Abstract
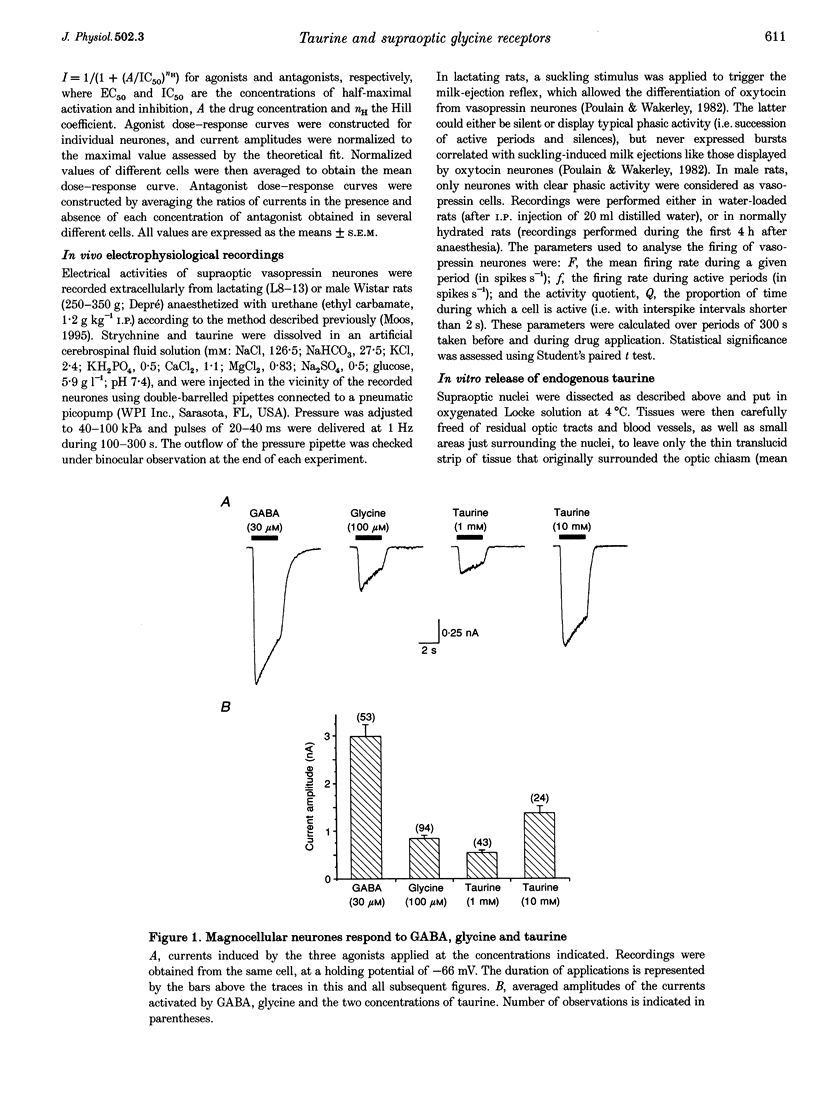
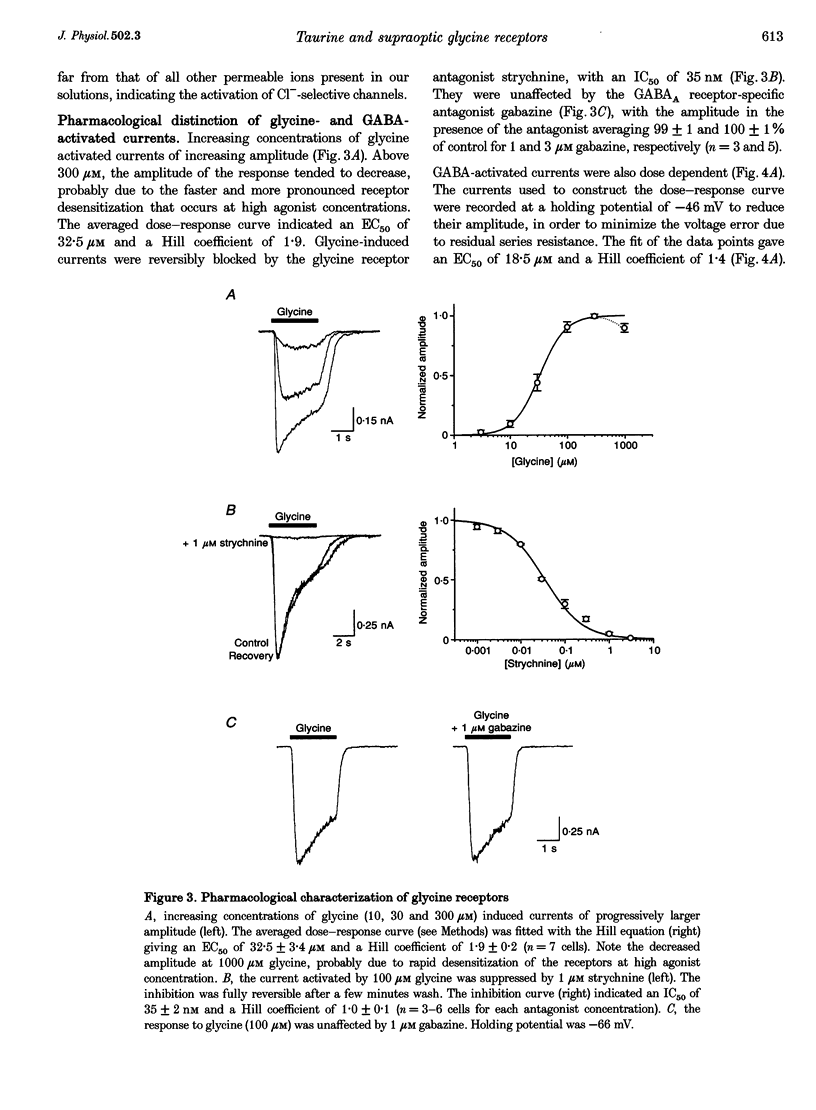
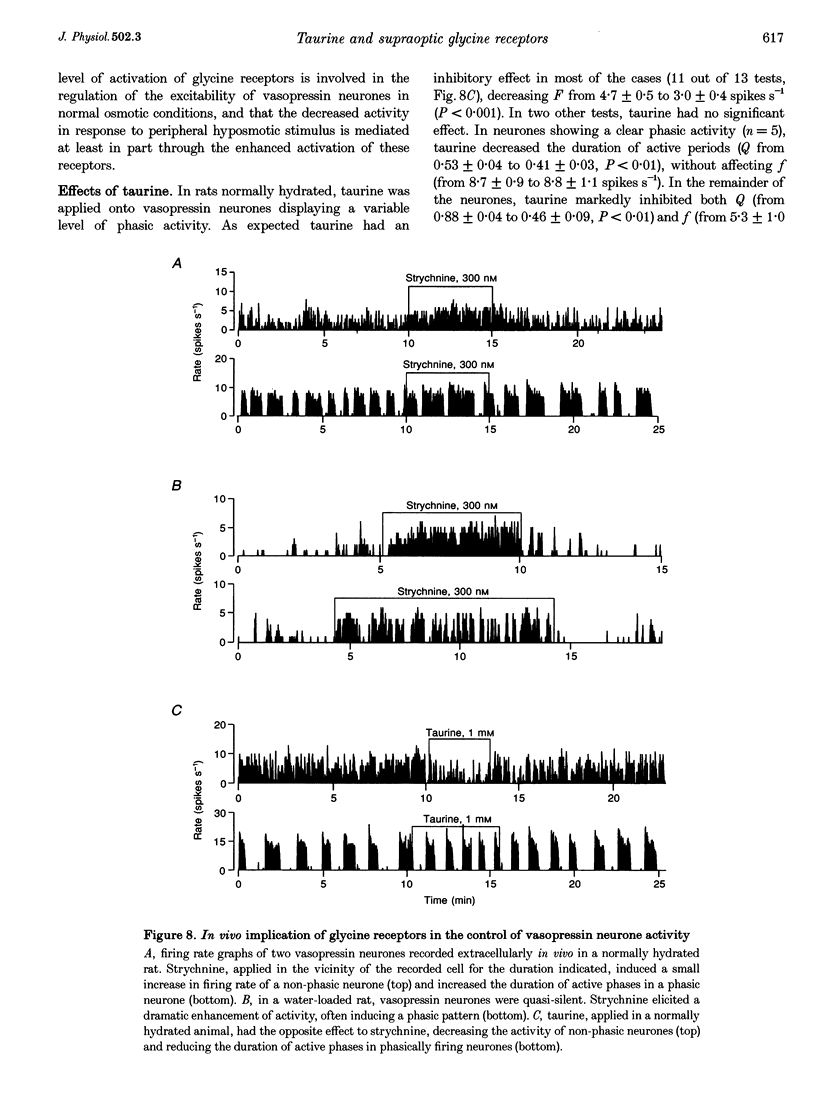
1. To evaluate the implication of taurine in the physiology of supraoptic neurones, we (i) investigated the agonist properties of taurine on glycine and GABAA receptors of supraoptic magnocellular neurones acutely dissociated from adult rats, using whole-cell voltage clamp, (ii) studied the effects of taurine and strychnine in vivo by extracellular recordings of supraoptic vasopressin neurones in anaesthetized rats, and (iii) measured the osmolarity-dependent release of endogenous taurine from isolated supraoptic nuclei by HPLC. 2. GABA, glycine and taurine evoked rapidly activating currents that all reversed close to the equilibrium potential for Cl-, indicating activation of Cl(-)-selective channels. Glycine-activated currents were reversibly blocked by strychnine (IC50 of 35 nM with 100 microM glycine), but were unaffected by the GABAA antagonist gabazine (1-3 microM). GABA-activated currents were reversibly antagonized by 3 microM gabazine, but not by strychnine (up to 1 microM). 3. Responses to 1 mM taurine were blocked by strychnine but not by gabazine and showed no additivity with glycine-induced currents, indicating selective activation of glycine receptors. Responses to 10 mM taurine were partially antagonized by gabazine, the residual current being blocked by strychnine. Thus, taurine is also a weak agonist of GABAA receptors. 4. In the presence of gabazine, taurine activated glycine receptors with an EC50 of 406 microM. Taurine activated at most 70% of maximal glycine currents, suggesting that it is a partial agonist of glycine receptors. 5. In vivo, locally applied strychnine (300 nM) increased and taurine (1 mM) decreased the basal electrical activity of vasopressin neurones in normally hydrated rats. The effect of strychnine was markedly more pronounced in water-loaded rats. 6. Taurine, which is concentrated in supraoptic glial cells, could be released from isolated supraoptic nuclei upon hyposmotic stimulation. Decreases in osmolarity of 15 and 30% specifically enhanced basal release of taurine by 42 and 124%, respectively. 7. We conclude that supraoptic neurones express high amounts of glycine receptors, of which taurine may be regarded as a major natural agonist. We postulate that taurine, which can be released in hyposmotic situations, acts on glycine receptors to exert an inhibitory control on magnocellular neurones during alterations of body fluid homeostasis, implicating an active participation of glial cells in this neuroendocrine regulatory loop.