Abstract
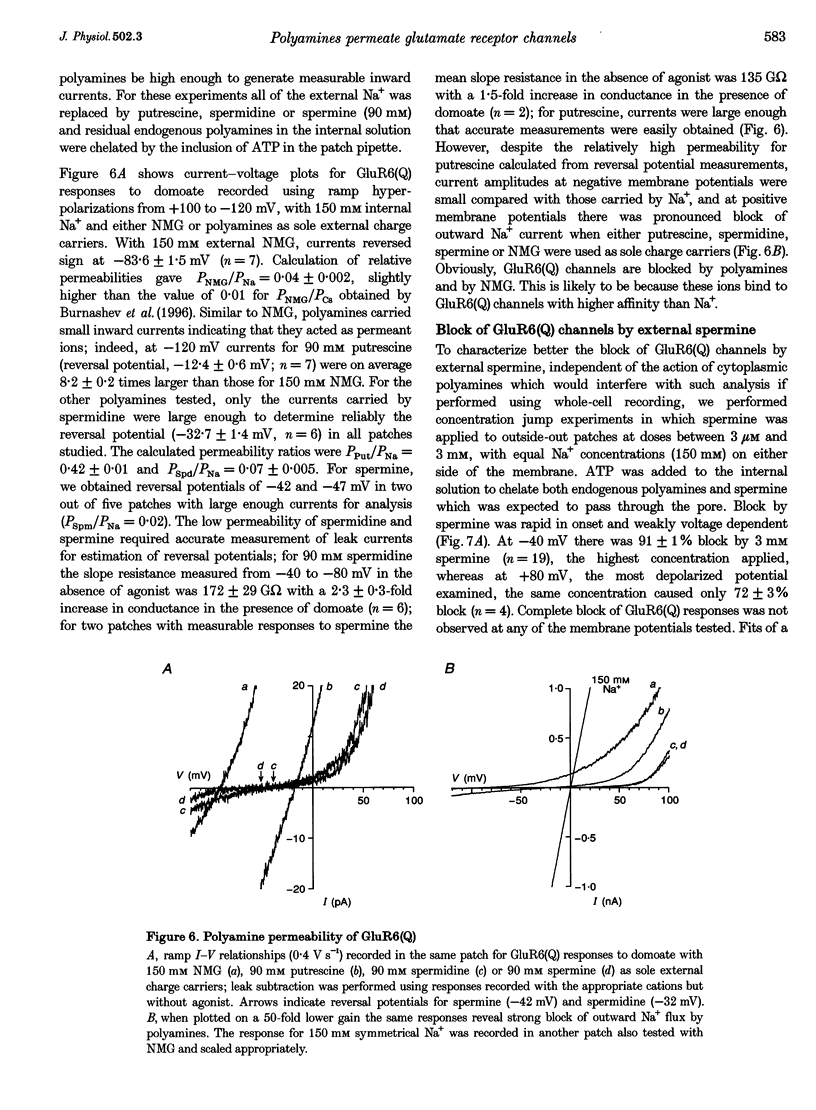
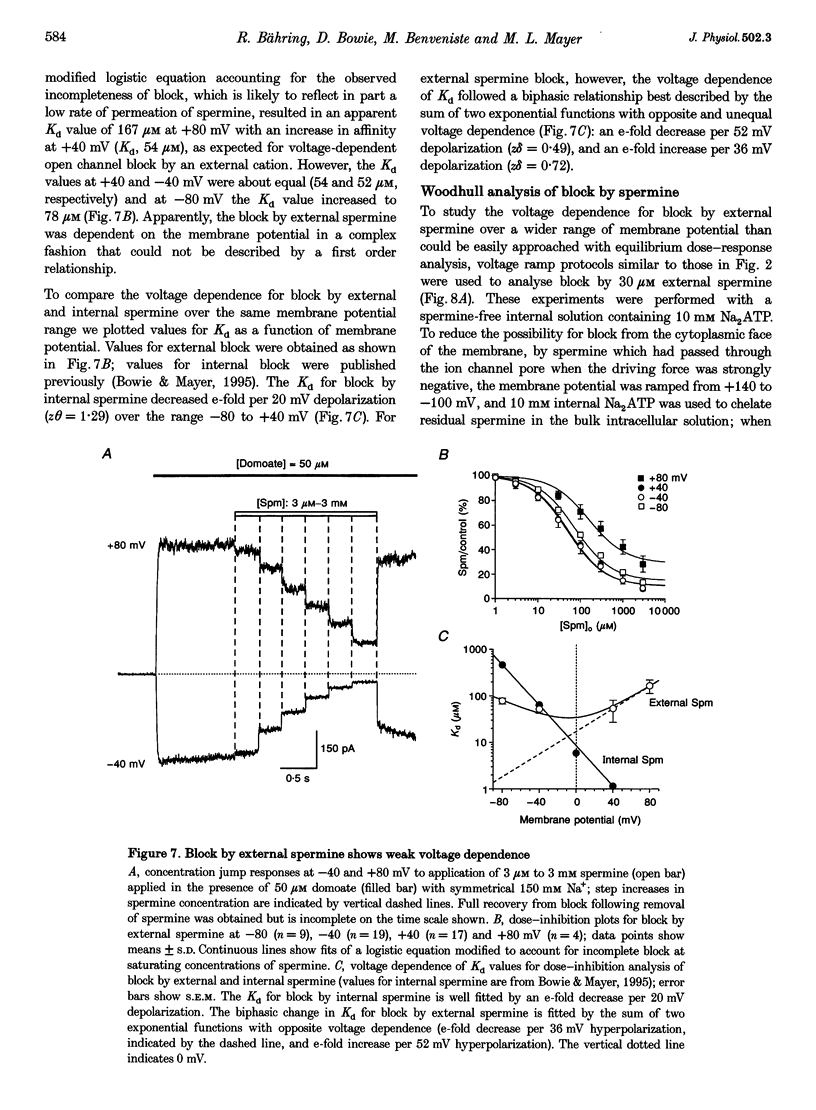
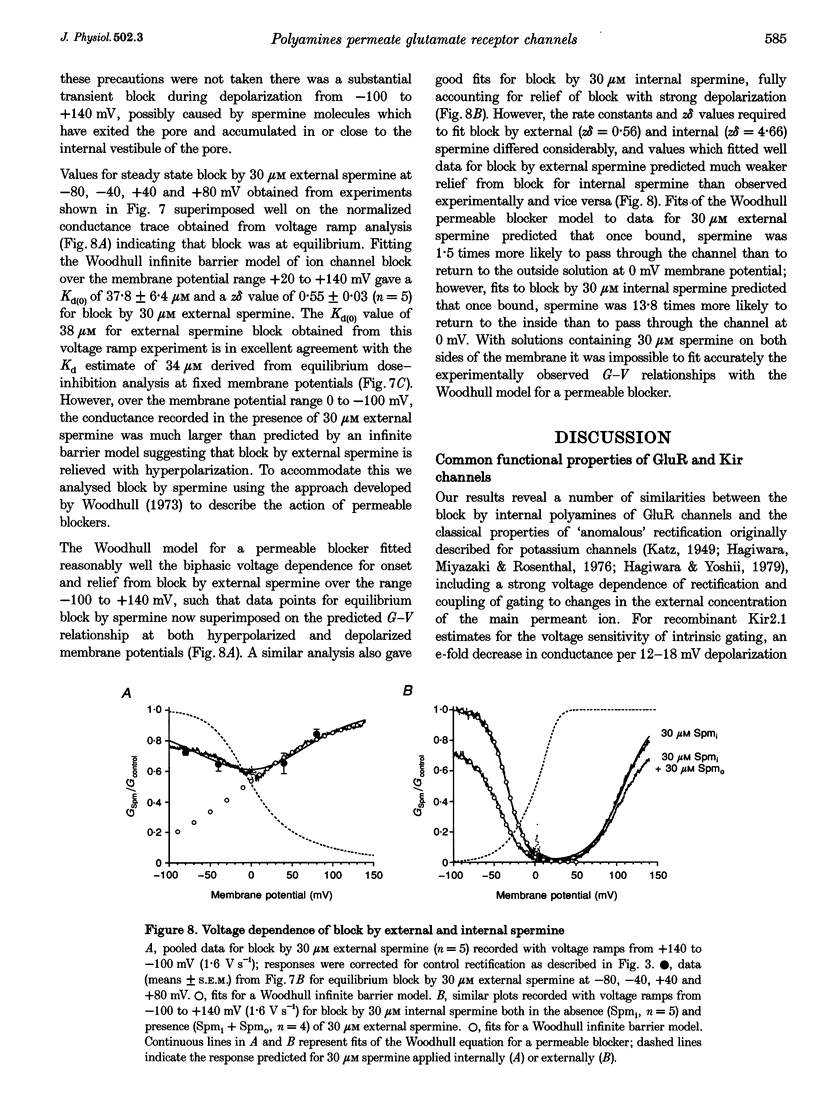
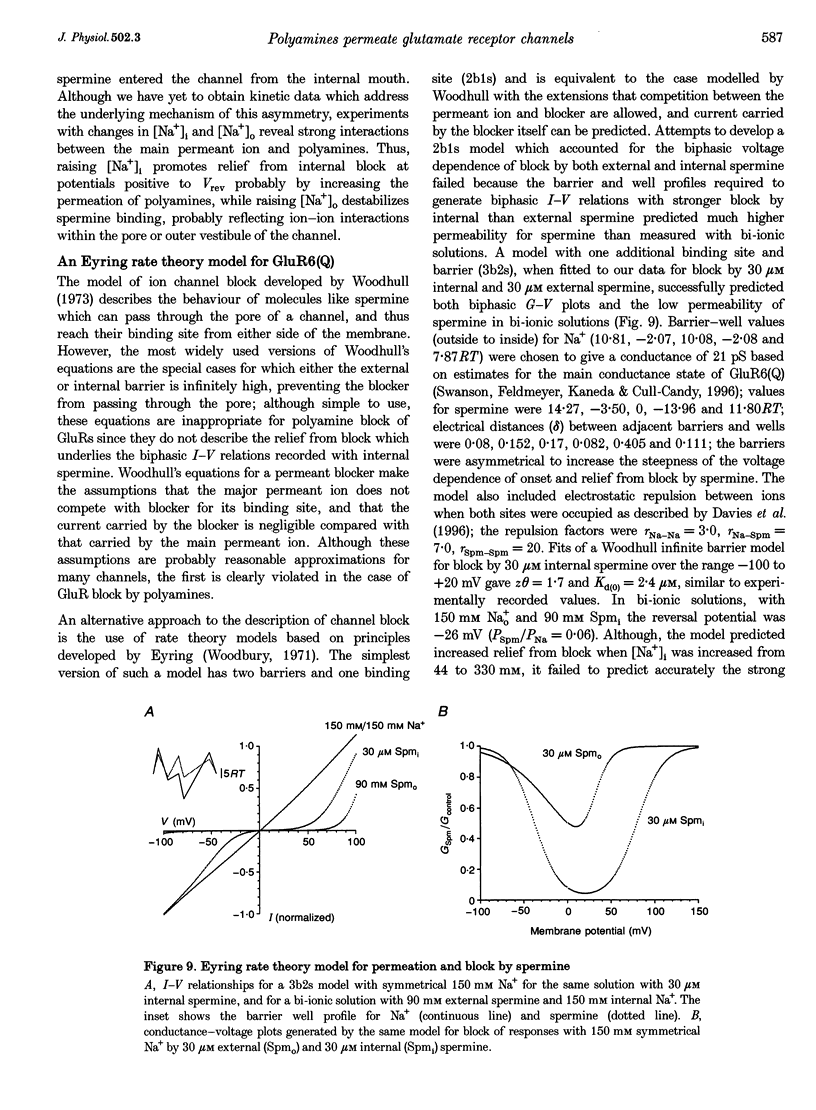
1. Polyamine block of rat GluR6(Q) glutamate receptor channels was studied in outside-out patches from transiently transfected HEK 293 cells. With symmetrical 150 mM Na+ and 30 microM internal spermine there was biphasic voltage dependence with 95% block at +40 mV but only 20% block at +140 mV. Dose-inhibition analysis for external spermine also revealed biphasic block; the Kd at +40 mV (54 microM) was lower than at +80 (167 microM) and -80 mV (78 microM). 2. For internal polyamines relief from block was most pronounced for spermine, weaker for N-(4-hydroxyphenylpropanoyl)-spermine (PPS), and virtually absent for philanthotoxin 343 (PhTX 343), suggesting that permeation of polyamines varies with cross-sectional width (spermine, 0.44 nm; PPS, 0.70 nm; PhTX 343, 0.75 nm). 3. With putrescine, spermidine, or spermine as sole external cations, inward currents at -120 mV confirmed permeation of polyamines. For bi-ionic conditions with 90 mM polyamine and 150 mM Na+i, reversal potentials were -12.4 mV for putrescine (permeability ratio relative to Na+, PPut/PNa = 0.42) and -32.7 mV for spermidine (PSpd/PNa = 0.07). Currents carried by spermine were too small to analyse accurately in the majority of patches. 