in Xenopus oocytes. J Biol Chem. 1994 Dec 23;269(51):32246–32253. [PubMed] [Google Scholar]