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akaike N., Kaneda M. Glycine-gated chloride current in acutely isolated rat hypothalamic neurons. J Neurophysiol. 1989 Dec;62(6):1400–1409. doi: 10.1152/jn.1989.62.6.1400. [DOI] [PubMed] [Google Scholar]
- Betz H. Structure and function of inhibitory glycine receptors. Q Rev Biophys. 1992 Nov;25(4):381–394. doi: 10.1017/s0033583500004340. [DOI] [PubMed] [Google Scholar]
- Bourque C. W., Oliet S. H., Richard D. Osmoreceptors, osmoreception, and osmoregulation. Front Neuroendocrinol. 1994 Sep;15(3):231–274. doi: 10.1006/frne.1994.1010. [DOI] [PubMed] [Google Scholar]
- Bureau M. H., Olsen R. W. Taurine acts on a subclass of GABAA receptors in mammalian brain in vitro. Eur J Pharmacol. 1991 May 25;207(1):9–16. doi: 10.1016/s0922-4106(05)80031-8. [DOI] [PubMed] [Google Scholar]
- Decavel C., Hatton G. I. Taurine immunoreactivity in the rat supraoptic nucleus: prominent localization in glial cells. J Comp Neurol. 1995 Mar 27;354(1):13–26. doi: 10.1002/cne.903540103. [DOI] [PubMed] [Google Scholar]
- Decavel C., Van den Pol A. N. GABA: a dominant neurotransmitter in the hypothalamus. J Comp Neurol. 1990 Dec 22;302(4):1019–1037. doi: 10.1002/cne.903020423. [DOI] [PubMed] [Google Scholar]
- Enz R., Bormann J. Expression of glycine receptor subunits and gephyrin in single bipolar cells of the rat retina. Vis Neurosci. 1995 May-Jun;12(3):501–507. doi: 10.1017/s0952523800008403. [DOI] [PubMed] [Google Scholar]
- Holopainen I., Kontro P., Oja S. S. Release of taurine from cultured cerebellar granule cells and astrocytes: co-release with glutamate. Neuroscience. 1989;29(2):425–432. doi: 10.1016/0306-4522(89)90069-9. [DOI] [PubMed] [Google Scholar]
- Horikoshi T., Asanuma A., Yanagisawa K., Anzai K., Goto S. Taurine and beta-alanine act on both GABA and glycine receptors in Xenopus oocyte injected with mouse brain messenger RNA. Brain Res. 1988 Sep;464(2):97–105. doi: 10.1016/0169-328x(88)90002-2. [DOI] [PubMed] [Google Scholar]
- Huxtable R. J. Physiological actions of taurine. Physiol Rev. 1992 Jan;72(1):101–163. doi: 10.1152/physrev.1992.72.1.101. [DOI] [PubMed] [Google Scholar]