4. Increasing [Na+]i from 44 to 330 mM had no effect on the potential for 50% block (V1/2) by 30 microM internal spermine; however, relief from block at positive membrane potentials increased with [Na+]i. In contrast, raising [Na+]o from 44 to 330 mM resulted in a depolarizing shift in V1/2, indicating a strong interaction between internal polyamines and external permeant ions. 5. The Woodhull infinite barrier model of ion channel block adequately described the action of spermine at membrane potentials insufficient to produce relief from block. For 30 microM internal spermine such analysis gave Kd(O) = 2.5 microM, z theta = 1.97; block by 30 microM external spermine was weaker and less voltage dependent (Kd(O) = 37.8 microM and z delta = 0.55); delta and theta are electrical distances measured from the outside and inside, respectively. 6. Fits of the Woodhull equation for a permeable blocker adequately described both onset and relief from block by spermine over a wide range of membrane potentials. However, the rate constants and z delta values estimated for block by internal spermine predicted much stronger external block than was measured experimentally, and vice versa. 7. An Eyring rate theory model with two energy wells and three barriers explained qualitatively many characteristic features of the action of polyamines on GluRs, including biphasic I-V relationships, weaker block by external than internal spermine and low permeability.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aleksandrov A., Velimirovic B., Clapham D. E. Inward rectification of the IRK1 K+ channel reconstituted in lipid bilayers. Biophys J. 1996 Jun;70(6):2680–2687. doi: 10.1016/S0006-3495(96)79837-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begenisich T. B., Cahalan M. D. Sodium channel permeation in squid axons. I: Reversal potential experiments. J Physiol. 1980 Oct;307:217–242. doi: 10.1113/jphysiol.1980.sp013432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowie D., Mayer M. L. Inward rectification of both AMPA and kainate subtype glutamate receptors generated by polyamine-mediated ion channel block. Neuron. 1995 Aug;15(2):453–462. doi: 10.1016/0896-6273(95)90049-7. [DOI] [PubMed] [Google Scholar]
- Brooks S. P., Storey K. B. Bound and determined: a computer program for making buffers of defined ion concentrations. Anal Biochem. 1992 Feb 14;201(1):119–126. doi: 10.1016/0003-2697(92)90183-8. [DOI] [PubMed] [Google Scholar]
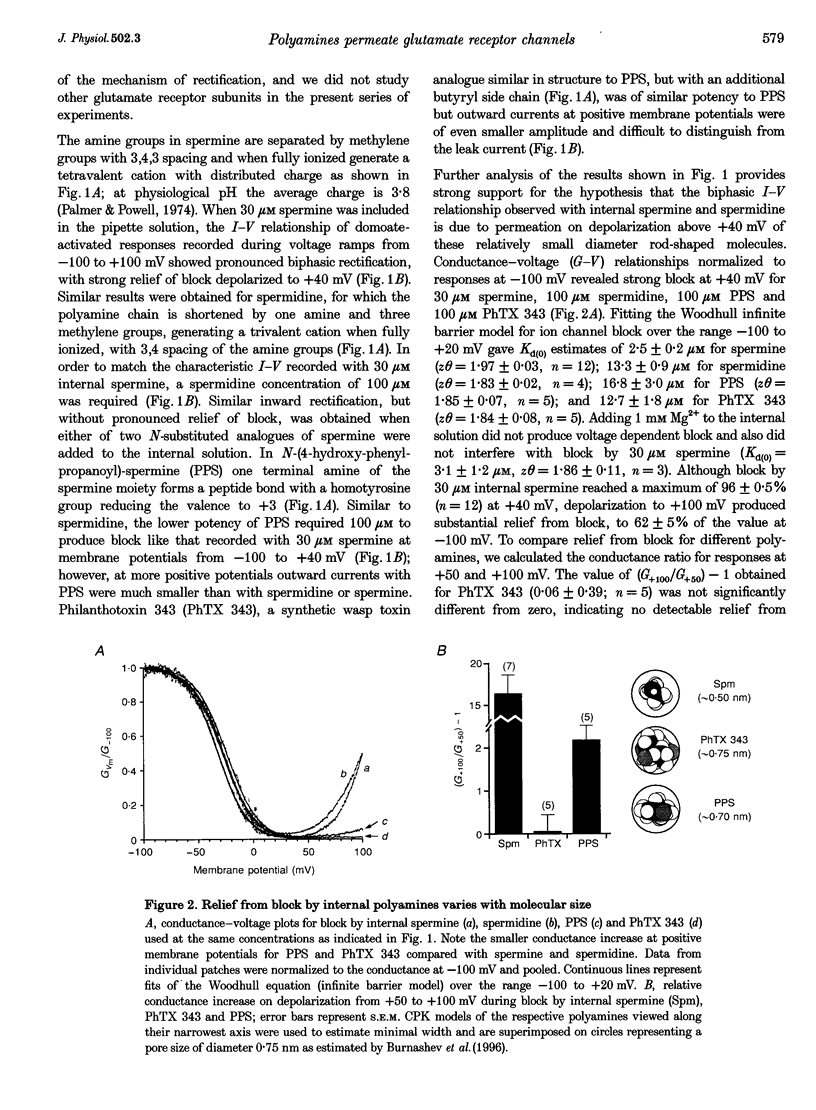
- Burnashev N., Villarroel A., Sakmann B. Dimensions and ion selectivity of recombinant AMPA and kainate receptor channels and their dependence on Q/R site residues. J Physiol. 1996 Oct 1;496(Pt 1):165–173. doi: 10.1113/jphysiol.1996.sp021674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Hawkes A. G. A note on correlations in single ion channel records. Proc R Soc Lond B Biol Sci. 1987 Feb 23;230(1258):15–52. doi: 10.1098/rspb.1987.0008. [DOI] [PubMed] [Google Scholar]
- Davies N. W., McKillen H. C., Stanfield P. R., Standen N. B. A rate theory model for Mg2+ block of ATP-dependent potassium channels of rat skeletal muscle. J Physiol. 1996 Feb 1;490(Pt 3):817–826. doi: 10.1113/jphysiol.1996.sp021189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingledine R., Hume R. I., Heinemann S. F. Structural determinants of barium permeation and rectification in non-NMDA glutamate receptor channels. J Neurosci. 