- Kaneda M., Farrant M., Cull-Candy S. G. Whole-cell and single-channel currents activated by GABA and glycine in granule cells of the rat cerebellum. J Physiol. 1995 Jun 1;485(Pt 2):419–435. doi: 10.1113/jphysiol.1995.sp020739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimelberg H. K., Goderie S. K., Higman S., Pang S., Waniewski R. A. Swelling-induced release of glutamate, aspartate, and taurine from astrocyte cultures. J Neurosci. 1990 May;10(5):1583–1591. doi: 10.1523/JNEUROSCI.10-05-01583.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishtal O. A., Osipchuk YuV, Vrublevsky S. V. Properties of glycine-activated conductances in rat brain neurones. Neurosci Lett. 1988 Feb 3;84(3):271–276. doi: 10.1016/0304-3940(88)90519-8. [DOI] [PubMed] [Google Scholar]
- Kudo Y., Akiyoshi E., Akagi H. Identification of two taurine receptor subtypes on the primary afferent terminal of frog spinal cord. Br J Pharmacol. 1988 Aug;94(4):1051–1056. doi: 10.1111/j.1476-5381.1988.tb11621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert R. C., Dayanithi G., Moos F. C., Richard P. A rise in the intracellular Ca2+ concentration of isolated rat supraoptic cells in response to oxytocin. J Physiol. 1994 Jul 15;478(Pt 2):275–287. doi: 10.1113/jphysiol.1994.sp020249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malminen O., Kontro P. Modulation of the GABA-benzodiazepine receptor complex by taurine in rat brain membranes. Neurochem Res. 1986 Jan;11(1):85–94. doi: 10.1007/BF00965168. [DOI] [PubMed] [Google Scholar]
- Martin D. L., Madelian V., Seligmann B., Shain W. The role of osmotic pressure and membrane potential in K(+)-stimulated taurine release from cultured astrocytes and LRM55 cells. J Neurosci. 1990 Feb;10(2):571–577. doi: 10.1523/JNEUROSCI.10-02-00571.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton J. E., Nattie E. E. Brain and CSF water and ions during dilutional and isosmotic hyponatremia in the rat. Am J Physiol. 1983 May;244(5):R724–R732. doi: 10.1152/ajpregu.1983.244.5.R724. [DOI] [PubMed] [Google Scholar]
- Melton J. E., Patlak C. S., Pettigrew K. D., Cserr H. F. Volume regulatory loss of Na, Cl, and K from rat brain during acute hyponatremia. Am J Physiol. 1987 Apr;252(4 Pt 2):F661–F669. doi: 10.1152/ajprenal.1987.252.4.F661. [DOI] [PubMed] [Google Scholar]
- Moos F. C. GABA-induced facilitation of the periodic bursting activity of oxytocin neurones in suckled rats. J Physiol. 1995 Oct 1;488(Pt 1):103–114. doi: 10.1113/jphysiol.1995.sp020949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabekura J., Omura T., Akaike N. Alpha 2 adrenoceptor potentiates glycine receptor-mediated taurine response through protein kinase A in rat substantia nigra neurons. J Neurophysiol. 1996 Oct;76(4):2447–2454. doi: 10.1152/jn.1996.76.4.2447. [DOI] [PubMed] [Google Scholar]
- Neher E. Correction for liquid junction potentials in patch clamp experiments. Methods Enzymol. 1992;207:123–131. doi: 10.1016/0076-6879(92)07008-c. [DOI] [PubMed] [Google Scholar]
- Oliet S. H., Bourque C. W. Osmoreception in magnocellular neurosecretory cells: from single channels to secretion. Trends Neurosci. 1994 Aug;17(8):340–344. doi: 10.1016/0166-2236(94)90177-5. [DOI] [PubMed] [Google Scholar]
- Oliet S. H., Bourque C. W. Properties of supraoptic magnocellular neurones isolated from the adult rat. J Physiol. 1992 Sep;455:291–306. doi: 10.1113/jphysiol.1992.sp019302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottersen O. P., Storm-Mathisen J., Somogyi P. Colocalization of glycine-like and GABA-like immunoreactivities in Golgi cell terminals in the rat cerebellum: a postembedding light and electron microscopic study. Brain Res. 1988 May 31;450(1-2):342–353. doi: 10.1016/0006-8993(88)91573-9. [DOI] [PubMed] [Google Scholar]
- Pasantes Morales H., Schousboe A. Volume regulation in astrocytes: a role for taurine as an osmoeffector. J Neurosci Res. 1988 Aug;20(4):503–509. doi: 10.1002/jnr.490200415. [DOI] [PubMed] [Google Scholar]
- Pasantes-Morales H., Moran J., Schousboe A. Volume-sensitive release of taurine from cultured astrocytes: properties and mechanism. Glia. 1990;3(5):427–432. doi: 10.1002/glia.440030514. [DOI] [PubMed] [Google Scholar]
- Pasantes-Morales H., Schousboe A. Release of taurine from astrocytes during potassium-evoked swelling. Glia. 1989;2(1):45–50. doi: 10.1002/glia.440020105. [DOI] [PubMed] [Google Scholar]
- Poulain D. A., Wakerley J. B. Electrophysiology of hypothalamic magnocellular neurones secreting oxytocin and vasopressin. Neuroscience. 1982 Apr;7(4):773–808. doi: 10.1016/0306-4522(82)90044-6. [DOI] [PubMed] [Google Scholar]
- Quinn M. R., Miller C. L. Taurine allosterically modulates flunitrazepam binding to synaptic membranes. J Neurosci Res. 1992 Sep;33(1):136–141. doi: 10.1002/jnr.490330117. [DOI] [PubMed] [Google Scholar]
- Rampon C., Luppi P. H., Fort P., Peyron C., Jouvet M. Distribution of glycine-immunoreactive cell bodies and fibers in the rat brain. Neuroscience. 1996 Dec;75(3):737–755. doi: 10.1016/0306-4522(96)00278-3. [DOI] [PubMed] [Google Scholar]