1992 Oct;12(10):4080–4087. doi: 10.1523/JNEUROSCI.12-10-04080.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donevan S. D., Rogawski M. A. Intracellular polyamines mediate inward rectification of Ca(2+)-permeable alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors. Proc Natl Acad Sci U S A. 1995 Sep 26;92(20):9298–9302. doi: 10.1073/pnas.92.20.9298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doupnik C. A., Davidson N., Lester H. A. The inward rectifier potassium channel family. Curr Opin Neurobiol. 1995 Jun;5(3):268–277. doi: 10.1016/0959-4388(95)80038-7. [DOI] [PubMed] [Google Scholar]
- Fakler B., Brändle U., Bond C., Glowatzki E., König C., Adelman J. P., Zenner H. P., Ruppersberg J. P. A structural determinant of differential sensitivity of cloned inward rectifier K+ channels to intracellular spermine. FEBS Lett. 1994 Dec 19;356(2-3):199–203. doi: 10.1016/0014-5793(94)01258-x. [DOI] [PubMed] [Google Scholar]
- Fakler B., Brändle U., Glowatzki E., Weidemann S., Zenner H. P., Ruppersberg J. P. Strong voltage-dependent inward rectification of inward rectifier K+ channels is caused by intracellular spermine. Cell. 1995 Jan 13;80(1):149–154. doi: 10.1016/0092-8674(95)90459-x. [DOI] [PubMed] [Google Scholar]
- Ficker E., Taglialatela M., Wible B. A., Henley C. M., Brown A. M. Spermine and spermidine as gating molecules for inward rectifier K+ channels. Science. 1994 Nov 11;266(5187):1068–1072. doi: 10.1126/science.7973666. [DOI] [PubMed] [Google Scholar]
- French R. J., Wells J. B. Sodium ions as blocking agents and charge carriers in the potassium channel of the squid giant axon. J Gen Physiol. 1977 Dec;70(6):707–724. doi: 10.1085/jgp.70.6.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Miyazaki S., Rosenthal N. P. Potassium current and the effect of cesium on this current during anomalous rectification of the egg cell membrane of a starfish. J Gen Physiol. 1976 Jun;67(6):621–638. doi: 10.1085/jgp.67.6.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
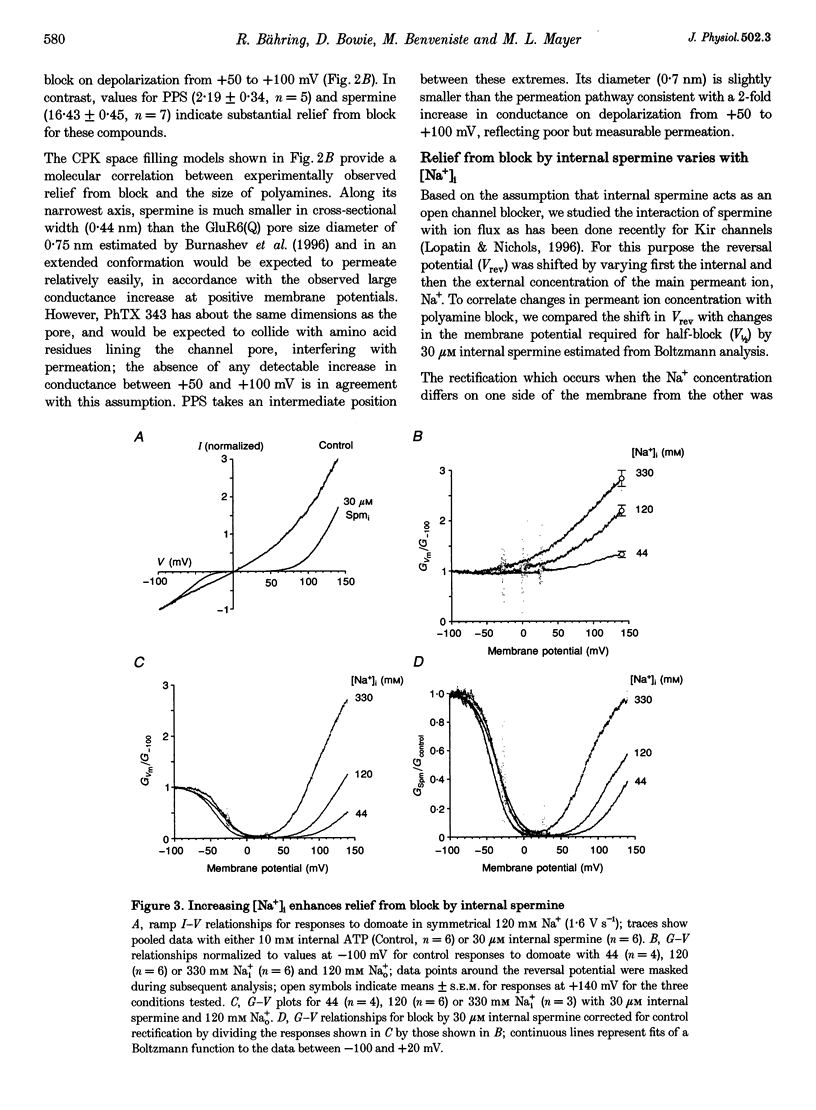
- Hagiwara S., Yoshii M. Effects of internal potassium and sodium on the anomalous rectification of the starfish egg as examined by internal perfusion. J Physiol. 1979 Jul;292:251–265. doi: 10.1113/jphysiol.1979.sp012849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Potassium channels in myelinated nerve. Selective permeability to small cations. J Gen Physiol. 1973 Jun;61(6):669–686. doi: 10.1085/jgp.61.6.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isa T., Iino M., Itazawa S., Ozawa S. Spermine mediates inward rectification of Ca(2+)-permeable AMPA receptor channels. Neuroreport. 1995 Oct 23;6(15):2045–2048. doi: 10.1097/00001756-199510010-00022. [DOI] [PubMed] [Google Scholar]
- Kamboj S. K., Swanson G. T., Cull-Candy S. G. Intracellular spermine confers rectification on rat calcium-permeable AMPA and kainate receptors. J Physiol. 1995 Jul 15;486(Pt 2):297–303. doi: 10.1113/jphysiol.1995.sp020812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh D. S., Burnashev N., Jonas P. Block of native Ca(2+)-permeable AMPA receptors in rat brain by intracellular polyamines generates double rectification. J Physiol. 