- Randle J. C., Bourque C. W., Renaud L. P. Characterization of spontaneous and evoked inhibitory postsynaptic potentials in rat supraoptic neurosecretory neurons in vitro. J Neurophysiol. 1986 Dec;56(6):1703–1717. doi: 10.1152/jn.1986.56.6.1703. [DOI] [PubMed] [Google Scholar]
- Randle J. C., Renaud L. P. Actions of gamma-aminobutyric acid on rat supraoptic nucleus neurosecretory neurones in vitro. J Physiol. 1987 Jun;387:629–647. doi: 10.1113/jphysiol.1987.sp016592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard D., Bourque C. W. Synaptic control of rat supraoptic neurones during osmotic stimulation of the organum vasculosum lamina terminalis in vitro. J Physiol. 1995 Dec 1;489(Pt 2):567–577. doi: 10.1113/jphysiol.1995.sp021073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmieden V., Kuhse J., Betz H. Agonist pharmacology of neonatal and adult glycine receptor alpha subunits: identification of amino acid residues involved in taurine activation. EMBO J. 1992 Jun;11(6):2025–2032. doi: 10.1002/j.1460-2075.1992.tb05259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokutomi N., Kaneda M., Akaike N. What confers specificity on glycine for its receptor site? Br J Pharmacol. 1989 Jun;97(2):353–360. doi: 10.1111/j.1476-5381.1989.tb11961.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl P., Elster L., Schousboe A. Identification and function of glycine receptors in cultured cerebellar granule cells. J Neurochem. 1994 Jun;62(6):2457–2463. doi: 10.1046/j.1471-4159.1994.62062457.x. [DOI] [PubMed] [Google Scholar]
- Winder D. G., Ritch P. S., Gereau R. W., 4th, Conn P. J. Novel glial-neuronal signalling by coactivation of metabotropic glutamate and beta-adrenergic receptors in rat hippocampus. J Physiol. 1996 Aug 1;494(Pt 3):743–755. doi: 10.1113/jphysiol.1996.sp021529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuarin J. P., Dudek F. E. Patch-clamp analysis of spontaneous synaptic currents in supraoptic neuroendocrine cells of the rat hypothalamus. J Neurosci. 1993 Jun;13(6):2323–2331. doi: 10.1523/JNEUROSCI.13-06-02323.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Pol A. N., Gorcs T. Glycine and glycine receptor immunoreactivity in brain and spinal cord. J Neurosci. 1988 Feb;8(2):472–492. doi: 10.1523/JNEUROSCI.08-02-00472.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]