1995 Jul 15;486(Pt 2):305–312. doi: 10.1113/jphysiol.1995.sp020813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuner T., Wollmuth L. P., Karlin A., Seeburg P. H., Sakmann B. Structure of the NMDA receptor channel M2 segment inferred from the accessibility of substituted cysteines. Neuron. 1996 Aug;17(2):343–352. doi: 10.1016/s0896-6273(00)80165-8. [DOI] [PubMed] [Google Scholar]
- Li-Smerin Y., Johnson J. W. Effects of intracellular Mg2+ on channel gating and steady-state responses of the NMDA receptor in cultured rat neurons. J Physiol. 1996 Feb 15;491(Pt 1):137–150. doi: 10.1113/jphysiol.1996.sp021202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopatin A. N., Makhina E. N., Nichols C. G. Potassium channel block by cytoplasmic polyamines as the mechanism of intrinsic rectification. Nature. 1994 Nov 24;372(6504):366–369. doi: 10.1038/372366a0. [DOI] [PubMed] [Google Scholar]
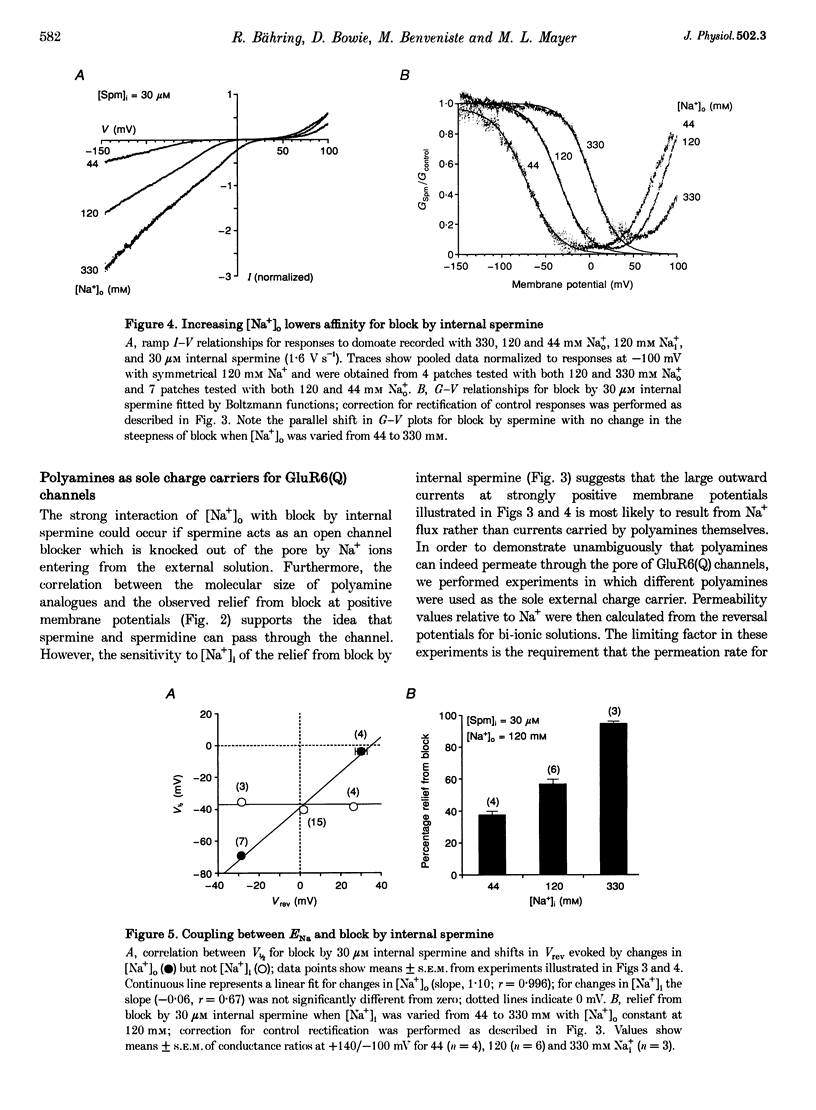
- Lopatin A. N., Nichols C. G. [K+] dependence of polyamine-induced rectification in inward rectifier potassium channels (IRK1, Kir2.1). J Gen Physiol. 1996 Aug;108(2):105–113. doi: 10.1085/jgp.108.2.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z., MacKinnon R. Electrostatic tuning of Mg2+ affinity in an inward-rectifier K+ channel. Nature. 1994 Sep 15;371(6494):243–246. doi: 10.1038/371243a0. [DOI] [PubMed] [Google Scholar]
- Neher E. Correction for liquid junction potentials in patch clamp experiments. Methods Enzymol. 1992;207:123–131. doi: 10.1016/0076-6879(92)07008-c. [DOI] [PubMed] [Google Scholar]
- Ozawa S., Iino M., Tsuzuki K. Suppression by extracellular K+ of N-methyl-D-aspartate responses in cultured rat hippocampal neurons. J Neurophysiol. 1990 Nov;64(5):1361–1367. doi: 10.1152/jn.1990.64.5.1361. [DOI] [PubMed] [Google Scholar]
- Stanfield P. R., Davies N. W., Shelton P. A., Khan I. A., Brammar W. J., Standen N. B., Conley E. C. The intrinsic gating of inward rectifier K+ channels expressed from the murine IRK1 gene depends on voltage, K+ and Mg2+. J Physiol. 1994 Feb 15;475(1):1–7. doi: 10.1113/jphysiol.1994.sp020044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanfield P. R., Davies N. W., Shelton P. A., Sutcliffe M. J., Khan I. A., Brammar W. J., Conley E. C. A single aspartate residue is involved in both intrinsic gating and blockage by Mg2+ of the inward rectifier, IRK1. J Physiol. 1994 Jul 1;478(Pt 1):1–6. doi: 10.1113/jphysiol.1994.sp020225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson G. T., Feldmeyer D., Kaneda M., Cull-Candy S. G. Effect of RNA editing and subunit co-assembly single-channel properties of recombinant kainate receptors. J Physiol. 1996 Apr 1;492(Pt 1):129–142. doi: 10.1113/jphysiol.1996.sp021295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdoorn T. A., Burnashev N., Monyer H., Seeburg P. H., Sakmann B. Structural determinants of ion flow through recombinant glutamate receptor channels. Science. 1991 Jun 21;252(5013):1715–1718. doi: 10.1126/science.1710829. [DOI] [PubMed] [Google Scholar]
- Vyklický L., Jr, Benveniste M., Mayer M. L. Modulation of N-methyl-D-aspartic acid receptor desensitization by glycine in mouse cultured hippocampal neurones. J Physiol. 1990 Sep;428:313–331. doi: 10.1113/jphysiol.1990.sp018214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washburn M. S., Dingledine R. Block of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors by polyamines and polyamine toxins. J Pharmacol Exp Ther. 1996 Aug;278(2):669–678. [PubMed] [Google Scholar]
- Wible B. A., Taglialatela M., Ficker E., Brown A. M. Gating of inwardly rectifying K+ channels localized to a single negatively charged residue. Nature. 1994 Sep 15;371(6494):246–249. doi: 10.1038/371246a0. [DOI] [PubMed] [Google Scholar]
- Wo Z. G., Oswald R. E. Unraveling the modular design of glutamate-gated ion channels. Trends Neurosci. 1995 Apr;18(4):161–168. doi: 10.1016/0166-2236(95)93895-5. [DOI] [PubMed] [Google Scholar]
- Woodhull A. M. Ionic blockage of sodium channels in nerve. J Gen Physiol. 1973 Jun;61(6):687–708. doi: 10.1085/jgp.61.6.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita T., Horio Y., Yamada M., Takahashi N., Kondo C., Kurachi Y. Competition between Mg2+ and spermine for a cloned IRK2 channel expressed in a human cell line. J Physiol. 1996 May 15;493(Pt 1):143–156. doi: 10.1113/jphysiol.1996.sp021370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Jan Y. N., Jan L. Y. Control of rectification and permeation by residues in two distinct domains in an inward rectifier K+ channel. Neuron. 1995 May;14(5):1047–1054. doi: 10.1016/0896-6273(95)90343-7. [DOI] [PubMed] [Google Scholar]